In this issue of Blood, Nixon and colleagues report on the impact of HIV infection on hematopoiesis. Hematopoietic stem cells (HSCs)/progenitor cells were subjected to HIV infection in vitro and in vivo using a humanized mouse model. They conclude that direct infection of intermediate progenitor cells by HIV adversely affects their hematopoietic potential, resulting in the observed cytopenias in HIV patients.1
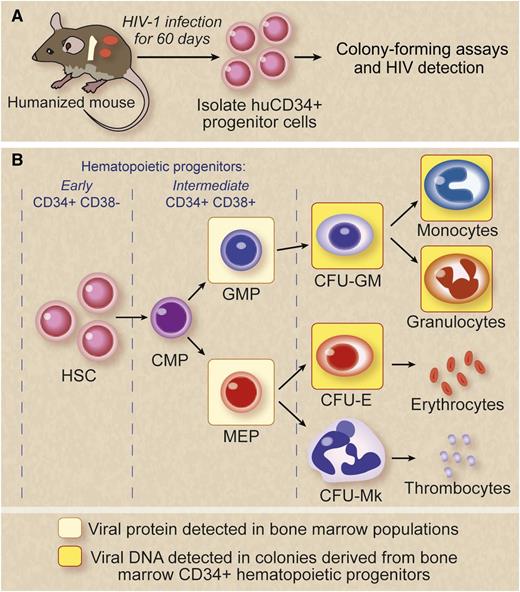
HIV infection impairs HPCs. A combination of colony-forming and viral detection assays were used on in vitro– and in vivo–infected HPCs to demonstrate hematological abnormalities. Marked viral-induced suppression was seen on intermediate progenitor cells. (A) For in vivo studies, a humanized BM, liver, and thymus mouse model that supports human hematopoiesis and is susceptible to HIV infection was used. Sixty days postinfection, HPCs were examined for the presence of viral protein expression by flow cytometry and viral DNA by PCR. (B) Viral protein expression was detected in the GMP and the MEP cells. Colony assays showed HPC impairment. HIV-1 infection was detected in multiple populations of intermediate HPCs and their progeny from BM-derived cells of HIV-1–exposed mice. HIV genomes were detected in a granulocyte, macrophage, and erythroid colonies. Viral protein/DNA detection is indicated in colored boxes. CFU-E, colony-forming unit erythroid; CFU-GM, colony-forming unit granulocyte macrophage; CFU-Mk, colony-forming unit megakaryocyte. Professional illustration by Debra T. Dartez.
HIV infection impairs HPCs. A combination of colony-forming and viral detection assays were used on in vitro– and in vivo–infected HPCs to demonstrate hematological abnormalities. Marked viral-induced suppression was seen on intermediate progenitor cells. (A) For in vivo studies, a humanized BM, liver, and thymus mouse model that supports human hematopoiesis and is susceptible to HIV infection was used. Sixty days postinfection, HPCs were examined for the presence of viral protein expression by flow cytometry and viral DNA by PCR. (B) Viral protein expression was detected in the GMP and the MEP cells. Colony assays showed HPC impairment. HIV-1 infection was detected in multiple populations of intermediate HPCs and their progeny from BM-derived cells of HIV-1–exposed mice. HIV genomes were detected in a granulocyte, macrophage, and erythroid colonies. Viral protein/DNA detection is indicated in colored boxes. CFU-E, colony-forming unit erythroid; CFU-GM, colony-forming unit granulocyte macrophage; CFU-Mk, colony-forming unit megakaryocyte. Professional illustration by Debra T. Dartez.
Patients with long-term HIV infection often exhibit multiple hematopoietic syndromes that encompass anemia, granulocytopenia, and thrombocytopenia, suggesting a central deficiency in hematopoiesis.2 A number of previous studies attempted to delineate the mechanism by which these conditions ensue.3 However, clear understanding of the impairment mechanism(s) remained an intractable problem because of the paucity of studies using a suitable experimental animal model that closely recapitulates human hematopoiesis during an ongoing HIV infection in vivo.
Three main possibilities exist for the observed hematological abnormalities.2,3 The first being the direct productive HIV infection of the early HSCs themselves with resultant deleterious effects. Although many previous studies were unable to detect HIV infection of CD34+ HSCs, more recent evidence pointed to infection in at least a subset of individuals.2-6 However, its impact other than in viral latency is unclear. Effects of HIV on intermediate progenitors were not fully evaluated until the present study. Second, even without direct productive infection, it is possible that HIV proteins such as the envelope protein and/or abnormal levels of cytokine milieu in the bone marrow (BM) of infected individuals may have indirect effects on hematopoietic progenitors, with many previous studies attesting to this. Alternatively, prolonged antiretroviral therapy in conjunction with other frequently used drugs in these patients may compromise the BM microenvironment, with resultant adverse effects on differentiating hematopoietic cells of various lineages. Accumulated evidence thus far suggests that a combination of these factors may play a role and contribute to overall hematopoietic deficiency. However, teasing out the individual contribution of each of these factors in vivo has proven to be difficult.
The studies of Nixon et al1 exploited a humanized mouse model to extend the results seen with direct in vitro exposure/infection of CD34+ HSCs and their later intermediate progenitor cells. The new-generation humanized mice derived by transplantation of human HSCs are capable of multilineage hematopoiesis and are susceptible to HIV infection showing chronic viremia and associated CD4 helper T-cell loss.7 In these mice, the transplanted HSCs home to the BM and set up residence. Here a BM, liver, and thymus humanized mouse version was used (see figure) because it is more appropriate than the previously used severe combined immunodeficiency-hu thy/liv (SCID-hu) mice that lack the human HSC engraftment in BM.8
Effects of HIV infection on hematopoietic progenitor cells (HPCs) were evaluated in vivo in the absence of long-term highly active antiretroviral treatment, thus precluding the potential impact of antiretroviral drugs on hematopoiesis. Another important aspect of the current study is the systematic correlation of in vitro data with that of the results obtained from in vivo data. The first set of experiments evaluated infection of a mixed population of CD34+ HSCs with an envelope glycoprotein of the vesicular stomatitis virus–pseudotyped HIV devoid of env and vpr genes, thus precluding their potential direct/indirect effects on hematopoiesis. Colony-forming unit (CFU) assays revealed both a decrease in their colony size and number. Yields of erythroid, megakaryocyte, and macrophage colonies were found to be negatively impacted. Subpopulations of intermediate HSCs—common myeloid progenitor (CMP) and granulocyte-monocyte progenitor (GMP) but not megakaryocyte-erythroid progenitor (MEP)—were shown to coexpress HIV co-receptors, either CCR5 or CXCR4 together with the primary receptor CD4. Infection of purified populations of CMP, GMP, and MEP with a dual-tropic HIV resulted in infection of these cells (albeit a small proportion), indicating their virus susceptibility. MEPs not coexpressing CD4 with either CCR5 or CXCR4 were found to be even more infection-prone however, suggesting CD4-independent entry that needs to be further investigated. In the in vivo experiments, CD34+ cells isolated from HIV-infected humanized mice with 3 different viral strains were positive for viral sequences by polymerase chain reaction (PCR) irrespective of viral tropism. When CD34+ cells isolated from these infected mice BM were evaluated by CFU assays, generation of all lineages were found to be adversely affected, erythroid cells in particular, with the exception of granulocytes. In vivo–infected CD34+ cells showed full-length viral DNA, demonstrating that these cells (although impaired) do survive and give rise to colonies in vitro, albeit smaller (see figure).
Although these studies made a good start in developing an experimentally amenable in vivo system, several critical questions remain and further studies are needed to validate this system further. (1) Are the respective cell lineages harboring the proviral DNA productively infected and contribute to the spread the virus? (2) Why are certain lineages more profoundly affected than others and what is the mechanism? (3) The current studies are performed with a limited number of viral clones representing only a few viral variants; therefore, how does the infection affect HPCs with patient-derived primary isolates (consisting of viral swarms with mutated sequences) from early stages vs late stages of infection when the hematological abnormalities are more severe? It is essential that results obtained from these mouse studies be verified by comparing them with those from the HIV-infected individuals at different stages of the disease. For example, it needs to be determined if intermediate hematopoietic progenitor cell populations obtained from highly active antiretroviral treatment-naive patients harbor HIV provirus and are consequently impaired in their development.
In summary, these studies broke new ground and established that certain intermediate hematopoietic cells are virus-susceptible during an ongoing HIV infection in vivo, resulting in their impairment. In addition, this work also demonstrated the utility of a humanized mouse model to further evaluate important questions on HIV-mediated hematological abnormalities.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal