To the editor:
The most frequent mature aggressive B-cell lymphomas are diffuse large B-cell lymphoma (DLBCL) and Burkitt lymphoma (BL). Patients suffering from molecularly defined BL (mBL) but treated with a regimen developed for DLBCL show an unfavorable outcome compared with mBL treated with chemotherapy regimens for BL.1 Distinguishing BL from DLBCL by conventional histopathology is challenging in lymphomas that have features common to both diseases (aggressive B-cell lymphoma unclassifiable with features of DLBCL and BL [intermediates]).2 Moreover, DLBCLs are a heterogeneous group of lymphomas comprising distinct molecular subtypes: the activated B-cell–like (ABC), the germinal center B-cell–like (GCB), and the unclassifyable subtype as defined by gene expression profiling (GEP).3 Attempts to replace GEP with techniques applicable to formalin-fixed paraffin-embedded (FFPE) tissue led to algorithms for immunohistochemical staining (IHS).4 Disappointingly, the algorithms yielded conflicting results with respect to their prognostic potential, raising concerns about their validity.5 Furthermore, IHS algorithms did not provide a fully resolved classification: they did not identify mBL nor did they separate ABC from unclassified DLBCLs.4
We used digital multiplexed gene expression (DMGE) with FFPE-derived RNA to classify agressive B-cell lymphomas. Our assay comprised only 30 genes (10 for the detection of mBL and 20 for the detection of ABC and GCB). We chose these genes by reanalysis of the microarray data reported in a previous study.6 A detailed description of the methods is provided in the supplemental Materials on the Blood website. Thirty-nine samples from mature aggressive B-cell lymphomas were analyzed using DMGE (nCounter; NanoString Technologies, Seattle, WA; see supplemental Materials for detailed methods) of FFPE- and fresh-frozen–derived RNA. All cases were previously characterized by the Molecular Mechanisms of Malignant Lymphoma6 consortium using the Affymetrix GeneChip technology (gold standard of classification).
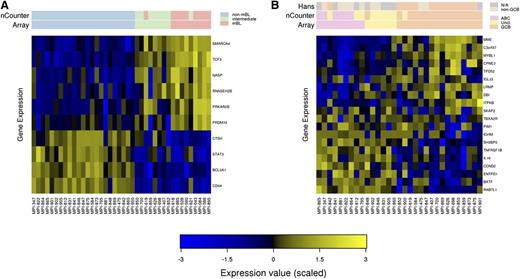
For FFPE-derived RNA, the classification of only 2 of 39 samples (5%) differed, when comparing DMGE-based predictions with the gold standard (array) (Figure 1A). The 2 divergent classifications were 1 case of mBL that was classified as intermediate and 1 case of an intermediate that was classified as mBL [both lymphomas carried a t(8;14) translocation]. No major mistake (a classification of a non-mBL as mBL or vice versa) was observed. This performance is comparable with that of DMGE data from fresh-frozen tissue blocks (supplemental Materials). Compared with the array, 5 of 31 (16%) non-mBL/DLBCLs received different molecular classifications using DMGE and FFPE-derived RNA (Figure 1B). All discrepancies comprised lymphomas that switched between the unclassified and ABC or unclassified and GCB labels. Again, no major mistake (a classification of a GCB as an ABC or vice versa) was observed. In contrast, the Hans IHS algorithm7 led to major mistakes (2 GCB classified as non-GCB and 1 ABC as GCB; Figure 1B).
Digital multiplexed gene expression of mature aggressive B-cell lymphomas. (A) Molecular classification of mature aggressive B-cell lymphomas as mBL, non-mBL, and intermediates using FFPE-derived RNA. The expression of the genes is color coded such that high expression is shown in yellow and low expression in blue. The molecular labels assigned by the array using fresh-frozen RNA and DMGE (nCounter) assay using FFPE-derived RNA are indicated as colored labels in the top bars. (B) Molecular classification of non-mBL using FFPE-derived RNA. The classification according to IHS (Hans), the array using fresh-frozen RNA and the DMGE assay using FFPE-derived RNA are indicated as colored labels in the top bars.
Digital multiplexed gene expression of mature aggressive B-cell lymphomas. (A) Molecular classification of mature aggressive B-cell lymphomas as mBL, non-mBL, and intermediates using FFPE-derived RNA. The expression of the genes is color coded such that high expression is shown in yellow and low expression in blue. The molecular labels assigned by the array using fresh-frozen RNA and DMGE (nCounter) assay using FFPE-derived RNA are indicated as colored labels in the top bars. (B) Molecular classification of non-mBL using FFPE-derived RNA. The classification according to IHS (Hans), the array using fresh-frozen RNA and the DMGE assay using FFPE-derived RNA are indicated as colored labels in the top bars.
GEP-based molecular classification of mature aggressive B-cell lymphomas is possible with FFPE-derived RNA at a reasonable cost (<100 Euro) and within a reasonable period of time (24 hours). To the best of our knowledge, this is the first molecular method for identifying mBL among mature aggressive B-cell lymphomas using FFPE-derived RNA.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Olivera Batic for excellent technical support.
This work was supported by the KinderKrebsInitiative Buchholz/Holm-Seppensen, an intramural grant from the Medical Faculty of the University of Kiel to W.K. (grant F343911), and the Molecular Mechanisms of Malignant Lymphoma with MYC-Dysregulation - A System Biology Approach for Genetics, Evolution, Signaling and Clinical Care (MMML-MYC-SYS) funded by the German Ministry of Health (grant 0316166).
Contribution: N.M.-S. and M.S. performed DMGE analysis; C.W.K. and R.S. performed bioinformatic analysis; W.K. designed research and provided funding; and N.M.-S., M.S., C.W.K., R.S., and W.K. were involved in the writing of the manuscript and approved the final manuscript.
Conflict of interest: The authors declare no competing financial interests.
Correspondence: Wolfram Klapper, Department of Pathology, Hematopathology Section and Lymph Node Registry, University Hospital Schleswig-Holstein, Campus Kiel/Christian-Albrecht University, Kiel, Arnold-Heller-Str 3, Haus 14, 24105 Kiel, Germany; e-mail: wklapper@path.uni-kiel.de.
References
Author notes
N.M.-S., M.S., and C.W.K. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal