Key Points
Current WHO criteria are inadequate for diagnosing “early-stage” PV.
Hemoglobin and hematocrit values are inadequate surrogate markers for erythrocytosis.
Abstract
We prospectively evaluated the accuracy of the 2007 World Health Organization (WHO) criteria for diagnosing polycythemia vera (PV), especially in “early-stage” patients. A total of 28 of 30 patients were diagnosed as PV owing to an elevated Cr-51 red cell mass (RCM), JAK2 positivity, and at least 1 minor criterion. A total of 18 PV patients did not meet the WHO criterion for an increased hemoglobin value and 8 did not meet the WHO criterion for an increased hematocrit value. Bone marrow morphology was very valuable for diagnosis. Low serum erythropoietin (EPO) values were specific for PV, but normal EPO values were found at presentation (20%). We recommend revision of the WHO criteria, especially to distinguish early-stage PV from essential thrombocythemia. Major criteria remain JAK2 positivity and increased red cell volume, but Cr-51 RCM is mandatory for patients who do not meet the defined elevated hemoglobin or hematocrit value (>18.5 g/dL and 60% in men and >16.5 g/dL and 56% in women, respectively). Minor criteria remain bone marrow histology or a low serum EPO value. For patients with a normal EPO value, marrow examination is mandatory for diagnostic confirmation. Because the therapies for myeloproliferative disorders differ, our data have major clinical implications.
Introduction
In 2007, the World Health Organization (WHO) published consensus criteria for the diagnosis of Philadelphia-negative myeloproliferative neoplasms (MPNs), of which the most common are polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).1 However, these criteria have never been objectively evaluated prospectively.2 The importance of distinguishing these diseases in their early phase rests upon differences in treatment and prognosis.3,4 A recent study,5 for example, showed that patients with PV should have a target hematocrit of less than 45% which significantly lowers the risk of cardiovascular death and major thrombosis.
The 2007 WHO criteria for the diagnosis of PV include 2 major criteria and 3 minor criteria. Major criteria include (1) hemoglobin >18.5 g/dL in men, 16.5 g/dL in women, or other evidence of increased red cell volume (RCV); and (2) presence of JAK2V617F or other functionally similar mutation such as JAK2 exon 12 mutation. Minor criteria include (1) bone marrow biopsy specimen showing hypercellularity for age with trilineage growth (panmyelosis) with prominent erythroid, granulocytic, and megakaryocytic proliferation; (2) serum erythropoietin (EPO) level below the reference range for normal; and (3) endogenous erythroid colony formation in vitro. Diagnosis requires the presence of both major criteria and 1 minor criteria or the presence of the first major criterion together with 2 minor criteria.
It is generally accepted that the JAK2V617F mutation or other JAK2 mutations (exon 12) is present in virtually all PV patients.6
The most important criterion after detection of a JAK2 mutation is the demonstration of an increased RCV. A red cell mass (RCM) value >25% above mean normal predicted value has been repeatedly demonstrated to indicate an absolute increase in RCV.7-11 However, this test is used less frequently since the days of the Polycythemia Vera Study Group (PVSG), when it was mandatory in all cases, irrespective of hematocrit values.12 According to the WHO criteria, the basis of an increased RCV arbitrarily includes a hemoglobin concentration >18.5 g/dL in men and >16.5 g/dL in women, hemoglobin or hematocrit value >99th percentile of institutional normal range for age, sex, altitude of residence, or hemoglobin >17g/dL in men and >15 g/dL in women if a true sustained increase ≥2 g/dL from baseline exists.1 In current clinical practice, hemoglobin values are most often used for estimating RCV, despite previously expressed concerns regarding its limitations as a surrogate marker. Johansson et al9 noted in their study of 77 PV patients that 65% of male PV patients and 37% of female PV patients did not meet the WHO criterion of an elevated hemoglobin value, despite an increased Cr-51 RCM. Similarly, Alvarez-Larran et al10 observed that 46% of their PV patients did not meet the WHO criterion of an elevated hemoglobin value, despite an increased Cr-51 RCM in an evaluation of 71 patients classified as ET owing to normal hemoglobin and hematocrit values. Likewise Cassinat et al11 found that 46.5% of their patients had an elevated RCM and, in fact, had PV. The inaccuracy of using the hematocrit for diagnosing PV has been noted for more than 40 years,12 a fact that has recently been emphasized by others.11,13 Furthermore, an internet search for the normal range for hematocrit values showed variation among institutions.
Alternative methods for measuring RCM other than using Cr-51 have been proposed, especially using plasma volume to derive the RCM. Although Fairbanks et al14 found a high correlation between 125I-albumin-derived RCM and Cr-51 RCM in their study, Moralidis et al15 noted a relatively wide range (±19.5%) of the 95% prediction intervals between the 2 methods. Balga et al16 reported both false positives and false negatives in their study of 264 patients. Of their 146 PV patients, 17 would have been misdiagnosed using 125I-albumin–derived RCM. Conversely, of their 118 patients with normal Cr-51 or 99mTc RCM, 29 would have been misdiagnosed as PV using 125I-albumin–derived RCM. Thus, estimating RCV using 125I-albumin plasma volume has been reported to be inaccurate.
The accuracy of the minor criteria has likewise been debated. Although a low serum EPO value (<4 mU/mL) is of value, such values are found in approximately 85% of PV patients and normal EPO values are observed in the remainder.17
The value of bone marrow histology in differentiating the MPNs has been vigorously discussed, particularly in distinguishing ET from PMF.18,19 In contrast, Thiele et al20 found that bone marrow histopathology can be useful in distinguishing PV from secondary polycythemia and the other MPNs, an observation first noted by the PVSG years ago.21
The last minor criterion, endogenous erythroid colony (EEC) assay, is rarely performed in most countries, except as a research procedure in experimental laboratories. It is not considered useful routinely in recent diagnostic algorithms.17
We therefore evaluated the accuracy of the WHO criteria in diagnosing PV, especially in “early-stage” patients. Unlike any other study, our patients were all followed regularly for 5 years so that the initial diagnosis could be confirmed and the diagnostic criteria used could thus be evaluated.
Materials and methods
We received IRB approval to collect blood, serum, and bone marrow aspirate samples from patients with MPNs to better understand their disease. We received permission to collect medical information from patient charts and to store samples of blood and bone marrow aspirate for future research. Informed consent was obtained in accordance with the Declaration of Helsinki.
We evaluated and followed 30 patients because they had laboratory findings of 2 or more of the following: hemoglobin >16.5 g/dL in men or >14.5 g/dL in women, white blood count >12 000 /uL, platelets >400 000 /uL, or mild splenomegaly. These points were chosen because review of our database showed approximately 75% of our PV patients presented with these findings, consistent with the 2001 WHO criteria for PV.22 The aforementioned characteristics were supplemented by a routine complete blood count and differential distribution, review of the peripheral blood smear, and blood chemistry determinations.
JAK2V617F allele burden was determined both qualitatively by amplification refractory mutation system polymerase chain reaction assay and quantitatively by pyrosequencing.23
RCM determination using Cr-51–labeled red cells and plasma volume determination using radioiodine-labeled serum albumin (125I) were performed as recommended by the International Council for Standardization in Haematology.24 RCM results were expressed as a percentage above mean normal predicted value using the standard formula of Pearson et al.7 The results were also expressed in terms of body weight (mL/kg), which were corrected for an “ideal” body mass for overweight patients (body mass index >25 kg/m2).25
A hematocrit value >99th percentile of the institutional normal range was interpreted as any value outside the normal range and was determined to be >50% for men and >45% for women at our institution.
Bone marrow biopsy slides were prepared using routine methods. Marrow sections were stained with hematoxylin and eosin, Gomori silver stain for reticulin, and trichrome stain for collagen. The Manoharan scale and the European Consensus System were used for evaluating fibrosis.26,27 Marrow interpretation by 2 hematopathologists was “blinded.”
Serum EPO values were obtained within 3 months of diagnosis for 21 patients. Of the remaining 9 patients, serum EPO values were obtained beyond the 3-month window; 5 had normal EPO values but were not counted because of possible treatment effects.28
EEC assays were not performed.
As part of the history, inquiry was made of geographic variations in altitude prior to the study and any changes in hemoglobin and hematocrit values associated with iron supplementation.
All patients have been seen at regular intervals at our institution for a median of 5 years to follow the course of their disease and thus allow confirmation of the original clinical diagnosis.
Results
Clinical characteristics of the 30 MPN patients at the time of diagnostic evaluation are shown in Table 1. There were 17 men and 13 women. The median age of the 30 patients was 53 years (men: 49 years, women: 57 years). A total of 12 patients had an enlarged spleen palpable a median of 2 cm below left costal margin (range: 1-9 cm). No change in altitude or oral iron supplementation was reported in any of our patients. No patient had a true sustained increase ≥2 g/dL from baseline on iron or other hematinics.
Laboratory findings of 30 evaluated MPN patients before diagnosis
| Patients . | Age (y) . | HGB (g/dL) . | HCT % . | WBC (K/μL) . | RBC (M/μL) . | PLT (K/μL) . |
|---|---|---|---|---|---|---|
| Men (n = 17) | 49 (28-73) | 17.2 (15.6-20.2) | 50.3 (45.7-54.9) | 10.5 (7.4-22.4) | 5.71 (4.92-6.69) | 772 (189-1082) |
| Women (n = 13) | 57 (26-72) | 16.8 (14.4-19.3) | 49.7 (44.3-56.0) | 11.2 (7.0-22.8) | 5.65 (4.47-7.07) | 659 (250-1435) |
| Patients . | Age (y) . | HGB (g/dL) . | HCT % . | WBC (K/μL) . | RBC (M/μL) . | PLT (K/μL) . |
|---|---|---|---|---|---|---|
| Men (n = 17) | 49 (28-73) | 17.2 (15.6-20.2) | 50.3 (45.7-54.9) | 10.5 (7.4-22.4) | 5.71 (4.92-6.69) | 772 (189-1082) |
| Women (n = 13) | 57 (26-72) | 16.8 (14.4-19.3) | 49.7 (44.3-56.0) | 11.2 (7.0-22.8) | 5.65 (4.47-7.07) | 659 (250-1435) |
Data are presented as the mean (range).
HCT, hematocrit; HGB, hemoglobin; PLT, platelet count; RBC, red blood cell count; WBC, white blood cell count.
Major criteria analysis
JAK2 mutation
Of the 30 patients, all were JAK2V617F positive. The median allele burden, determined in 29 patients, was 26.5% (range: 2.9%-93.6%).
Increased RCV
Of the 30 patients, 28 had an increased Cr-51 RCM whereas the other 2 patients had normal values. Of these 28 patients, 27 were shown to have increased RCM by both the Pearson method and the body-weight method. One patient with a RCM 118% of the mean normal predicted value had an increased RCM of 36 mL/kg according to the body-weight method. The 2 patients with normal RCM had normal values according to both methods. Although RCM generally increased with increasing hemoglobin and hematocrit, it was not possible to correlate a RCM with a unique hemoglobin or hematocrit value.
Of the 28 patients with an increased RCM, 18 (4 women and 14 men) did not meet the WHO criteria for an increased hemoglobin value, as shown in Figure 1. The median hemoglobin count was 15.2 g/dL (range: 14.4-16.4) for the 4 women and 17.2 g/dL (range: 15.6-18.1) for the 14 men. Likewise, of the 28 patients with an increased RCM, 8 (1 woman and 7 men) did not meet the WHO criteria for an increased hematocrit value. The hematocrit value was 44.3% for the woman, and the median hematocrit value for the 7 men was 48.5% (range: 45.7-49.4). All 8 patients who did not meet the hematocrit criterion also did not meet the hemoglobin criterion. In summary, of the 28 patients with an elevated RCM, 10 (35.7%) met the WHO criterion for an increased hemoglobin value, 20 (71.4%) met the WHO criterion for an increased hematocrit value, and 20 (71.4%) met the WHO criterion for either an increased hemoglobin or hematocrit value.
Comparison of RCM criterion vs hemoglobin criterion for 30 MPN patients. HGB and Hgb, hemoglobin.
Comparison of RCM criterion vs hemoglobin criterion for 30 MPN patients. HGB and Hgb, hemoglobin.
Minor criteria analysis
Marrow morphology
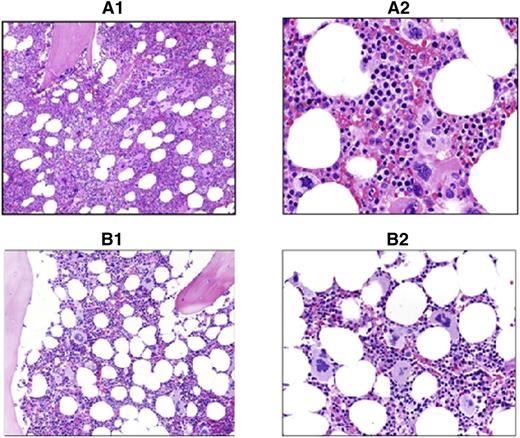
The 28 patients with JAK2V617F positivity and an increased RCM showed marrow findings consistent with PV, which included age-corrected hypercellularity due to panmyelosis, associated with an increased number of megakaryocytes displaying cytologic pleomorphism and mild atypia (Figure 2), and variably increased reticulin fibers up to grade 2 (Manoharan scale), MF-1 (European Consensus System).
Comparison of PV vs ET marrow morphology. Shown are hematoxylin and eosin sections of study cases showing typical PV vs ET morphology. (A1) PV: ×200 magnification. Hypercellular marrow with panmyelosis. (A2) PV: ×400 magnification of the same case. Note the pleomorphic megakaryocytes in loose clusters. (B1) ET: ×200 magnification. Normocellular marrow with increased megakaryopoiesis. (B2) ET: ×400 magnification of the same case. Note the large hyperlobulated megakaryocytes lacking significant pleomorphism.
Comparison of PV vs ET marrow morphology. Shown are hematoxylin and eosin sections of study cases showing typical PV vs ET morphology. (A1) PV: ×200 magnification. Hypercellular marrow with panmyelosis. (A2) PV: ×400 magnification of the same case. Note the pleomorphic megakaryocytes in loose clusters. (B1) ET: ×200 magnification. Normocellular marrow with increased megakaryopoiesis. (B2) ET: ×400 magnification of the same case. Note the large hyperlobulated megakaryocytes lacking significant pleomorphism.
Of the 2 patients with a normal RCM, 1 was interpreted as PMF due to the presence of significant fibrosis (+2 to +3), tight clusters of large pleomorphic megakaryocytes, and other typical findings.19 The biopsy specimen of the other patient was consistent with a myeloproliferative disorder (most likely ET) owing to morphologic findings that included minimal hypercellularity, an increase in megakaryocytes lacking cytologic pleomorphism and/or atypia, and no increase in reticulin.
Serum EPO values
Of the 23 patients with an increased RCM and JAK2V617F positivity, 18 had low EPO values (median: 2.0 mU/mL), whereas the other 5 patients had normal EPO values (median: 7.0 mU/mL). Of the 2 patients with a normal RCM, 1 with a marrow consistent with PMF had a low EPO value of 3.0 and 1 with a marrow consistent with ET had a normal EPO value of 8.0 mU/mL.
Final diagnosis
The analysis of the criteria is shown in Table 2. In summary, 28 patients were diagnosed as PV after fulfilling the 2 major criteria and at least 1 minor criterion, subsequently confirmed by the need for phlebotomy during the first year of follow-up. Of these 28 patients, only 7 met every criterion (JAK2 positivity, increased RCM, increased hemoglobin, increased hematocrit, low serum EPO, and bone marrow consistent with PV). Only 10 PV patients met the elevated hemoglobin criterion and only 20 PV patients met the elevated hematocrit criterion. Furthermore, 3 PV patients (JAK2 positivity, increased RCM, and bone marrow consistent with PV) with normal hemoglobin and hematocrit values also had normal serum EPO values.
Diagnostic findings for the diagnosis of MPNs (n = 30)
| Parameters . | Number of patients . | Final diagnosis . | |||||
|---|---|---|---|---|---|---|---|
| JAK2 . | RCM . | HGB . | HCT . | BM biopsy result . | EPO . | ||
| + | + | + | + | + | + | 7 | PV |
| + | + | − | + | + | + | 7 | PV |
| + | + | − | − | + | + | 4 | PV |
| + | + | − | + | + | − | 2 | PV |
| + | + | − | − | + | − | 3 | PV |
| + | + | + | + | + | * | 3 | PV |
| + | + | − | + | + | * | 1 | PV |
| + | + | − | − | + | * | 1 | PV |
| + | − | − | + | −† | + | 1 | ET |
| + | − | − | − | −‡ | − | 1 | PMF |
| Parameters . | Number of patients . | Final diagnosis . | |||||
|---|---|---|---|---|---|---|---|
| JAK2 . | RCM . | HGB . | HCT . | BM biopsy result . | EPO . | ||
| + | + | + | + | + | + | 7 | PV |
| + | + | − | + | + | + | 7 | PV |
| + | + | − | − | + | + | 4 | PV |
| + | + | − | + | + | − | 2 | PV |
| + | + | − | − | + | − | 3 | PV |
| + | + | + | + | + | * | 3 | PV |
| + | + | − | + | + | * | 1 | PV |
| + | + | − | − | + | * | 1 | PV |
| + | − | − | + | −† | + | 1 | ET |
| + | − | − | − | −‡ | − | 1 | PMF |
EPO obtained 3 months after diagnosis (see “Results” section).
Marrow consistent with ET (see “Results” section).
Marrow consistent with PMF (see “Results” section).
+, criterion met for PV; −, criterion not met for PV.
The remaining 2 patients with normal RCM were diagnosed as PMF and ET, respectively, due to bone marrow morphology and other hematologic parameters. The patient diagnosed as PMF progressed rapidly and died 4 years after diagnosis whereas the patient diagnosed as ET has remained stable.
Discussion
Discovery of JAK2V617F and exon 12 abnormalities ushered in a new era in our understanding of the pathophysiology of myeloproliferative neoplasms and permitted an opportunity for more accurate diagnosis. In 2007, the World Health Organization published consensus criteria for the diagnosis of PV. Although Cr-51 RCM is listed as a method to determine increased RCV, only a limited number of centers have continued employing this technique,29 presumably because of reasons of cost and time.14 Using hemoglobin and hematocrit values as surrogates has become more and more frequent despite cautionary studies,9-11,13 and this has led to the obfuscation of distinguishing MPNs, especially between early-stage PV and ET.3,30 Appropriate early diagnosis is important because early treatment may prevent complications and progression of the MPNs.31-33
Our study is the first prospective evaluation of the parameters of the WHO criteria (except EEC) performed simultaneously and is the only one with a median 5-year follow-up to confirm clinical diagnoses. We found that using surrogate markers is inadequate for determining an increased RCV for early cases, because 64.3%, 28.5%, and 28.5% of our patients would not have been diagnosed as PV using hemoglobin, hematocrit, and either hemoglobin or hematocrit values, respectively. These findings are consistent with recent studies,9-11,34 but in fact were emphasized by the PVSG more than 40 years ago.12 However, in contrast to the PVSG, we agree with Pearson et al34 that for men with a hemoglobin >18.5 g/dL or a hematocrit >60% and for women with a hemoglobin >16.5 g/dL or a hematocrit >56%, Cr-51 RCM determinations are not necessary.
The hematocrit value was more frequently abnormal than the hemoglobin concentration in our PV patients. This relates to the fact that the ambiguity of the 99th percentile (which in our institution is 50% for men and 45% for women) led to an arbitrary cutoff point for the hematocrit that is relatively lower than the hemoglobin cutoff.
In contrast to Fairbanks et al,14 our study of 125I-albumin plasma volume–derived RCM led to the misdiagnosis of PV (data not shown), also consistent with other studies.15,16 Our analysis indicates this is due to the use by Fairbanks et al of a constant f ratio (ratio of total body hematocrit to venous hematocrit) of 0.86, which proved incorrect in clinical practice. For individual MPN patients, the f ratio spans a wide range owing to variability of plasma volume. In our cases, the f ratio ranged from 0.74 to 1.05, similar to others15,16 who have also reported this degree of variation. Thus, Cr-51 RCM determination remains the best test for distinguishing absolute erythrocytosis and the subsequent diagnosis of PV. It should be noted, however, that Cr-51 RCM interpretations are not unequivocally accurate in all cases. The RCM of one PV patient was interpreted as 118% of the mean normal predicted value according to Pearson’s method and as 36 mL/kg (consistent with PV) in terms of corrected body weight. In this case, the diagnosis of PV was confirmed by JAK2 positivity, an EPO of 1 mU/mL, hypercellular marrow, a history of Budd-Chiari syndrome, and need for phlebotomy (4 in the first year), thus highlighting the importance of using both calculations to establish erythrocytosis in borderline cases.
Our data indicate that marrow evaluation is very valuable for the diagnosis of PV, an observation first noted by the PVSG nearly 40 years ago.21 Whereas morphologic distinction of ET from PMF has led to much debate,18,19 the bone marrow findings of PV are more distinct. The presence of an age-adjusted hypercellular bone marrow with increased trilineage proliferation (ie, showing panmyelosis) is very characteristic of PV even in its early phase.20,35,36 In these early phase cases, like in all stages of PV, megakaryocyte morphology is quite characteristic and helps in the differential diagnosis of this disease. The presence of marked pleomorphism of the megakaryocytes is not a feature present in ET. Conversely, the lack of marked cytologic atypia and tight clusters, findings restricted to PMF, facilitates its separation from PV. The presence of only mild to moderate marrow fibrosis in PV also allows separation from ET (absence of fibrosis) and from fibrotic cases of PMF. The lack of stainable bone marrow iron in the majority of cases of PV is another useful diagnostic criterion.20,35,36 Additionally, marrow biopsy specimens obtained at the time diagnosis are useful in establishing reticulin and collagen content, serving as a basis for subsequent analysis of potential disease progression. Marrow cytogenetics obtained from our patients showed a similar incidence of chromosomal abnormalities, as others have reported.37 However, we did not present our analysis in detail as cytogenetics are not included in current or prior WHO criteria for diagnosing PV, and the relationship between chromosomal abnormalities and JAK2V617F remains uncertain.38 Moreover, the abnormalities that occur are not specific to the MPNs.38 Thus, cytogenetic study at diagnosis is useful in establishing a baseline karyotype to provide a potential marker for disease progression, but it is not helpful as a specific diagnostic criterion.
We and others39 caution against an overreliance on serum EPO values as a diagnostic criterion in view of the relatively high frequency of normal EPO values in unquestioned cases of PV. Whereas a low serum EPO is very specific in PV and clinically useful, approximately 20% of our PV patients had normal EPO values, consistent with others.28 In addition, our 1 patient interpreted as PMF presented with a low EPO. Review of his records showed no antecedent history of PV or ET.
We recommend revision of the WHO criteria for the diagnosis of PV patients, especially those with early-stage disease. Our data indicate the hazard of using consensus criteria based on expert opinions and not validated by fact. Major criteria should remain JAK2 positivity and an increased RCV. However, for patients with lesser values, only a Cr-51 RCM determination will unequivocally demonstrate an increased RCV. Minor criteria should remain bone marrow findings and low serum EPO levels. However, a normal EPO level does not eliminate the diagnosis of PV, and marrow biopsy becomes mandatory for confirmation if 1 of the 2 minor criteria is to be satisfied. We agree with current WHO criteria that diagnosis requires the presence of both major criteria and either minor criterion in order to minimize consequences of incorrect molecular test results and to optimize diagnostic specificity.1 In the absence of JAK2 positivity (including exon 12 mutation), the diagnosis of PV should be made with trepidation and the other JAK2-negative MPNs should be considered.40 The difficulty in diagnosis of PV indicates there is an error of varying frequency in all our tests. If the diagnosis remains in doubt, patients should be followed closely to determine whether there is an increase in hematocrit values requiring phlebotomy. In the instances where the hemoglobin and hematocrit criteria are not met and Cr-51 RCM remains unavailable, marrow examination may be useful in identifying early-stage PV. However, bone marrow biopsy results are not a substitute for establishing erythrocytosis, and a definitive diagnosis cannot be made in such a situation. Because the therapies for the myeloproliferative disorders differ, our findings have major clinical implications.
Presented in part at the 53rd annual meeting of the American Society of Hematology, San Diego, CA, 2011; the 101st annual meeting of the United States and Canadian Academy of Pathology, Vancouver, BC, Canada, 2012; and the annual meeting of the Society of Nuclear Medicine and Molecular Imaging Annual Meeting, Miami, FL, 2012.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Amy V. Jones and Nicholas C.P. Cross for JAK2 quantitative allele burden determinations; Dr Y. Lynn Wang for qualitative JAK2 determinations; Drs Maria De Sancho, Ellen Ritchie, Gail Roboz, and Babette Weksler for contributing patients to the study; and Mr Vasilios Avlonitis for technical support.
This work was supported in part by the William and Judy Higgins Memorial Trust of the Cancer Research and Treatment Fund Inc., New York, NY.
Authorship
Contribution: R.T.S. designed the study, cared for patients, reviewed histopathology slides and isotope data, analyzed all patient data, and wrote the manuscript; W.C. collected patient data, analyzed data, and wrote the manuscript; A.O. reviewed histopathology slides, provided microphotographs, analyzed data, and wrote the manuscript; S.P.A. and S.J.G. performed and analyzed Cr-51 RCM and I-125 plasma volume determinations and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard T. Silver, Weill Cornell Medical College, Weill Greenberg Center, 1305 York Ave, New York, NY 10021; e-mail: rtsilve@med.cornell.edu