Key Points
AML induction with liposomal daunorubicin (80 mg/m2 per day for 3 days) shows antileukemic activity comparable to idarubicin (12 mg/m2 per day for 3 days).
Liposomal daunorubicin promises to be more active in the t(8;21) subgroup and causes less treatment-related toxicity.
Abstract
Outcomes of patients with acute myeloid leukemia (AML) improve significantly by intensification of induction. To further intensify anthracycline dosage without increasing cardiotoxicity, we compared potentially less cardiotoxic liposomal daunorubicin (L-DNR) to idarubicin at a higher-than-equivalent dose (80 vs 12 mg/m2 per day for 3 days) during induction. In the multicenter therapy-optimization trial AML-BFM 2004, 521 of 611 pediatric patients (85%) were randomly assigned to L-DNR or idarubicin induction. Five-year results in both treatment arms were similar (overall survival 76% ± 3% [L-DNR] vs 75% ± 3% [idarubicin], Plogrank = .65; event-free survival [EFS] 59% ± 3% vs 53% ± 3%, Plogrank = .25; cumulative incidence of relapse 29% ± 3% vs 31% ± 3%, P(Gray) = .75), as were EFS results for standard (72% ± 5% vs 68% ± 5%, Plogrank = .47) and high-risk (51% ± 4% vs 46% ± 4%, Plogrank = .45) patients. L-DNR resulted in significantly better probability of EFS in patients with t(8;21). Overall, treatment-related mortality was lower with L-DNR than idarubicin (2/257 vs 10/264 patients, P = .04). Grade 3/4 cardiotoxicity was rare after induction (4 L-DNR vs 5 idarubicin). Only 1 L-DNR and 3 idarubicin patients presented with subclinical or mild cardiomyopathy during follow-up. In conclusion, at the given dose, L-DNR has overall antileukemic activity comparable to idarubicin, promises to be more active in subgroups, and causes less treatment-related mortality. This trial was registered at www.clinicaltrials.gov as #NCT00111345.
Introduction
Intensification of induction treatment has had the greatest impact on improving the rate of first remission and overall survival (OS) in adult and pediatric acute myeloid leukemia (AML).1,2 Recently, Löwenberg et al3 reported the effect of increasing anthracycline doses during induction in adults. However, dose-related anthracycline toxicity, especially acute and late cardiotoxicity, might limit further treatment intensification, particularly in children.4-6
Standard AML induction therapy in children and adults comprises 3 days of anthracycline administration [eg, daunorubicin ≥60 mg/m2, idarubicin 10-12 mg/m2, or the anthracenedione mitoxantrone 10-12 mg/m2] and 7−10 days of cytarabine (100-200 mg/m2). With these regimens, 80%-90% of children and adolescents achieve complete remission (CR).7
In children, the risk of anthracycline cardiotoxicity is higher than in adults, owing to the developing heart muscle, and there are limits to increasing pediatric anthracycline doses during induction or total cumulative doses.6 According to animal testing and experiences in first human clinical studies, a liposomal formulation of daunorubicin (L-DNR) offers the possibility to increase single and cumulative dosages of anthracyclines without increasing cardiotoxicity.8-12
Here we report on the randomized introduction of L-DNR during induction in the Multicenter Therapy-Optimization Trial AML-BFM 2004 for the Treatment of Acute Myeloid Leukemias in Children and Adolescents at a dose of 80 mg/m2 per day for 3 days, which is higher than the dose of idarubicin that is provided in the control arm (standard 12 mg/m2 per day for 3 days, corresponding to 60 mg/m2 per day for 3 days L-DNR, equivalent dose conversion of 1:5),13,14 both in combination with cytarabine and etoposide. A dose equivalent of 1:1 was assumed for daunorubicin vs L-DNR, because the active substances are chemically identical, and only the pharmacokinetics are different. The aim was (1) to improve outcome by increasing anthracycline dosage during induction; and (2) to evaluate treatment-related mortality (TRM) and toxicity, including acute and long-term cardiotoxicity.
Patients and methods
Patients
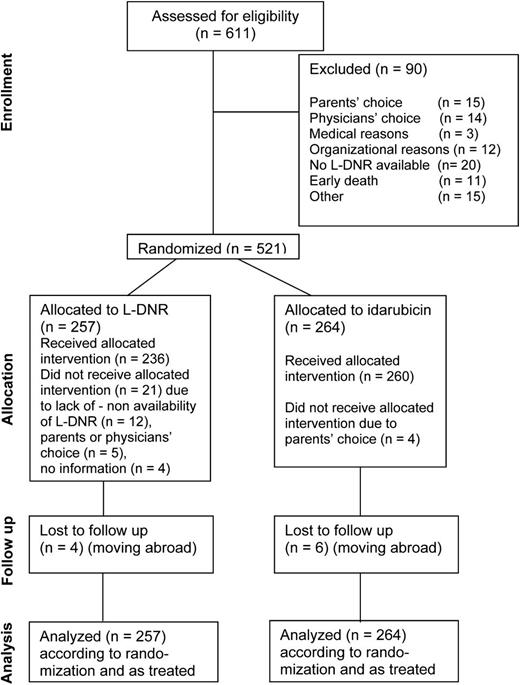
Between March 2004 and April 2010, 611 AML patients <18 years of age were enrolled in the population-based study AML-BFM 2004. Of these, 521 were randomized (257 L-DNR induction vs 264 idarubicin induction) and 90 (15%) were not randomized (for details, see Figure 1 CONSORT [Consolidated Standards of Reporting Trials] diagram). Patient outcome was analyzed as randomized on an intent-to-treat basis, and toxicities were analyzed as treated. Patients with acute promyelocytic leukemia (APL) were included in the protocol, because the chemotherapy regimen was similar to that of standard-risk (SR) AML patients (except for additional all-trans-retinoic acid courses). Outcome results with and without APL patients were calculated. Patients with Down syndrome, to whom a reduced anthracycline dosage is given a priori, were intentionally excluded from this analysis.
CONSORT diagram of AML-BFM 2004 showing the flow of participants from enrollment to randomization, therapy, follow-up, and analysis.
CONSORT diagram of AML-BFM 2004 showing the flow of participants from enrollment to randomization, therapy, follow-up, and analysis.
The AML-BFM 2004 study was performed in Germany, Austria, Switzerland, and the Czech Republic. The study was approved by the national ethics committees and institutional review boards in each country. Written informed consent from patients, parents, or guardians was obtained at study entry in accordance with the Declaration of Helsinki. Central review of bone marrow morphology and cytogenetics was performed. Comprehensive cytogenetic data from 485 of the 521 patients (93%) were available. Cytogenetic analysis was performed as previously described.15 Further, routine testing for internal tandem duplications of FLT3 (FLT3-ITD) was done at initial diagnosis, as stratification to the high-risk (HR) group was based on positivity for FLT3-ITD, among other criteria.
Treatment plan
The main study objective of AML-BFM 2004 was the improvement of prognosis by intensification of chemotherapy without increasing toxicity by (1) randomized introduction of L-DNR (80 mg/m2 per day for 3 days) in a dose higher than the assumed equivalent dose of idarubicin (12 mg/m2 per day for 3 days) during induction, each combined with cytarabine and etoposide; and (2) randomized introduction of 2-chloro-2-deoxyadenosine (6 mg/m2 per day for 2 days) as intensification during the cytarabine/idarubicin consolidation for HR patients (Figure 2). Apart from that, the treatment schedule of AML-BFM 2004 was for the most part similar to the AML-BFM 98 protocol.16,17 An exception was the second induction with HAM, which was administered generally to all patients in the AML-BFM 98 trial, but in AML-BFM 2004 to HR patients only. Two additional short cycles with 0.5 g/m2 cytarabine and HDAC (1 g/m2) plus anthracyclines were given after first induction (SR patients) or second induction (HR patients), followed by intensification with HDAC/etoposide and maintenance. The indication for allogeneic hematopoietic stem cell transplantation (HSCT) from sibling donors in CR1 was restricted to HR patients17 and, since 2006, recommended only for HR patients with bone marrow blasts >5% after second induction.18 Intrathecal cytarabine was given 11-12 times according to age-dependent dosage regimens. Randomized cranial irradiation (12 vs 18 Gy), which was performed over 2 study periods, was stopped in May 2009.17
Treatment schedule of AML-BFM 2004 induction. First induction, AIE (cytarabine/idarubicin/etoposide) or randomized (R1) with ADxE (cytarabine/L-DNR/etoposide); second induction, (high-dose cytarabine [3g/m2]/mitoxantrone) (HAM); consolidation, HAM or randomized (R2) AI/2-CDA (cytarabine [0.5 g/m2]/idarubicin/2-chloro-2-deoxyadenosine) vs AI (cytarabine [0.5 g/m2]/idarubicin); intensification: HAE (high-dose cytarabine [3 g/m2]/etoposide), CNS-RT (central nervous system [cranial] irradiation randomized [R3] to 12 or 18 Gy). APL patients received additional all-trans-retinoic acid during all therapy courses. The cumulative anthracycline dosages in SR and HR patients were, with AIE induction, 350 and 450 mg/m2, respectively; with ADxE, 410 and 510 mg/m2. IT, intrathecal.
Treatment schedule of AML-BFM 2004 induction. First induction, AIE (cytarabine/idarubicin/etoposide) or randomized (R1) with ADxE (cytarabine/L-DNR/etoposide); second induction, (high-dose cytarabine [3g/m2]/mitoxantrone) (HAM); consolidation, HAM or randomized (R2) AI/2-CDA (cytarabine [0.5 g/m2]/idarubicin/2-chloro-2-deoxyadenosine) vs AI (cytarabine [0.5 g/m2]/idarubicin); intensification: HAE (high-dose cytarabine [3 g/m2]/etoposide), CNS-RT (central nervous system [cranial] irradiation randomized [R3] to 12 or 18 Gy). APL patients received additional all-trans-retinoic acid during all therapy courses. The cumulative anthracycline dosages in SR and HR patients were, with AIE induction, 350 and 450 mg/m2, respectively; with ADxE, 410 and 510 mg/m2. IT, intrathecal.
Definitions and statistics
Patients were stratified into either a SR or HR group. SR was defined as FAB (French-American-British) M1/M2 with Auer rods; FAB M4eo or favorable cytogenetics [t(8;21)/AML1-ETO or inv(16) or t(16;16) and/or CBFB/MYH1)]; bone marrow blasts <5% on day 15; or FAB M3 (all patients). HR was defined as all others. SR patients were reclassified to the HR group if FLT3-ITD positive.
Early death (ED) was defined as death before or within the first 6 weeks (42 days) of treatment and further subdivided into (1) ED before or within the first 15 days of therapy, which mainly reflects lethal events due to hyperleukocytosis/leukostasis and bleeding; and (2) ED within days 16-42 of treatment, which mainly reflects deaths due to infectious complications during aplasia after induction.19 CR was defined according to the CALGB (Cancer and Leukemia Group B) criteria20 achieved at the end of intensification treatment. OS was calculated from date of diagnosis to death of any cause, and event-free survival (EFS) from diagnosis until first event (failure to achieve remission [calculated as an event at t = 0], relapse, secondary malignancy, or death by any cause) or last follow-up. Probabilities of survival were estimated using the Kaplan–Meier method with standard errors according to Greenwood21 and compared with the log-rank test. Cumulative incidence functions of relapse, secondary malignancy, and incidence of cardiac toxicity were constructed by the method of Kalbfleisch and Prentice.21 Competing events for cardiac toxicity were HSCT in CR1, relapse, and death. Functions were compared by Gray test. Toxicity was assessed according to the National Cancer Institute common toxicity criteria (CTC) version 2.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf). Cardiotoxicity of grade 1/2 was called subclinical, and grade 3/4, clinical. During follow-up, patients had to be evaluated once a year for symptoms and echocardiographic changes suggestive of cardiac toxicity.
Univariate analysis was conducted by Wilcoxon test for quantitative variables and Fisher exact test or χ2 statistics for qualitative variables. Computations were performed using SAS (Statistical Analysis System, version 9.1; SAS Institute, Cary, NC). Date of evaluation was March 15, 2012, and median follow-up time was 5.0 (range 0.5-8.5) years.
The study was designed to have a power of 80% to detect a 13% difference in 5-year EFS probability (pEFS, baseline 50%) by including 245 patients per group. Two interim analyses were planned after 3 and 5 years. Sample size calculation was done according to Freedman22 and corrected for interim analysis using the R procedure gsDesign.
Results
Patient characteristics
Table 1 shows characteristics of the cohorts randomized in the L-DNR or idarubicin arm. Patient characteristics were comparable in the randomized groups and also in the corresponding groups as treated (data not shown).
Baseline data for patients randomized to L-DNR vs idarubicin
| . | L-DNR . | Idarubicin . | P* . |
|---|---|---|---|
| n | 257 | 264 | |
| Age, y, median (Q1-Q3) | 9.8 (2.4-14.5) | 8.3 (2.2-14.1) | .28 |
| Leukocytes, ×103/µL, median (range) | 15 050 (4785-61 700) | 16 300 (4550-56 350) | .88 |
| Gender | .66 | ||
| Male | 135 (52) | 133 (50) | |
| Female | 122 (48) | 131 (50) | |
| CNS involvement, n/total n (%) | 22/252 (9) | 39/259 (15) | .03† |
| Extramedullary organ involvement | 67 (26) | 56 (21) | .19 |
| Cytogenetic data available, n/total n (%) | 239/257 (93) | 247/264 (94) | |
| Cytogenetic data | .11 | ||
| t(8;21) | 29 (12) | 29 (12) | |
| t(15;17) | 24 (10) | 14 (6) | |
| inv(16) | 24 (10) | 20 (8) | |
| Normal | 41 (17) | 63 (26) | |
| Other | 121 (51) | 121 (49) | |
| Risk groups | .68 | ||
| SR | 94 (37) | 92 (35) | |
| HR | 163 (63) | 172 (65) |
| . | L-DNR . | Idarubicin . | P* . |
|---|---|---|---|
| n | 257 | 264 | |
| Age, y, median (Q1-Q3) | 9.8 (2.4-14.5) | 8.3 (2.2-14.1) | .28 |
| Leukocytes, ×103/µL, median (range) | 15 050 (4785-61 700) | 16 300 (4550-56 350) | .88 |
| Gender | .66 | ||
| Male | 135 (52) | 133 (50) | |
| Female | 122 (48) | 131 (50) | |
| CNS involvement, n/total n (%) | 22/252 (9) | 39/259 (15) | .03† |
| Extramedullary organ involvement | 67 (26) | 56 (21) | .19 |
| Cytogenetic data available, n/total n (%) | 239/257 (93) | 247/264 (94) | |
| Cytogenetic data | .11 | ||
| t(8;21) | 29 (12) | 29 (12) | |
| t(15;17) | 24 (10) | 14 (6) | |
| inv(16) | 24 (10) | 20 (8) | |
| Normal | 41 (17) | 63 (26) | |
| Other | 121 (51) | 121 (49) | |
| Risk groups | .68 | ||
| SR | 94 (37) | 92 (35) | |
| HR | 163 (63) | 172 (65) |
Data are n (%) unless stated otherwise. Q, quartile; CNS, central nervous system.
By χ2 test.
No differences were found in OS, EFS, or cumulative incidence of relapse in CNS-positive or -negative patients.
Overall results
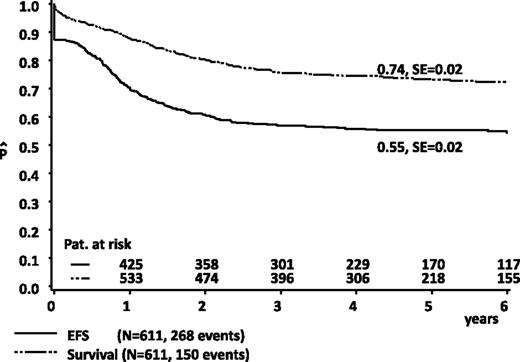
Five-year pOS and pEFS values in all patients (n = 611) were 74% ± 2% and 55% ± 2%, respectively (Figure 3). Survival rates were improved compared with the previous study, AML-BFM 98 (n = 473, 65% ± 2%, Plogrank = .001), whereas pEFS results were in the same range (51% ± 2%, Plogrank = .24, results updated).16 Results for the 217 SR patients were favorable: pOS 89% ± 2%, pEFS 71% ± 3%, and results for the 394 HR patients were suitable, pOS 65% ± 3%, pEFS 46% ± 3%. In comparing patients who were randomized (n = 516) and nonrandomized (n = 90) (patients with ED before day 15 excluded), pEFS results were similar (56% ± 2% vs 54% ± 6%, Plogrank = .88).
Estimated probability of 5-year OS and EFS in all patients from AML-BFM 2004. SE, standard error.
Estimated probability of 5-year OS and EFS in all patients from AML-BFM 2004. SE, standard error.
Randomization of L-DNR vs idarubicin
Results for L-DNR (n = 257) vs idarubicin (n = 264) patients were similar: 5-year pOS 76% ± 3% vs 75% ± 3%, Plogrank = .65, pEFS 59% ± 3% vs 53% ± 3%, Plogrank = .25 (Figure 4) and cumulative incidence of relapse at 5 years 29% ± 3% vs 31% ± 3%, PGray = .79 (Table 2). Results in SR or HR patients were also similar (5-year pEFS SR 72% ± 5% vs 68% ± 5%, Plogrank = .47; HR 51% ± 4% vs 46% ± 4%, Plogrank = .45). TRM was lower in the L-DNR group than the idarubicin group; for details see “Acute toxicity” below, and Table 2. Results according to treatment as randomized were similar to those as given (data not shown). When APL patients were excluded, results were similar to those of the total group (5-year pOS 74% ± 3% vs 74% ± 3%, Plogrank = .75, pEFS 56% ± 3% vs 51% ± 3%, Plogrank = .33). There was 1 ED from intracerebral bleeding in an APL patient.
Estimated 5-year pEFS in patients randomized to L-DNR (ADxE) vs idarubicin (AIE). SE, standard error.
Estimated 5-year pEFS in patients randomized to L-DNR (ADxE) vs idarubicin (AIE). SE, standard error.
Results of randomized patients
| . | L-DNR . | Idarubicin . | P* . |
|---|---|---|---|
| n | 257 | 264 | |
| ED (before d 42) | 5 (2) | 7 (3) | .59 |
| ED (before d 15) related to initial complications | 3 | 2 | |
| ED/TRM (d 15 or later) after induction | 2 | 5 | |
| Death in CCR (TRM) | 1 (0.4) | 6 (3) | .02 |
| TRM during intensification courses | 0 | 5 | |
| TRM after HSCT in first CR | 1 | 1 | |
| Total ED/TRM following induction treatment | 2 | 10 | .04 |
| Blasts >5% at d 15, n/total n (%) | 59/239 (25) | 35/235 (15) | .01 |
| Blasts >5% at d 28, n/total n (%) | 46/202 (23) | 37/206 (18) | .27 |
| Nonresponders | 24 (9) | 25 (10) | .97 |
| CR achieved | 228 (89) | 232 (88) | .79 |
| Relapse, cumulative incidence, n (% ± SE) | 74 (29 ± 3) | 79 (31 ± 3) | .79 |
| Secondary malignancies, cumulative incidence, n (% ± SE) | 1 (1 ± 1) | 3 (1 ± 1) | .29 |
| 5-y pOS, % ± SE | 76 (3) | 75 (3) | .65 |
| 5-y pEFS, % ± SE | 59 (3) | 53 (3) | .25 |
| . | L-DNR . | Idarubicin . | P* . |
|---|---|---|---|
| n | 257 | 264 | |
| ED (before d 42) | 5 (2) | 7 (3) | .59 |
| ED (before d 15) related to initial complications | 3 | 2 | |
| ED/TRM (d 15 or later) after induction | 2 | 5 | |
| Death in CCR (TRM) | 1 (0.4) | 6 (3) | .02 |
| TRM during intensification courses | 0 | 5 | |
| TRM after HSCT in first CR | 1 | 1 | |
| Total ED/TRM following induction treatment | 2 | 10 | .04 |
| Blasts >5% at d 15, n/total n (%) | 59/239 (25) | 35/235 (15) | .01 |
| Blasts >5% at d 28, n/total n (%) | 46/202 (23) | 37/206 (18) | .27 |
| Nonresponders | 24 (9) | 25 (10) | .97 |
| CR achieved | 228 (89) | 232 (88) | .79 |
| Relapse, cumulative incidence, n (% ± SE) | 74 (29 ± 3) | 79 (31 ± 3) | .79 |
| Secondary malignancies, cumulative incidence, n (% ± SE) | 1 (1 ± 1) | 3 (1 ± 1) | .29 |
| 5-y pOS, % ± SE | 76 (3) | 75 (3) | .65 |
| 5-y pEFS, % ± SE | 59 (3) | 53 (3) | .25 |
Data are n or n (%) unless stated otherwise. CCR, continuous complete remission.
By Gray test (except for 5-year pOS and pEFS, which are by log rank test).
Cytogenetic subgroups
Results in 29 patients with t(8;21) and treated with L-DNR were significantly better than in those who received idarubicin (n = 29): pEFS 76% ± 8% vs 54% ± 9%, Plogrank = .04. This difference for pEFS was not seen for the whole group of core-binding-factor leukemias (Plogrank = .28), and there were no significant differences after administration of either L-DNR or idarubicin in patients with inv(16). No further differences were seen in the cohort analysis of patients with t(15;17), normal karyotype, or MLL rearrangements (data not shown).
Bone marrow blast count on days 15 and 28
Data of early response to induction treatment evaluated by analysis of d 15 bone marrow showed a significantly higher number of patients with >5% blasts in the L-DNR arm (59/239 = 25% vs 35/235 = 15%, P = .008); however, the difference was not evident in day 28 blast counts (Table 2).
Acute toxicity
Time to recovery of neutrophils to 500/µL after induction was similar in both arms (L-DNR median 20.0 days, quartile 1 [Q1]-Q3 14-26 days, vs idarubicin median 21.0 days, Q1-Q3 12-27 days; P = .73), as was time to recovery of platelets to 20 000/µL (L-DNR median 9.0 days, Q1-Q3 2-17 days, vs idarubicin median 12.0 days, Q1-Q3 3-17 days; P = .16).
The rate of severe infections was slightly, but not significantly, lower with L-DNR (PFisher = .21) (Table 3). After induction, transaminases were more often elevated in L-DNR patients than in those treated with idarubicin; however, this was transient and not of clinical importance. Skin toxicity was low in both groups. Four L-DNR patients suffered from either arrhythmia (n = 1) or reduced cardiac function grade 3/4 (n = 3), which occurred during sepsis in 2 patients. In 5 idarubicin patients, cardiac function was reduced, and 3 of those also had arrhythmia. Because 3 of these patients suffered from additional severe infections, these cases cannot be considered as cardiotoxicity caused solely by anthracyclines. One of these patients died on d 3 of induction with life-threatening bleeding and acute respiratory distress syndrome (ARDS); another 2 patients died on days 17 and 22, respectively, from severe infections. The other 2 patients presented with grade 3 cardiotoxicity after induction, one of them with concurrent pneumonia. There was no grade 1-4 cardiotoxicity in the patients of the L-DNR and idarubicin groups after the HAM course.
Toxicity (CTC score III/IV) after induction in patients treated with L-DNR and idarubicin
| . | L-DNR . | Idarubicin . | P . |
|---|---|---|---|
| General condition | 86/223 (39) | 116/260 (45) | .18 |
| SGOT, SGPT | 32/225 (14) | 20/258 (8) | .02 |
| Arrhythmia | 1/215 (0.5) | 3/252 (1.2) | .40 |
| Cardiac function (congestive heart failure) | 3/183 (1.6) | 5/221 (2.3)* | .65 |
| Echocardiography | 0/186 (0) | 0/214 (0) | — |
| Cardiac total | 4/183 (2.1)* | 5/214 (2.3)† | 1.0 |
| Central neurotoxicity | 4/226 (1.8) | 7/261 (2.7) | .50 |
| Infection | 70/226 (31.0) | 95/261 (36.4) | .21 |
| Pulmonary toxicity | 33/204 (16) | 35/248 (14) | .54 |
| Mucositis | 58/225 (26) | 64/259 (25) | .79 |
| Skin toxicity | 8/223 (3.6) | 7/256 (2.7) | .58 |
| . | L-DNR . | Idarubicin . | P . |
|---|---|---|---|
| General condition | 86/223 (39) | 116/260 (45) | .18 |
| SGOT, SGPT | 32/225 (14) | 20/258 (8) | .02 |
| Arrhythmia | 1/215 (0.5) | 3/252 (1.2) | .40 |
| Cardiac function (congestive heart failure) | 3/183 (1.6) | 5/221 (2.3)* | .65 |
| Echocardiography | 0/186 (0) | 0/214 (0) | — |
| Cardiac total | 4/183 (2.1)* | 5/214 (2.3)† | 1.0 |
| Central neurotoxicity | 4/226 (1.8) | 7/261 (2.7) | .50 |
| Infection | 70/226 (31.0) | 95/261 (36.4) | .21 |
| Pulmonary toxicity | 33/204 (16) | 35/248 (14) | .54 |
| Mucositis | 58/225 (26) | 64/259 (25) | .79 |
| Skin toxicity | 8/223 (3.6) | 7/256 (2.7) | .58 |
Data are n/total n (%). SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
One patient died associated with sepsis.
Three patients died associated with ARDS and sepsis (see “Acute toxicity”).
ED and TRM in patients treated with L-DNR and idarubicin are illustrated in Table 2. ED before day 15 was mostly due to cerebral bleeding, related to initial hyperleukocytosis and coagulation disturbance or to ARDS. TRM following induction treatment occurred in 2 patients due to fungal and bacterial infections after L-DNR induction and in 5 patients of the idarubicin group (severe infections/septicemia/ARDS). Another 5 patients of the idarubicin group died after HAM or the subsequent 3 intensive courses due to bacterial and fungal septicemia, abscess, or thromboembolism. Considering also TRM during intensification, the TRM rate of the L-DNR group was significantly lower compared with the idarubicin group: 2 of 257 vs 10 of 264, P = .04.
Late toxicity
Secondary malignancies.
One patient in the L-DNR arm developed a secondary malignancy (acute lymphoblastic leukemia), as did 3 patients in the idarubicin arm (1 acute lymphoblastic leukemia and 2 myelodysplastic syndrome).
Late cardiotoxicity.
Five-year cumulative incidence of late and persistent (subclinical and clinical) cardiotoxicity in CR1 was assessed in median after 5 (0.9–8.5) years, excluding those with transient cardiac dysfunction (ie, CTC grade 1 recorded at only 1 time point) and those with either HSCT in CR1 or relapse. The incidence values were 0.7% (n = 1) in the L-DNR group and 1.8% (n = 3) in the idarubicin group (Table 4). In detail, the sole patient in the L-DNR cohort was a 3-year-old girl who suffered from mild therapeutically compensated clinical cardiomyopathy (grade 3). Treatment with angiotensin-converting enzyme (ACE) inhibitors was stopped 7.6 years after diagnosis.
Late cardiotoxicity in patients treated with L-DNR and idarubicin
| . | L-DNR . | Idarubicin . |
|---|---|---|
| n | 170 | 185 |
| CTC score I | 9 | 10 |
| After relapse/HSCT* | 4 | 3 |
| No relapse/no HSCT and CTC score I only once* | 5 | 6† |
| No relapse/no HSCT and CTC score I present >6 mo | 0 | 1 |
| CTC score III/IV | 2 | 3 |
| After relapse/HSCT* | 1‡ | 1 |
| No relapse/no SCT and CTC score III/IV present >6 mo | 1 | 2 |
| Total: No relapse/no HSCT and CTC score I-IV present >6 mo | 1 | 3 |
| . | L-DNR . | Idarubicin . |
|---|---|---|
| n | 170 | 185 |
| CTC score I | 9 | 10 |
| After relapse/HSCT* | 4 | 3 |
| No relapse/no HSCT and CTC score I only once* | 5 | 6† |
| No relapse/no HSCT and CTC score I present >6 mo | 0 | 1 |
| CTC score III/IV | 2 | 3 |
| After relapse/HSCT* | 1‡ | 1 |
| No relapse/no SCT and CTC score III/IV present >6 mo | 1 | 2 |
| Total: No relapse/no HSCT and CTC score I-IV present >6 mo | 1 | 3 |
There were no CTC II cardiotoxicities.
Excluded for the calculation of induction-related cardiotoxicity.
One patient refused follow-up.
Patient died 7 days after HSCT with sepsis and cardiac failure.
In patients treated with idarubicin, 1 patient suffered from subclinical cardiomyopathy after 2.3 and 4.0 years from diagnosis, presenting with reduced shortening fraction (SF) CTC grade 1. Later controls were without pathological findings. Another case with subclinical cardiotoxicity remained unclear, as follow-ups were refused (see Table 4). Two girls showed mild therapeutically compensated clinical cardiomyopathy in CR1. One developed cardiac functional impairment (CTC grade 3) during consolidation, suffered from hypotension 1.5 years after diagnosis, and 6 months later from arrhythmia with a reduced SF CTC grade 1. She recovered during follow-up and remained without signs of cardiotoxicity 4.5 years after diagnosis. The second girl showed reduced SF CTC grade 2 after induction and received no further idarubicin, but mitoxantrone. After 4 years of follow-up, she had mildly reduced SF CTC at 2 occasions, and ACE inhibitor treatment was indicated. In all patients of both treatment groups with controls, SF improved during times of follow-up.
Multivariate analysis
Cox regression analysis was performed including the following variables: treatment arm, cytogenetic risk group [SR: t(8;21), inv(16), t(15;17) vs other], MLL rearrangements, age (< vs ≥10 years), leukocytes (< vs ≥20 000 × 103/µL), and an interaction term between therapy (randomized treatment with L-DNR) and t(8;21). Only unfavorable cytogenetics was an independent risk factor (hazard risk ratio = 2.64, 95% confidence interval 1.72-4.04, Pχ2 = .000; the interaction between treatment and t(8;21) was not significant (Pχ2 = .97).
Discussion
Dose intensity during induction, along with the cumulative dose of anthracyclines, plays a major role in the treatment of AML.2,3,13 Based on several randomized trials comparing anthracyclines in AML therapy, idarubicin appeared to be very efficacious.13,14 However, children are more susceptible to anthracycline cardiotoxicity than adults because of ongoing heart muscle growth. It is thus of high importance to minimize cardiotoxicity in anthracycline-based regimens.
L-DNR is a liposomally entrapped daunorubicin with a more favorable pharmacology than free daunorubicin.23 Low accumulation of L-DNR in the heart has been proven in animals, and minimal cardiotoxicity despite significant antileukemic activity was found in a mouse model.8,9 In 277 AIDS patients with Kaposi sarcoma, no cardiotoxicity of L-DNR was seen, after cumulative doses of up to 1700 mg/m2.10 Hence, liposomal anthracyclines are believed to cause less cardiotoxicity at higher cumulative doses than conventional anthracyclines.10,11,24-26
Based on these data, we assumed that higher doses of this liposomal anthracycline could be administered safely. Notably, we found previously that idarubicin was somewhat superior to conventional DNR in induction at a conversion factor of 1:5,14 but observations of the Children’s Cancer Group27 made us doubt that the idarubicin dose could be further increased. Thus, in AML-BFM 2004, we randomized L-DNR at a dose of 80 mg/m2 per day for 3 days vs standard induction with idarubicin at 12 mg/m2 per day for 3 days (corresponding to L-DNR 60 mg/m2/ per day for 3 days). Overall, we found similar response rates after induction and comparable long-term outcome. Hence, this surmised mild dose increase did not translate into largely different efficacy. Notably, our study was underpowered to discriminate small differences: to show a pEFS improvement from 53% to 59% with 80% power, α = 5% 2-sided, 1050 patients per group would have been needed. However, our results in subgroups of patients and TRM show that L-DNR still proved to be more effective and favorable.
For example, results by randomization were better for patients with t(8;21) treated with L-DNR vs idarubicin. We suggest that this might be due to the prolonged active plasma level of the liposomal drug. We found it interesting to note that at day 15, a typical response assessment time point,28,29 the idarubicin cohort showed lower blast counts, whereas the 2 cohorts were no longer different at d 28 before the second treatment course. Again, this could be indicative of a prolonged effect of L-DNR compared with idarubicin, and could be important for t(8;21) leukemias, which have a relatively slow blast-cell clearance over time compared with other cytogenetic subtypes. Notably, AML-BFM 2004 was designed without HAM treatment in all SR patients, including those with t(8;21), unlike AML-BFM 98. We have shown30 that second induction with HAM is beneficial for these patients. Nevertheless, we consider now that the more favorable activity of L-DNR over idarubicin in first induction could compensate at least partly for the lack of HAM. Overall, our 2004 results even improved over those of 1998. This can mainly be seen by the high 5-year survival rates (74% ± 2% vs 65% ± 2%, Plogrank = .001).
Improved survival rates in pediatric AML, similar to those in our study, have recently been reported by different treatment groups from Japan, Europe, and the United States.31-34 In view of these good results, which have been achieved with different but always intensive strategies, it is even more important to reduce acute and long-term toxicities to ensure the highest possible quality of life. In our study, most acute toxicities, including hematological and infectious complications, were in the expected range or tended to be lower after treatment with L-DNR compared with idarubicin. This is explicitly underpinned by a lower TRM rate in patients of the L-DNR arm. Cardiotoxicity was low after induction in both arms, and during follow-up, only 1 L-DNR patient and 2 idarubicin patients required treatment with ACE inhibitors. However, the assessment of long-term anthracycline cardiotoxicity requires longer follow-up.6 Results from the prior AML-BFM trials (mainly with idarubicin treatment) showed a relatively low rate of early clinical and subclinical cardiotoxicity, and the 11-year cumulative incidence of late clinical cardiotoxicity was 2.5%.35 However, our current data on acute and long-term cardiotoxicity, although preliminary, clearly reveal that an induction dose of 3 × (80 mg/m2 per day) L-DNR does not increase cardiotoxicity. Cardioprotection with dexrazoxane might be another option to reduce anthracycline cardiotoxicity.36 However, the application of that drug in those <18 years old is discouraged owing to the risk of secondary malignancies.37
In summary, L-DNR proves to be an effective drug within a multiagent approach for treating de novo AML in children and adolescents in general, and has specific activity in patients with t(8:21). This specific effectiveness of L-DNR was also found in Relapsed AML 2001/01,38 suggesting that core-binding-factor AML is much more sensitive to anthracyclines in general (or more specific to L-DNR) than other subtypes of AML. L-DNR shows a favorable overall profile, as well as a favorable cardiac toxicity profile, and eventually allows an increased anthracycline dose without increasing cardiotoxicity. The AML-BFM study group will therefore continue to use L-DNR in their forthcoming de novo AML trial.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the colleagues, data managers, and technicians of the participating hospitals for their valuable cooperation and Jans-Enno Müller (Hannover) and Nora Mühlegger (Vienna) for competent data management. The authors acknowledge gratefully the help of Ursula Bernsmann (Hannover) and Gesche Tallen (Berlin) in preparing the manuscript.
This work was supported by the Deutsche Krebshilfe e.V. and partly by the project (Ministry of Health, Czech Republic) for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic).
Authorship
Contribution: U.C., M.Z., J.-P.B., M.N.D., and D.R. designed and performed research and wrote and edited the paper; M.Z., U.C., and D.R. analyzed the data; C.v.N., A. Sander, and J.S. performed diagnostic studies and edited the paper; G.F., N.G., T.K., B.K., T.L., J.R., A. Schrauder, A.v.S., and J.S. provided study materials or patients and edited the manuscript; and all authors reviewed the final version of the manuscript.
Conflict-of-interest disclosure: D.R. is a member of the advisory board from Galen. The remaining authors declare no competing financial interests.
A complete listing of the participating AML-BFM institutions and investigators appears in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Ursula Creutzig, AML-BFM Trial Centre, Department of Pediatric Hematology and Oncology, Hannover Medical School, Children’s Hospital, Carl-Neuberg-Strasse 1, D-30625 Hannover, Germany; e-mail: ursula@creutzig.de.

![Figure 2. Treatment schedule of AML-BFM 2004 induction. First induction, AIE (cytarabine/idarubicin/etoposide) or randomized (R1) with ADxE (cytarabine/L-DNR/etoposide); second induction, (high-dose cytarabine [3g/m2]/mitoxantrone) (HAM); consolidation, HAM or randomized (R2) AI/2-CDA (cytarabine [0.5 g/m2]/idarubicin/2-chloro-2-deoxyadenosine) vs AI (cytarabine [0.5 g/m2]/idarubicin); intensification: HAE (high-dose cytarabine [3 g/m2]/etoposide), CNS-RT (central nervous system [cranial] irradiation randomized [R3] to 12 or 18 Gy). APL patients received additional all-trans-retinoic acid during all therapy courses. The cumulative anthracycline dosages in SR and HR patients were, with AIE induction, 350 and 450 mg/m2, respectively; with ADxE, 410 and 510 mg/m2. IT, intrathecal.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/1/10.1182_blood-2013-02-484097/4/m_37f2.jpeg?Expires=1767704419&Signature=B8NWTbfCrJso4nWgrohqAJbC5TVZnuDXzfjKAfj5T3o9GpLSABaeMnpyST7LbNqgrpLMlfaQU06FZFCm-00xkC4~N-vvUUXU8iiib50N23X5P4HjqRffuYSdbdAvY-GB0CUih7vfwH~ql95OFZ3wt2~Kch1g4AwK5b4Tz2eaBlpBEOJKjBruks32Sm7QjEvotZ9D7WR8jDICPvL5Vh2qmiCVMHOovXQGLeh6oUz5NK-7ZBRRGPe3CXOzxlgRrEA-rFcSfUvWGcIlbgG6idlF5CwD1~S01zl9TFON-wDDegwdGfMn7f0P5OwsgkIUPhM-vPCpHTwpVoJ32EgoTkGNQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)