Key Points
In canine S aureus pneumonia, first randomized blinded trial showing blood transfused at end of storage period increases mortality.
Increased in vivo hemolysis, cell-free hemoglobin, pulmonary hypertension, tissue damage, and gas exchange abnormalities each contributed.
Abstract
Two-year-old purpose-bred beagles (n = 24) infected with Staphylococcus aureus pneumonia were randomized in a blinded fashion for exchange transfusion with either 7- or 42-day-old canine universal donor blood (80 mL/kg in 4 divided doses). Older blood increased mortality (P = .0005), the arterial alveolar oxygen gradient (24-48 hours after infection; P ≤ .01), systemic and pulmonary pressures during transfusion (4-16 hours) and pulmonary pressures for ∼ 10 hours afterward (all P ≤ .02). Further, older blood caused more severe lung damage, evidenced by increased necrosis, hemorrhage, and thrombosis (P = .03) noted at the infection site postmortem. Plasma cell–free hemoglobin and nitric oxide (NO) consumption capability were elevated and haptoglobin levels were decreased with older blood during and for 32 hours after transfusion (all P ≤ .03). The low haptoglobin (r = 0.61; P = .003) and high NO consumption levels at 24 hours (r = −0.76; P < .0001) were associated with poor survival. Plasma nontransferrin-bound and labile iron were significantly elevated only during transfusion (both P = .03) and not associated with survival (P = NS). These data from canines indicate that older blood after transfusion has a propensity to hemolyze in vivo, releases vasoconstrictive cell-free hemoglobin over days, worsens pulmonary hypertension, gas exchange, and ischemic vascular damage in the infected lung, and thereby increases the risk of death from transfusion.
Introduction
Transfusion of red blood cells (RBCs) is one of the most commonly used, potentially lifesaving medical therapies. Each year, some 80.7 million units of blood are collected in 167 countries worldwide, and approximately 15 million units are collected and transfused in the United States alone.1,2 RBCs can be stored for up to 42 days to meet inventory needs, and by standard practice the oldest blood is usually used first (“first in, first out”). Food and Drug Administration (FDA) regulations only stipulate that at the end of the storage period 75% of the cells remain in the circulation at 24 hours after transfusion and that hemolysis in the storage bag does not exceed 1%,3 no other product specification of quality is required. Although 6-week-old stored blood meets current FDA standards, laboratory and clinical studies have raised concerns that “older” blood may not be as safe as blood stored for a shorter duration.4-8
Refrigerated storage of blood results in a “storage lesion” characterized by rheologic changes, metabolic derangements, changes in oxygen affinity and delivery, oxidative injury to lipids and proteins, RBC shape change, loss of membrane carbohydrates, and reduced RBC lifespan.8-10 The storage lesion results in increased adhesion of RBCs to endothelial cells, and hemolysis with release into circulating plasma of cell-free hemoglobin (CFH), potassium, shed proteins, lipids, and microvesicles.8-10 Many clinical studies have raised concerns that patients receiving older blood have an increased risk of death.6 However, there are insufficient data from randomized controlled trials (RCTs) to warrant changing blood-banking practice in the United States. Whereas a recently published RCT conducted during 5 years in premature neonates did not demonstrate a difference in outcome between those who received fresher or older red cells, the age difference between the fresh and old blood was small.11 Several other studies being conducted in Canada, Australia, and the United States are not expected to be completed and analyzed for several years.12-14 These clinical studies also may not be optimally designed or powered to provide definitive results.11-14 Clinically relevant animal models can be used to corroborate risks, help determine mechanisms, and facilitate development of new therapeutic approaches to blood storage and transfusion practice.
In selecting an animal model, we hypothesized that the published observational studies conducted to date have an unaccounted for bias and that older stored blood is safe.6 Canines were chosen as a model because blood banking procedures for this species are similar to those for humans, making such a study both feasible and clinically relevant, and canine hemoglobin is functionally and immunologically indistinguishable from human hemoglobin.15-17 Given the widespread use of RBC transfusion over decades, and the relatively sparse evidence of toxicity, we chose to study critically ill animals near death and compare the freshest practical blood with blood at the very end of its shelf-life (42 days) using large volumes to mimic a massive transfusion of blood at the very end of its shelf-life (42 days) to maximize the chances of finding a clinical effect if one existed. Contrary to our hypothesis, we found 42-day-old blood profoundly increased mortality.
Methods
Overview of study size
Forty-eight purpose-bred beagles (12 to 28 months old, 10-15 kg) were used in 3 separate experiments. Eight of these animals were used for RBC chromium labeling (Cr51) studies to determine the viability at 7 and 42 days of commercially available canine universal donor stored blood used in these studies (DEA 1.1 ABRINT; see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Twenty-four of these animals were used for a bacterial dose finding study. This was done to determine the dose of intrapulmonary bacterial challenge needed in addition to exchange transfusion of 1 blood volume to the model to produce a ∼ 50% mortality (see supplemental Methods). Eight of these 24 animals at the dose that produced the desired mortality (1.25 × 109 CFU/kg S aureus) and 16 more animals studied at that same dose were compared to determine whether age of stored blood (7- vs 42-day-old) alters mortality associated with bacterial pneumonia. All studies were approved by the Animal Care and Use Committee of the Clinical Center at the National Institutes of Health.
Bacterial dose finding studies
Animals were challenged with increasing intrapulmonary doses of S aureus at time 0 [1 (n = 8), 1.25 (n = 8), 1.5 (n = 4), and 2.0 (n = 4) × 109 CFU/kg]. Each week, 4 challenged animals were randomized to receive an exchange transfusion of 1 blood volume of 7-day-old blood (n = 2) or 42-day-old blood (n = 2). Investigators and all other staff were blinded to animal treatment groups throughout the studies.
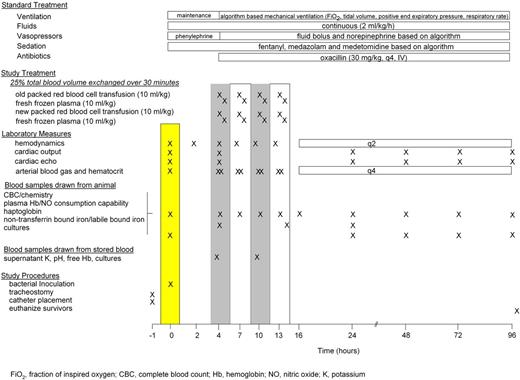
Before infectious challenge, animals had a tracheostomy, external jugular vein introducer, and percutaneous femoral artery and urinary catheters placed under general anesthesia.18 After these procedures, animals were weaned off anesthesia; continuous sedation (fentanyl, midazolam, and medetomidine), and mechanical ventilation via the tracheostomy tube were initiated (see supplemental Methods); and a balloon-tipped pulmonary arterial thermodilution catheter was placed via the introducer. At time 0 (T0), animals underwent bronchoscopy and inoculation of the right lower lobe with S aureus (see supplemental Methods).
During the first 4 hours after S aureus inoculation, phenylephrine was titrated to maintain MAP > 80 mmHg as sedation was optimized and sepsis developed. After 4 hours, when symptoms of sepsis were more fully developed, phenylephrine administration was discontinued and intravascular hemodynamic measures and blood samples were obtained (see Figure 1 and supplemental Methods). Treatment for sepsis was then initiated with fluid boluses and norepinephrine (NE) infusion titrated based on scheduled PAOP and MAP measurements, respectively, using algorithms (see supplemental Methods). Fractional inspired oxygen concentration (FiO2), respiratory rate (RR), and positive end expiratory pressure (PEEP) levels were titrated based on algorithms incorporating scheduled SaO2, and arterial blood gas measures as previously described.18 Oxacillin (30 mg/kg IV) was started 4 hours after bacterial inoculation and administered every 4 hours thereafter. Conventional intensive care unit support used during the ventilation of critically ill large animals was administered as previously described.18 Animals alive at 96 hours were considered survivors and then euthanized while still sedated (Beuthanol; 75 mg/kg IV).
Exchange transfusion study
This study is identical to the dosing study previously described, except only 1 dose of bacteria was used. The dose, 1.25 × 109 CFU/kg S aureus (n = 8), was selected because it was the dose closest to producing 50% overall mortality in the dose finding study. Sixteen more animals were then randomized to 7- vs 42-day-old stored blood and received this same dose before exchange transfusion for a total of 24 animals.
Exchange transfusion of 7- and 42-day-old stored blood
Canine universal donor blood (DEA 1.1 ABRINT) was refrigerated (1-6°C) and stored in plastic bags until use after either 7 or 42 days of storage. All blood products were processed and leukoreduced as described (see supplemental Methods). Starting at 4 hours after bacterial challenge, animals were randomized to 7- or 42-day-old stored blood to be exchange transfused. Each animal had 25% of total blood volume (20 mL/kg) removed via the femoral artery for 30 minutes and then replaced with an infusion of stored packed RBCs (10 mL/kg) followed by thawed fresh frozen canine universal donor plasma (10 mL/kg) through the side arm of a catheter introducer (8 Fr, Maxxim Medical) in the external jugular vein for 30 minutes. Blood and plasma was warmed to room temperature before transfusion. This procedure was done first at 4 hours after bacterial challenge and repeated at 7, 10, and 13 hours after bacterial challenge. Accounting for mixing, this will result in a 68% exchange transfusion of the animals' own blood with either 7- or 42-day-old stored blood. The packed RBCs and plasma was cultured and screened to detect contamination with each transfusion. All 48 blood storage bag cultures (2 blood storage bag cultures per animal) showed no growth except one of 2 bags of old blood of one animal with the recipient blood culture negative by 24 hours. Removing this animal the mortality results are essentially unchanged (P = .0009).
Statistical methods
Survival times were compared between older and newer blood groups using exact log rank tests (StatXact, Cytel Software), with stratified tests applied to account for potential cycle effect. To compare various continuous variables between the 2 groups, changes from baseline values were used. Linear mixed models (SAS PROC Mixed) were used for the analysis. Pearson correlation was used to assess the association between log-transformed plasma Hp, NO consumption, NTBI and LPI values, and survival times. Fisher exact test was used to compare the rates of right ventricular dilatation between the 2 groups. All P values are 2-tailed and considered significant if P ≤ .05. See supplemental Methods for complete statistical methods.
Results
Survival and gas exchange
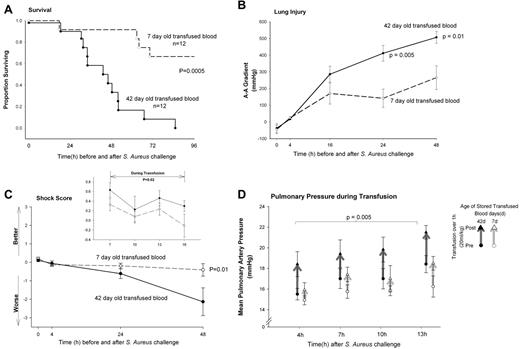
Comparing survival of animals (n = 24) challenged with 1.25 × 109 CFU/kg S aureus and then randomized to be exchange transfused with 1 blood volume of 42- or 7-day-old stored blood, the animals receiving older stored blood had a higher mortality rate (deaths/number studied; 12/12 [100%] vs 4/12 [33%], respectively, P = .0005, exact stratified log-rank test; Figure 2A). The arterial-alveolar oxygen gradient was also significantly worsened (higher) in the animals receiving 42- versus 7-day-old blood at 24 hours (P = .005) and 48 hours (P = .01) after bacterial challenge (Figure 2B).
Survival curves. (A) Kaplan-Meier plot over the 96 hours study comparing animals challenged with intrapulmonary S aureus and exchange transfused 42- (solid circle, solid line) or 7-day-old stored blood (open circle, dashed line). (B) Serial measures of lung injury. Arterial-alveolar oxygen gradient shows lung damage was significantly worse (higher) in the animals with pneumonia receiving 42- (solid circle, solid line) versus 7-day-old (open circle, dashed line) stored blood at 24 hours (P = .005) and 48 hours (P = .01) after bacterial challenge. (C) Degree of shock after exchange transfusion. Shock reversal score accounts for the level of vasopressor support (norepinephrine) needed to maintain the mean arterial pressure at a preset normal level for canines (mean 80 mmHg). Only after 48 hours did bacterial challenge and transfusion of 42-day-old blood produce a significantly worse (lower) shock reversal score (P = .01). (Inset) Degree of shock during exchange transfusion. In contrast, the shock reversal score was improved (higher) in the 42 compared with 7-day-old stored blood (P = .02) during the period of transfusion (7-16 hours). (D) Pulmonary artery pressures during transfusions. Mean pulmonary artery pressures (mPAP) immediately before (circle) and after (triangle) each of the 4 exchange transfusions of 20 mL/kg-stored blood. The difference between pre- and post-mPAP (thick line) was greater with transfusion of 42- (solid symbol, black line) compared with 7-day-old (open symbol, gray line) blood (P = .005, the 4 exchanges averaged). Data presented as mean ± SE.
Survival curves. (A) Kaplan-Meier plot over the 96 hours study comparing animals challenged with intrapulmonary S aureus and exchange transfused 42- (solid circle, solid line) or 7-day-old stored blood (open circle, dashed line). (B) Serial measures of lung injury. Arterial-alveolar oxygen gradient shows lung damage was significantly worse (higher) in the animals with pneumonia receiving 42- (solid circle, solid line) versus 7-day-old (open circle, dashed line) stored blood at 24 hours (P = .005) and 48 hours (P = .01) after bacterial challenge. (C) Degree of shock after exchange transfusion. Shock reversal score accounts for the level of vasopressor support (norepinephrine) needed to maintain the mean arterial pressure at a preset normal level for canines (mean 80 mmHg). Only after 48 hours did bacterial challenge and transfusion of 42-day-old blood produce a significantly worse (lower) shock reversal score (P = .01). (Inset) Degree of shock during exchange transfusion. In contrast, the shock reversal score was improved (higher) in the 42 compared with 7-day-old stored blood (P = .02) during the period of transfusion (7-16 hours). (D) Pulmonary artery pressures during transfusions. Mean pulmonary artery pressures (mPAP) immediately before (circle) and after (triangle) each of the 4 exchange transfusions of 20 mL/kg-stored blood. The difference between pre- and post-mPAP (thick line) was greater with transfusion of 42- (solid symbol, black line) compared with 7-day-old (open symbol, gray line) blood (P = .005, the 4 exchanges averaged). Data presented as mean ± SE.
Systemic and mean pulmonary artery pressure abnormalities
The shock reversal score (see supplemental information) takes into account the level of vasopressor support (norepinephrine) needed to maintain the mean arterial pressure at a preset normal level for canines (mean 80 mmHg). This score showed no significant differences at 4 hours after bacterial challenge comparing the 2 different storage age blood transfused groups (both P = NS; Figure 2C). However, older compared with newer blood during transfusion (7 to 16 hours) was associated with an improved (higher) shock reversal score (P = .02; Figure 2C inset). At 24 hours there was no longer a significant difference in shock score comparing treatment groups but by 48 hours after bacterial challenge, the older versus newer stored blood treatment group actually had a worse (lower) shock reversal score (P = .01).
To investigate this early vasoconstrictive effect during transfusion further, we examined mean pressures in the pulmonary artery (mPAPs), which is the closest blood vessel to where the stored blood was infused. The difference between pre and post-mPAP was greater with transfusion of older compared with newer blood (P = .005, the 4 exchanges averaged; Figure 2D).
Cardiac responses to increased vasoconstrictive properties of older stored blood
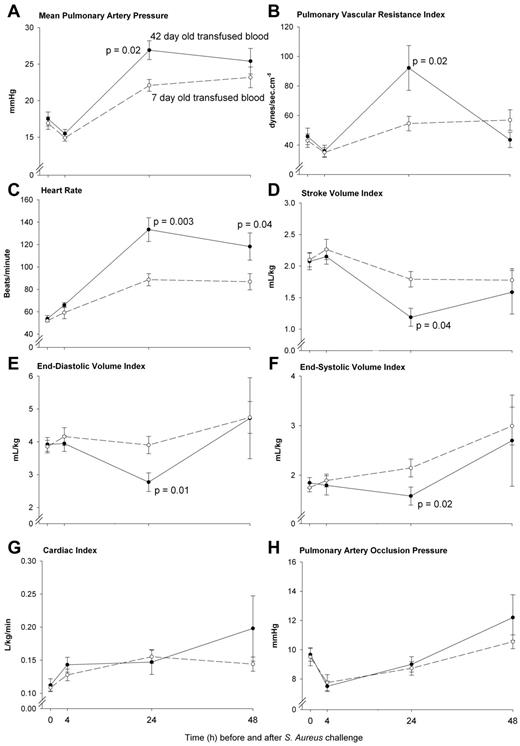
The pulmonary pressures were profoundly increased by 24 hours after transfusion of older compared with newer stored blood (mPAP; P = .02 and pulmonary vascular resistance index; P = .02; Figure 3A-B). In response to this acute pulmonary arterial hypertension at 24 hours associated with older blood transfusion, the size of the left ventricle decreased [left ventricular end diastolic (P = .01) and end systolic volume index (P = .02)], resulting in decreases in stroke volume index (P = .04; Figure 3D-F). A greater mean heart rate (HR) was observed in response to transfusion of older blood at 24 to 48 hours (both, P ≤ .04; Figure 3C). Despite a decrease in stroke volume index, this increase in HR with older blood resulted in animals maintaining at 24 hours, a cardiac index similar to those receiving newer stored blood (P = NS; Figure 3G). Cardiac filling pressures (mean PAOP) were not significantly different after transfusion of older compared with newer stored blood throughout this time period (P = NS; Figure 3H). Of note, at 24 hours the time of severe pulmonary hypertension associated with older blood, on echocardiogram the size of the right ventricle was estimated in comparison to the left ventricle in the 4 chambers view (the only view obtained). Right ventricular dilatation was considered present if the right ventricle was greater than 2/3 the size of the left ventricle read blinded to animal's treatment group by a cardiologist (M.A.S.). At 24 hours, examining all available echocardiograms the animals receiving older versus newer stored blood had more right ventricular dilatation (5/10 [50%] vs 0/8 [0%] respectively; P = .04).
Serial pulmonary vascular pressures and cardiac volumes, filling pressures, output, and heart rates. Serial cardiopulmonary parameters comparing 42-day-old (closed circle, solid line) and 7-day-old (open circle, dashed line) transfused blood. Measures of pulmonary vasoconstriction in animals (A-B) significantly increased after transfusion of 42- compared with 7-day-old stored blood (P = .02 for both) at 24 hours. This greater increase in 42-day-old blood mPAP was associated with a reduced cardiac end diastolic (E, P = .01) and end systolic (F, P = .02) volume index and a subsequent decrease in stroke volume index (D, P = .04) compared with 7-day-old blood. The decrease in these indexes coincided in a significant increased heart rate (C) with older blood at 24 hours (P = .003) that remained elevated to 48 hours (P = .04) resulting in a similar cardiac index (G, P = NS) between treatment groups. Pulmonary artery occlusion pressures (H) were not significantly different after transfusion of 42- compared with 7-day-old transfused blood (P = NS). Data presented as mean ± SE.
Serial pulmonary vascular pressures and cardiac volumes, filling pressures, output, and heart rates. Serial cardiopulmonary parameters comparing 42-day-old (closed circle, solid line) and 7-day-old (open circle, dashed line) transfused blood. Measures of pulmonary vasoconstriction in animals (A-B) significantly increased after transfusion of 42- compared with 7-day-old stored blood (P = .02 for both) at 24 hours. This greater increase in 42-day-old blood mPAP was associated with a reduced cardiac end diastolic (E, P = .01) and end systolic (F, P = .02) volume index and a subsequent decrease in stroke volume index (D, P = .04) compared with 7-day-old blood. The decrease in these indexes coincided in a significant increased heart rate (C) with older blood at 24 hours (P = .003) that remained elevated to 48 hours (P = .04) resulting in a similar cardiac index (G, P = NS) between treatment groups. Pulmonary artery occlusion pressures (H) were not significantly different after transfusion of 42- compared with 7-day-old transfused blood (P = NS). Data presented as mean ± SE.
Plasma CFH levels
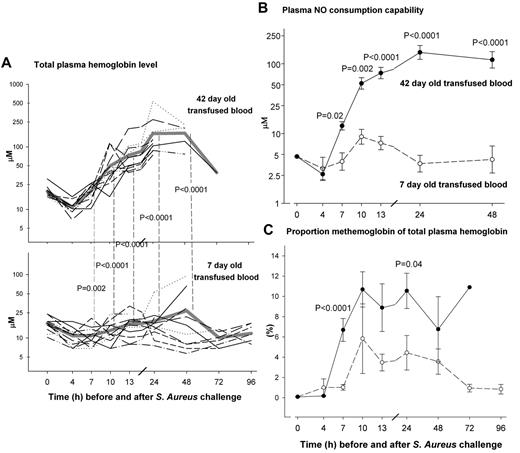
The observed increases in pressures led us to search for a vasoactive substance that could have been transfused more with older blood. CFH, a potentially vasoactive compound that can be found in plasma of animals, was not elevated in either treatment group before exchange transfusion (Figure 4A; 4 hours; P = NS). However, CFH was increased in animals receiving older blood during transfusion (7, 10, and 13 hours; P = .002 to P < .0001) and remained elevated up to 48 hours (24 and 48 hours; both P < .0001). To confirm that CFH was vasoactive, we next determined the nitric oxide (NO) scavenging capability of these plasma samples. Similar to the increases in total CFH levels, there were increases in NO consumption capability of plasma with older versus newer stored transfused blood from 7 to 48 hours (P = .02 to P < .0001; Figure 4B). We then used spectral deconvolution of absorption spectra to examine these plasma samples and found that little of the total CFH (5%-10%) was in the oxidized metHb form, and that most, as indicated by the NO consumption capability assay, was therefore in the reduced oxygenated vasoactive form, oxyhemoglobin (oxyHb).
Serial plasma cell–free hemoglobin levels (CFH), nitric oxide consumption capability, and metHb levels. (A) Serial values of total plasma cell–free hemoglobin (CFH) in 42-day-old (top plot) compared with 7-day-old (bottom plot) transfused stored blood. Mean values for CFH by treatment group over time are represented by a thick gray line. The various forms of dashed lines represent individual animals CFH values over time. The 42-day-old blood was similar to 7-day-old blood before exchange transfusion (4 hours; P = NS) and not elevated compared with baseline. However, CFH was increased in 42- versus 7-day-old blood during transfusion (7, 10, and 13 hours; P = .002 to P < .0001) and remained elevated to 48 hours (24 and 48 hours; both P < .0001). The accuracy of measuring CFH using Drabkins was confirmed by correlating at 24 hours (a time of a wide range of values) with measures using the Winterbourne38 and deconvolution methods (R2 > 0.995; see supplemental Figure 2). The slopes are not significantly different from 1 (0.987 ± 0.012, 0.980 ± 0.015), the intercepts are not significantly different from 0 (2.03 ± 1.68, −2.07 ± 2.20), and R2 are at least 0.995, confirming the accuracy of our methodology measuring CFH. (B) Similar to the increases in total CFH levels, NO consumption capability of plasma was increased in 42- (closed circle, solid line) versus 7-day-old (open circle, dashed line) from 7 to 48 hours (P = .02 to P < .0001). (C) To confirm the CFH was in the reduced species form, we measured the proportion of metHb in total plasma Hb using spectral de-convolution of absorption spectra. Less than 10% of CFH was in the metHb species form indicating approximately 90% of the CFH was in the reduced oxyHb species form consistent with the plasma NO consumption capability findings. Although low on a percentage basis, 42-day-old blood had significantly more metHb by the end of the first infusion (7 hours, P < .0001) that remained higher until 24 hours (P = .04) compared with 7-day-old blood. Data presented (B-C) as mean ± SE.
Serial plasma cell–free hemoglobin levels (CFH), nitric oxide consumption capability, and metHb levels. (A) Serial values of total plasma cell–free hemoglobin (CFH) in 42-day-old (top plot) compared with 7-day-old (bottom plot) transfused stored blood. Mean values for CFH by treatment group over time are represented by a thick gray line. The various forms of dashed lines represent individual animals CFH values over time. The 42-day-old blood was similar to 7-day-old blood before exchange transfusion (4 hours; P = NS) and not elevated compared with baseline. However, CFH was increased in 42- versus 7-day-old blood during transfusion (7, 10, and 13 hours; P = .002 to P < .0001) and remained elevated to 48 hours (24 and 48 hours; both P < .0001). The accuracy of measuring CFH using Drabkins was confirmed by correlating at 24 hours (a time of a wide range of values) with measures using the Winterbourne38 and deconvolution methods (R2 > 0.995; see supplemental Figure 2). The slopes are not significantly different from 1 (0.987 ± 0.012, 0.980 ± 0.015), the intercepts are not significantly different from 0 (2.03 ± 1.68, −2.07 ± 2.20), and R2 are at least 0.995, confirming the accuracy of our methodology measuring CFH. (B) Similar to the increases in total CFH levels, NO consumption capability of plasma was increased in 42- (closed circle, solid line) versus 7-day-old (open circle, dashed line) from 7 to 48 hours (P = .02 to P < .0001). (C) To confirm the CFH was in the reduced species form, we measured the proportion of metHb in total plasma Hb using spectral de-convolution of absorption spectra. Less than 10% of CFH was in the metHb species form indicating approximately 90% of the CFH was in the reduced oxyHb species form consistent with the plasma NO consumption capability findings. Although low on a percentage basis, 42-day-old blood had significantly more metHb by the end of the first infusion (7 hours, P < .0001) that remained higher until 24 hours (P = .04) compared with 7-day-old blood. Data presented (B-C) as mean ± SE.
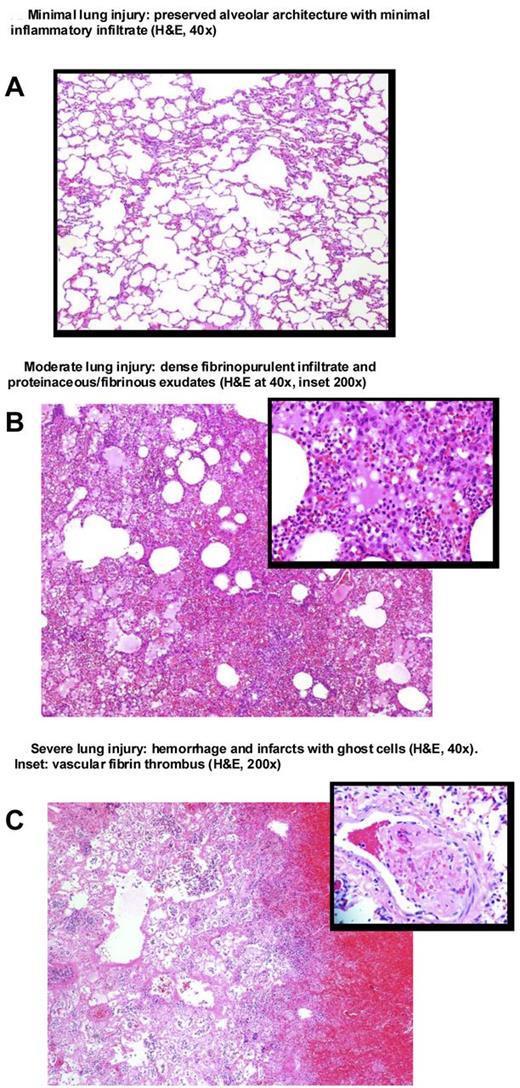
The degree of lung histopathology. Representative postmortem lung tissue samples showing typical minimal, moderate, and severe injury. The typical scores for tissue injury and inflammation in the right infected lung and left nonbacteria-challenged lung with older and newer blood transfusions are as follows: On the left, for nonbacteria-challenged lungs of these animals, the tissue injury scores (hemorrhage, necrosis, thrombosis) were median (range), older blood group 2 (1,2), and newer blood group 2 (1,4); on the right, for infected lungs, the scores were older blood group 3 (1,4) and newer blood group 2 (2,4). On the left, for nonbacteria-challenged lung of these animals, the inflammatory injury scores (neutrophils and fibrin deposition) were older blood group 4 (2,5) and newer blood group 4 (2,5). On the right, for infected lungs, the inflammatory injury scores were older blood group 4 (3-5) and newer blood group 4 (4-6).
The degree of lung histopathology. Representative postmortem lung tissue samples showing typical minimal, moderate, and severe injury. The typical scores for tissue injury and inflammation in the right infected lung and left nonbacteria-challenged lung with older and newer blood transfusions are as follows: On the left, for nonbacteria-challenged lungs of these animals, the tissue injury scores (hemorrhage, necrosis, thrombosis) were median (range), older blood group 2 (1,2), and newer blood group 2 (1,4); on the right, for infected lungs, the scores were older blood group 3 (1,4) and newer blood group 2 (2,4). On the left, for nonbacteria-challenged lung of these animals, the inflammatory injury scores (neutrophils and fibrin deposition) were older blood group 4 (2,5) and newer blood group 4 (2,5). On the right, for infected lungs, the inflammatory injury scores were older blood group 4 (3-5) and newer blood group 4 (4-6).
Markers of hemolysis in the circulation
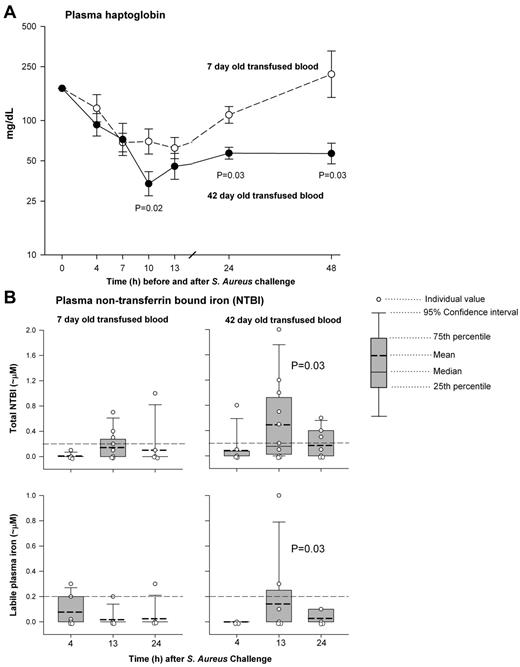
The haptoglobin (Hp) levels with transfusion of older blood were lower at 10 hours (P = .02), 24 hours (P = .03), and 48 hours (P = .03), compared with newer transfused stored blood (Figure 5 top panel). Nontransferrin-bound iron (NTBI) and labile plasma iron, 2 measures of circulating iron levels (iron bound to proteins that do not normally handle release of iron into the plasma versus the toxic iron moiety in plasma, respectively) were significantly increased during transfusion of older versus newer stored blood at 13 hours (both P = .03), but not after the transfusions were finished at 24 hours (both P = NS; Figure 5 bottom panel).
Serial plasma haptoglobin and nontransferrin-bound iron and labile iron levels. (A) Plasma haptoglobin levels were lower in 42 (closed circle, solid line) compared with 7 (open circle, dashed line) day-old blood at 10 hours (P = .02), 24 hours (P = .03), and 48 hours (P = .03). Data presented as mean ± SE. (B) Nontransferrin-bound iron (top plot) and labile plasma iron (bottom plot) were significantly increased with 42- (right plot) but not 7-day-old (left plot) stored blood at 13 hours (during transfusion) compared with 4 hours (just before transfusion; both P = .03). After the transfusions were finished at 24 hours with 7- and 42-day-old blood, the nontransferrin-bound and labile iron were not significantly elevated (both P = NS). Note: One data value at 4 hours of 42-day-old blood of nontransferrin-bound iron was excluded because of an very high value measured before transfusion (shown in figure), whereas the corresponding labile plasma iron level and CFH level for this animal was not elevated which would be inconsistent with our understanding of the biology. If we include this value, the nontransferrin-bound iron at 13 hours compared with 4 hours with 42-day-old blood is not significantly different (P = .20). Data are presented as individual values (open circle), mean (dark dash), median (solid line), 75th and 25th percentile (bounds of gray box) and 95th percentile (error bars).
Serial plasma haptoglobin and nontransferrin-bound iron and labile iron levels. (A) Plasma haptoglobin levels were lower in 42 (closed circle, solid line) compared with 7 (open circle, dashed line) day-old blood at 10 hours (P = .02), 24 hours (P = .03), and 48 hours (P = .03). Data presented as mean ± SE. (B) Nontransferrin-bound iron (top plot) and labile plasma iron (bottom plot) were significantly increased with 42- (right plot) but not 7-day-old (left plot) stored blood at 13 hours (during transfusion) compared with 4 hours (just before transfusion; both P = .03). After the transfusions were finished at 24 hours with 7- and 42-day-old blood, the nontransferrin-bound and labile iron were not significantly elevated (both P = NS). Note: One data value at 4 hours of 42-day-old blood of nontransferrin-bound iron was excluded because of an very high value measured before transfusion (shown in figure), whereas the corresponding labile plasma iron level and CFH level for this animal was not elevated which would be inconsistent with our understanding of the biology. If we include this value, the nontransferrin-bound iron at 13 hours compared with 4 hours with 42-day-old blood is not significantly different (P = .20). Data are presented as individual values (open circle), mean (dark dash), median (solid line), 75th and 25th percentile (bounds of gray box) and 95th percentile (error bars).
Correlations of survival with measures of hemolysis
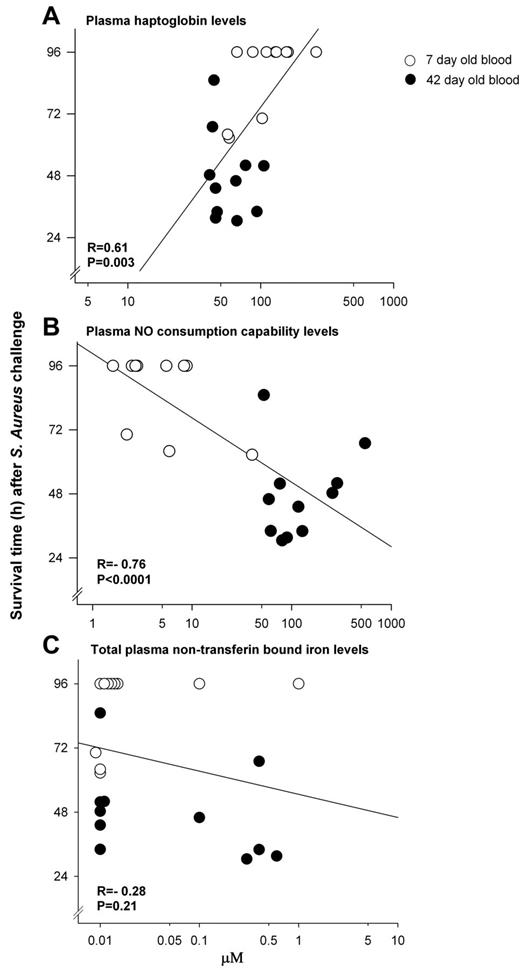
There was a moderately strong association among all animals at 24 hours between plasma Hp levels and survival time (r = 0.61, P = .003), as well as NO consumption capability of plasma and survival time (r = −0.76, P < .0001; Figure 6A-B). In contrast, among all animals receiving transfusion of stored blood, at 24 hours there was no significant association found between NTBI (Figure 6C) or LPI (data not shown) levels and survival times, or at any other time studied (all, P = NS).
Associations between survival and indicators of the degree of hemolysis. Correlations at 24 hours between survival time and (A) plasma haptoglobin levels (r = 0.61, P = .003); and (B) NO consumption capability of plasma (r = −0.76, P < .0001); were significant. In contrast, correlations between survival time and (C) total nontransferrin-bound iron levels (r = −0.28, P = .21) were not.
Associations between survival and indicators of the degree of hemolysis. Correlations at 24 hours between survival time and (A) plasma haptoglobin levels (r = 0.61, P = .003); and (B) NO consumption capability of plasma (r = −0.76, P < .0001); were significant. In contrast, correlations between survival time and (C) total nontransferrin-bound iron levels (r = −0.28, P = .21) were not.
Lung and kidney pathology
To help one understand the type of lung injury found and the range of injuries scores read blinded to treatment group by the pathologists (M.A., M.Q.) in the left nonbacteria-challenged and right bacteria-challenged lung, 3 representative postmortem examples are presented in Figure 7 and the range of tissue injury and inflammation scores found described in the figure legend (panel A, minimal injury; panel B, moderate injury; and panel C, severe injury; see supplemental information). The right directly challenged-with-bacteria lung postmortem was compared within each animal to the left nondirectly bacteria-challenged lung postmortem as a time-matched control. In animals that received older compared with newer stored blood, there was significantly greater lung tissue damage, as evidenced by greater increase in hemorrhage, necrosis, and thrombosis scores for the right infected versus left noninfected lung (1.0 ± 0.25 vs 0.25 ± 0.30; P = .03). There was a greater inflammatory injury score represented by the number of neutrophils per high power field and fibrin deposition in the right versus left lung also; however, in contrast to tissue damage, this increase in inflammation in the right versus left lung was not significantly different comparing the 2 stored blood transfusion groups (0.25 ± 0.35 vs 0.33 ± 0.26; P = NS). In the postmortem kidneys, there were no significant abnormalities in animals regardless of treatment (data not shown).
Quantitative laboratory parameters, sedation support, and fluids in/out
In supplemental Table 2 are shown mean ± SE and significance values for multiple variables at baseline (0 hours), 4, 7, 10, 13, 24, and 48 hours after bacterial challenge for the animals in this study receiving older versus newer stored blood. This is shown for completeness and some variables have significant differences at specific time points but none of this data can explain the overall findings in this study.
Discussion
For investigating the potential toxicity of prolonged storage of blood for transfusion, we used a validated canine model of septic pneumonia whose bacterial challenge dose was reoptimized to account for the effects of multiple transfusions on the mortality profile (see supplemental information). The blood used was collected and prepared by a commercial canine blood bank that is licensed by the FDA, and used procedures and technology similar to what is used for collecting and storing human RBCs.15,19 The canine RBCs used in these experiments had less than 1% hemolysis in the bag at the end of the storage period and ≥ 60% survival of the RBCs by the radio-chromium labeling technique 24 hours after transfusion, within the range of what is expected of stored human RBCs in clinical practice (see supplemental information).20-23 Critically ill canines infected with S aureus pneumonia enrolled in these experiments were all treated as patients with bacterial pneumonia in an intensive care unit. Treatment included, titrated to within normal physiologic parameters; mechanical ventilation, appropriate fluids, vasopressors and sedation, as well as antibiotics and laboratory monitoring. These critically ill animals were then randomized in a blinded fashion to be exchange transfused with 1 blood volume of 42- or 7-day-old stored commercially available, universal donor canine blood.
In these septic animals, transfusion of the older blood was associated with significantly increased mortality (Figure 2), lung injury, and degree of shock. The older blood was more vasoactive in that during transfusion, systemic pressures and pulmonary artery pressures (Figure 2) were higher, and pulmonary pressures remained elevated for at least 10 hours after transfusion (Figure 3), meeting criteria for acute pulmonary arterial hypertension.24,25 This pulmonary hypertension was sufficiently severe as to cause right ventricular dilatation and adversely affect left ventricular filling, resulting in marked tachycardia to maintain cardiac output. The concentration of plasma cell–free hemoglobin (CFH) increased progressively for days in these animals receiving older blood; CFH release from ongoing hemolysis of older blood was further documented by significantly decreased haptoglobin levels. In addition, with older blood there was a progressive increase of NO consumption capability of plasma over several days, indicating the presence of the reduced form of hemoglobin, oxyHb, which can scavenge NO and is therefore potentially vasoactive.8,26-32
The degree of decrease in haptoglobin, as well as increases in NO consumption levels at 24 hours, were both significantly associated with decreased survival. Further, with older blood transfusions, stained sections of lung obtained from the site of infection at necropsy showed more severe ischemic vascular damage, as evidenced by increased necrosis, hemorrhage, and thrombosis. Taken together, the above data suggest that the mechanism of increased risk of older blood involves a greater propensity after transfusion for ongoing hemolysis as stored blood ages in vivo, which during transfusion and for days afterward results in release into the plasma of substantial quantities of oxyHb (on average from 7-48 hours, a gradual increase from approximately 20-150μM).8,26-32 Prolonged exposure to oxyHb, a vasoconstrictive substance in the plasma, resulted in worsening of ischemic vascular damage in the lung at the site of tissue injury, resulting in more severe gas exchange abnormalities, and pulmonary arterial hypertension,29,30,33 which contributed to the increased risk of death from transfusion. It is not clear from our experiment whether the “first pass” of hemoglobin through the lung makes this organ particularly sensitive, or whether pre-existing pulmonary damage is necessary for the increase in mortality. However, necropsy findings did not indicate that other organs were specifically targeted or responsible for the animals' demise.
Transient increases in nontransferrin-bound iron (NTBI) and labile iron (the toxic iron moiety) were also noted with older blood during the exchange transfusions, but not afterward. The availability of circulating iron, at 8 to 12 hours after bacterial challenge, could promote bacterial growth of the S aureus challenge, exacerbating the infection in the lung, shock, pulmonary hypertension, gas exchange abnormalities, and the risk of mortality.5 However, exchange transfusions were begun only after administering a cidal antibiotic, Oxacillin, to which the S aureus challenge is sensitive, preventing bacterial growth.18 Consistent with this finding, blood culture in all animals at 8 to 10 hours after transfusion showed no growth. Nonetheless, the transient release of NTBI and labile iron during transfusion is a potential contributor to the increased risk of death in this model of S aureus pneumonia. Finally, S aureus can itself be hemolytic; however, the lack of hemolysis in animals who received only fresh blood is strong evidence that the specific bacterial species is not causally implicated.
This canine study is the first blinded randomized trial in humans or animals using standard blood banking techniques to show that blood transfused at the end of the storage period can increase mortality. The mechanism appears to be increased in vivo hemolysis with older stored blood that results in release of CFH, NO depletion and vascular injury and/or increased circulating iron, promoting bacterial growth and inflammation, causing injury. Supporting the NO depletion hypothesis, Baek et al have shown in a guinea pig model of transfusion, older but not newer stored blood led to hemolysis, vasoconstriction, vascular injury, and kidney dysfunction.7 Those effects were attenuated by forming a CFH and Hp complex thus isolating these injuries to release of CFH. Earlier studies in mice, consistent with this hypothesis, found high levels of CFH depletes haptoglobin and contributes to renal injury.34 In contrast, and in support of the iron hypothesis, Hod et al have shown in mice transfused with stored older blood produced increased NTBI levels and increased inflammation, as well as enhanced bacterial grown in vitro.5
Data from our study overall are more consistent with the NO depletion than the iron hypothesis: (1) There was a strong association with increases in NO consumption capability in plasma and decreased survival but no significant association between increases in NTBI or labile iron levels and survival. (2) The elevations of the vasoconstrictive CFH persisted for up to 48 hours in these animals, providing substantial time to aggravate vascular injury at sites of infection. In contrast, NTBI and labile iron elevations were only elevated transiently during transfusions in animals on cidal antibiotics preventing bacterial growth. (3) With older blood, lung pathology showed evidence of increased vascular injury with hemorrhage, necrosis, and thrombosis, consistent with the NO depletion, but no signs of worsening inflammation, which might have suggested more severe pulmonary infection because of increased bacterial growth from circulating iron.
We know of no evidence from critically ill septic patients receiving massive transfusions to extrapolate to our experiment, or that such serial observations have been made in transfused septic human subjects. Most chronically or massively transfused patients or exchange-transfused patients receive relatively fresh blood. In trauma patients, transfusing blood of average storage time (12.2 days) in large volumes, approximately 1 to 6 L, in the peritransfusion period, consistent with our data, produced decreased haptoglobin levels and increased circulating CFH.35 Even with small volumes of transfusion in stable patients with anemia, CFH can be detected and blood storage time correlates with CFH concentration.36 Moreover, transfusion of 2 units of RBCs produced higher CFH levels than of 1 unit of RBCs. The relationship between age of the stored blood, the volume of blood transfused, increases of CFH levels over time, and outcome, needs to be determined for septic patients and other critically ill patients.37
There are limitations to the interpretation of our data; we only examined septic canines with pneumonia receiving massive transfusions and our findings might not be applicable to noninfectious illnesses, different types of infections, variation in the severity of infection we studied, or transfusions that are not massive in quantity. Although hemoglobin and RBCs across species are believed well preserved,15-17 our results may be idiosyncratic to canines. Nonetheless, at least in 1 murine model, findings were somewhat comparable.7 The effects of age of stored blood as well, as critical illness on the development of CFH levels over days after transfusion, has not been fully elucidated in humans, and elevations we found after transfusion of older stored blood may represent only poorer storage of canine RBCs unrelated to human transfusion of stored blood. Finally, although our data show that older blood increases risks and implicate increased hemolysis with release of CFH, causing vascular injury, we cannot exclude other causative factors, some known (such as increases in labile iron) and some unknown, as contributing to this increased risk.
In conclusion, in a blinded, randomized trial in canines with pneumonia, animals randomized to massive transfusion of older stored blood had an increased mortality rate, thus documenting at least in some settings, as stored blood ages, the risk of death from transfusion increases. The data from this study support increased in vivo hemolysis and the NO depletion hypothesis as the mechanism of increased risks of older stored blood, in that with older blood, the increased in vivo hemolysis results in release of cell-free oxyHb over days, causing pulmonary hypertension and vascular damage at sites of injury, and gas exchange abnormalities, each contributing to the increased risk of death with older blood.8
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Juli Maltagliati and Kelly Byrne for excellent assistance in producing and submitting the paper, and John Hess, MD, of the University of Maryland, for advice on setting up canine blood banking procedures.
Intramural NIH funds and NIH external grants HL058091 and HL098032 were used to support this study. The work by the authors was done as part of US government–funded research; however, the opinions expressed are not necessarily those of the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: S.B.S. and D.W. performed experiments, helped write the first draft of the paper, and helped make figures; J.S. designed the study and performed statistical analysis; J.F. performed experiments and made figures; T.K. and C.H. performed laboratory analysis; M.A.S. read cardiac echoes; M.A. and M.Q. read pathology slides; M.T.G. designed the study, provided laboratory support, and edited the paper; D.B.K.-S. provided laboratory support and edited the paper; H.G.K. set up blood-banking procedures, designed the study, and edited the paper; and C.N. conceived and designed the study, wrote the first draft, and edited the paper.
Conflict-of-interest disclosure: D.K.-S. and M.T.G. are listed as coauthors on a patent application on methods of treating hemolysis and on patent applications related to development of blood substitutes using recombinant human neuroglobin. The remaining authors declare no competing financial intersts.
Correspondence: Charles Natanson, Critical Care Medicine Dept, NIH Clinical Center, 10 Center Dr, Rm 2C145, National Institutes of Health, Bethesda, MD 20892; e-mail: cnatanson@cc.nih.gov.
References
Author notes
S.B.S. and D.W. contributed equally to this work.