The report by Solomon et al in this issue of Blood is a welcome addition to understanding the clinical impact of prolonged red cell storage.1
A major advance in blood banking during the past several decades is the ability to successfully preserve red blood cells for up to 42 days. This has contributed to improved inventory management and decreased blood product wastage. It has also been long known that a “storage lesion” develops although only some changes have been identified, the storage lesion was never defined comprehensively, and few efforts were devoted to this issue. The National Heart, Lung, and Blood Institute (NHLBI) is funding several R01 projects to use contemporary techniques to determine these storage-related changes. An impetus for this basic science work is that one of the current major issues in transfusion medicine is whether red blood cells undergo changes during storage that generate cytokines, inflammatory mediators, or other agents that can create clinical problems in patients. Several clinical trials and laboratory studies have shown that long-stored red cells have a detrimental effect, particularly in patients undergoing cardiovascular surgery.2-5 However, each of these trials has shortcomings, and other trials have not found an effect on long-stored red blood cells.5-7 An NHLBI-funded large, multicenter, randomized trial comparing red cells stored for less than 10 days with cells stored for more than 21 days in patients undergoing cardiovascular surgery is under way in hopes of resolving this important clinical issue.8 However, even well-designed large, multicenter clinical trials have weaknesses and it will likely be very difficult to resolve this issue definitively with clinical trials.8 In addition, clinical trials involve specifically defined patient groups that will inevitably lead to the concern that the effects may be different in different kinds of patients. Thus, an animal model such as that described by Solomon et al1 will be extremely valuable in blending in vitro data developed in the basic science laboratory with information from complex clinical trials.
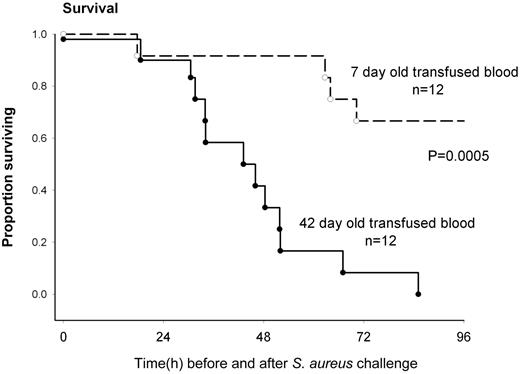
Solomon et al have developed a canine model with Staphylococcus aureus–induced pneumonia in 1 lung with its attendant sepsis.1 They then create 4 episodes of very substantial (25% blood volume) acute blood loss followed immediately by an equivalent red cell and plasma transfusion. Shed blood is replaced by either 7- or 42-day stored canine red cells. The animals were treated similar to human intensive care patients and thoroughly monitored. Animals who received longer-stored blood had poorer survival (as illustrated in the figure), greater degree of shock, higher pulmonary artery pressures, right ventricular dilatation, a poorer arterial-alveolar oxygen gradient, and greater tachycardia. Not unexpectedly, the concentration of cell-free hemoglobin increased, the haptoglobin decreased, and nitric oxide consumption increased in the recipients of longer-stored red cells. Using the noninfected lung as a control, the infected lungs showed more hemorrhage, necrosis, and thrombosis in animals who received longer-stored red cells.
Survival of Stapholococcus aureus–challenged animals who shed blood was replaced by 7- or 42-day stored red cells. See Figure 2A in the article by Solomon et al that begins on page 1663.
Survival of Stapholococcus aureus–challenged animals who shed blood was replaced by 7- or 42-day stored red cells. See Figure 2A in the article by Solomon et al that begins on page 1663.
Not all observations showed detrimental effects of longer-stored red cells. The shock reversal score was better in the group receiving longer-stored red cells and there was no difference in cardiac output between the 2 groups, possibly because tachycardia compensated for decreased stroke volume. A pulmonary inflammatory injury score involving neutrophils and fibrin was not different between the animals receiving longer- versus shorter-stored red cells.
Several methodologic issues affect thinking about these results. While probably necessary for hemostasis, plasma was also unfortunately transfused, complicating the picture. While plasma is used in massive transfusion, it is not routinely used in many other clinical transfusion situations in which the possible detrimental effects of longer-stored red cells have been raised. The Solomon model involves acute loss of 25% blood volume 4 times during 13 hours each followed by an episode of massive transfusion. This is major blood loss, but also a limited situation in contrast to a large but more continuous blood loss that would not necessarily involve the fluid shifts and other physiologic effects of acute large blood loss.
It is not clear whether canine red cells at the end of the storage period are equivalent to similarly stored human red cells. The reported posttransfusion recovery of canine red cells of 60% would not meet Food and Drug Administration requirements for human red cell preservation. Thus, it is possible that the canine red cells would be more severely damaged and lead to more severe or additional toxicities than those caused by human red cells stored for 42 days.
The S aureus model of pneumonia with resultant pulmonary and endothelial damage provides an excellent milieu in which to study the effects of red cell transfusion, but it is difficult to know the extent to which this can mimic the variety of human clinical circumstances in which stored red cells might have an adverse effect. Finally, because of the blood loss/transfusion strategy, the model may have a limited extrapolation to the human clinical situation because the risks of longer stored blood—if true—may apply to only certain patient populations.
Solomon and colleagues have provided valuable and interesting data that will add to the understanding of the clinical effects of red cell storage and a model that can serve as a bridge between in vitro data and human in vivo clinical trial results.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal