Key Points
This study identified a distinct subgroup of WAS patients with an early onset (before the age of 2 years) of severe, life-threatening manifestations.
HSCT is a curative strategy in this subgroup of patients and should be performed as early in life as possible, even when a fully matched donor is lacking.
Abstract
On the basis of a nationwide database of 160 patients with Wiskott-Aldrich syndrome (WAS), we identified a subset of infants who were significantly more likely to be attributed with an Ochs score of 5 before the age of 2 (n = 26 of 47 [55%], P = 2.8 × 10−7). A retrospective analysis revealed that these patients often had severe refractory thrombocytopenia (n = 13), autoimmune hemolytic anemia (n = 15), and vasculitis (n = 6). One patient had developed 2 distinct cancers. Hemizygous mutations predictive of the absence of WAS protein were identified in 19 of the 24 tested patients, and the absence of WAS protein was confirmed in all 10 investigated cases. Allogeneic hematopoietic stem cell transplantation (HSCT) was found to be a curative treatment with a relatively good prognosis because it was successful in 17 of 22 patients. Nevertheless, 3 patients experienced significant disease sequelae and 4 patients died before HSCT. Therefore, the present study identifies a distinct subgroup of WAS patients with early-onset, life-threatening manifestations. We suggest that HSCT is a curative strategy in this subgroup of patients and should be performed as early in life as possible, even when a fully matched donor is lacking.
Introduction
Wiskott-Aldrich syndrome (WAS, OMIM#301000) is a rare, X-linked, primary immune deficiency (PID) that leads to the classic triad of eczema, microthrombocytopenia, and combined immunodeficiency.1,2 The incidence of WAS has been estimated at less than 1 in 100 000 live births.3 The condition is caused by hemizygous mutations in the WAS gene (Xp11.22-23), which encodes the WAS protein (WASp).4 The latter is expressed exclusively in hematopoietic cells and has a major role in actin polymerization, regulation of cytoskeleton reorganization, signal transduction, and apoptosis.5 Mutations in the WAS gene lead to a wide spectrum of diseases, ranging from a mild form of WAS (X-linked thrombocytopenia or XLT)6 to the archetypal, severe form of WAS.7 Patients with a typical WAS phenotype can be cured by allogeneic hematopoietic stem cell transplantation (HSCT).8 Furthermore, gene-therapy protocols are currently being developed for patients lacking a satisfactory donor.9-11 Genotype-phenotype correlation studies have shown that patients not expressing WASp have a more severe phenotype than patients with residual WASp expression.7,12 However, it is currently impossible to predict a patient's individual outcome on the basis of their WAS genotype and/or WASp expression levels. In WAS, the widely used clinical severity score developed by Ochs ranges from 1-3 for XLT/mild WAS patients and 4-5 for patients with an archetypal phenotype.13 A score of 5 is associated with severe disease (autoimmunity and/or inflammation and/or malignancy).14,15 Although severe forms of WAS generally occur in patients displaying the full-blown WAS phenotype, severe autoimmunity and malignancy can also occur at any age (and with no forewarning) in XLT patients.12,13,16 Severe, life-threatening presentations in XLT/WAS may occur in young children and are particularly challenging.14,15 In the present study of a nationwide cohort of patients with WAS, we sought to better describe the presentations and outcomes of patients assigned with a score of 5 on the Ochs scale early in life.
Methods
Patients
The French National Center for Primary Immunodeficiencies (CEREDIH) has established a national network of 58 medical departments with experience in the care of pediatric and adult patients with PIDs. The CEREDIH collects data according to the European Society for Immunodeficiencies (ESID) core dataset and enters them into the ESID secure online database.17,18 In accordance with French regulatory requirements, informed consent (in accordance with the Declaration of Helsinki) was obtained when patients were registered in the CEREDIH registry. In this context, we carried out a retrospective, nationwide analysis of the medical records of French XLT/WAS patients. According to a published clinical classification,13 a score of 5 is defined as the presence of either clinical autoimmunity and/or inflammation and/or a tumor. We defined the specific feature of severe refractory thrombocytopenia (SRT) as a platelet count that never increased above 10 G/L (even 1 hour after platelet transfusions) and bleeding (regardless of the patient's antiplatelet antibody status). Given the life-threatening severity of this condition and the difficulty in establishing an autoimmune origin, patients with SRT were assigned a score of 5. Profound thrombocytopenia was defined as a recurrently measured platelet count of between 10 and 20 G/L, but did not prompt the assignment of a score of 5. We performed a detailed, retrospective analysis of the patients' medical records and collected and reviewed clinical, immunologic, and genetic data.
Immunologic investigations
Immunophenotyping results and serum Ig levels were retrieved from the CEREDIH registry. Autoantibody assay results were also retrospectively retrieved from the patients' medical records.
Genetic testing and WASp expression
Assessment of the severity of hemorrhage and vasculitis
Hemorrhagic episodes were considered to be severe when they: (1) involved a major organ (the CNS, lungs, or digestive tract), (2) were life-threatening or associated with significant morbidity (joints or eyes), or (3) required specific medical care (including hospitalization and RBC transfusions). Intraoral hemorrhage was not considered to be severe. In terms of vasculitis, we considered only unmistakably severe episodes involving major organs (eg, the brain, digestive tract, lungs, large areas of the skin, and/or many different joints) that were documented by either medical imaging and/or histology. No kidney vasculitis occurred in our patients below the age of 2 years.
Evaluation of therapy
The treatment outcome was classified as a complete response (CR) or a partial response (PR) on the basis of the complete or partial correction of clinical manifestations, respectively.
Statistical analysis
Differences in observed distributions were analyzed using a χ2 test or Fisher test, as appropriate. Survival was analyzed according to the Kaplan-Meier method and median follow-up was estimated with inverse Kaplan-Meier curves. The smoothed hazard function was estimated using a kernel-based approach. A piecewise exponential model of survival data were used to compare the risks of developing a score of 5 during or after the first 2 years of life, respectively. All tests were 2-sided and the threshold for statistical significance was set to P < .05. All statistical analyses were carried out using the survival and muhaz routines in the R Version 2.13.1 software package.
Results
Probability of assignment of a score of 5 in the WAS /XLT cohort (Figure 1)
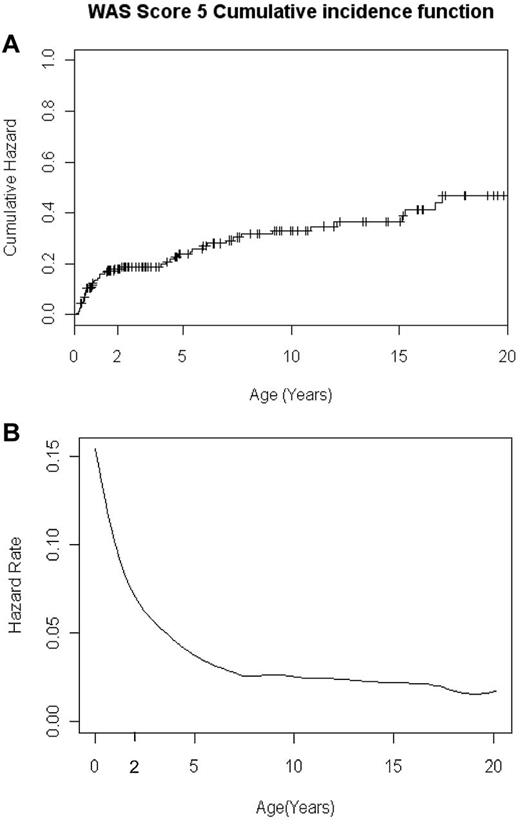
The cumulative probability of being assigned an Ochs score of 5 in the French national cohort of 160 WAS patients. (A) Cumulative incidence function of being assigned an Ochs score of 5 as a function of age. (B) Smoothed instantaneous hazard function of being assigned an Ochs score of 5 as a function of age.
The cumulative probability of being assigned an Ochs score of 5 in the French national cohort of 160 WAS patients. (A) Cumulative incidence function of being assigned an Ochs score of 5 as a function of age. (B) Smoothed instantaneous hazard function of being assigned an Ochs score of 5 as a function of age.
A total of 160 consecutive patients in the CEREDIH registry had a diagnosis of WAS (n = 135 patients) or XLT (n = 25 patients). The median age at diagnosis was 0.8 years. Forty-seven patients (29%) had autoimmunity, tumors, and/or SRT, prompting the assignment of an Ochs score of 5. The cumulative event probability and hazard function curves (among the 160 patients) of developing a score of 5 are depicted in Figure 1. These curves show heterogeneity in the likelihood of being assigned a score of 5 before or after the patient's second birthday and suggest the existence of 2 distinct subsets of WAS patients. The annual risk rate for being assigned a score of 5 was significantly higher during the first 2 years of life (11 cases per 100 subjects at risk) than after the second birthday (2.4 cases per 100 subjects at risk, P = 2.8 × 10−7). Therefore, we focused our analysis on the subgroup of patients with an early-onset, severe, life-threatening WAS phenotype.
Characteristics of WAS patients assigned a score of 5 in the first 2 years of life
Twenty-six (16%) unrelated patients born between 1983 and 2010 could be assigned a score of 5 at some point in their first 2 years of life (Table 1). The median age at onset in this subgroup was 6.2 months (range, 2-22.5). The revealing sign was autoimmune hemolytic anemia (AIHA) in 9 of the 26 patients (36%). Three of these 9 patients had AIHA alone (P4, P8, and P12), 3 of the others had AIHA with profound thrombocytopenia (P3, P13, and P10), and the remaining 3 had autoimmune pancytopenia (P2, P17, and P19). In 5 patients (19%), attribution of a score of 5 had been prompted by vasculitis; 4 of these 5 patients had vasculitis only (P5, P15, P18, and P17) and 1 (P9) had vasculitis with SRT.
The first clinical manifestations leading to an assignment of a score of 5 in the first 2 years of life (N = 26)
| Manifestation at onset of a score of 5 . | n . | % . |
|---|---|---|
| SRT only | 12 | 46 |
| AIHA only | 3 | 12 |
| AIHA + profound thrombocytopenia* | 3 | 12 |
| Autoimmune pancytopenia | 3 | 12 |
| Vasculitis only | 4 | 15 |
| Vasculitis + SRT | 1 | 4 |
| Tumor only | 0 | 0 |
| Manifestation at onset of a score of 5 . | n . | % . |
|---|---|---|
| SRT only | 12 | 46 |
| AIHA only | 3 | 12 |
| AIHA + profound thrombocytopenia* | 3 | 12 |
| Autoimmune pancytopenia | 3 | 12 |
| Vasculitis only | 4 | 15 |
| Vasculitis + SRT | 1 | 4 |
| Tumor only | 0 | 0 |
SRT indicates severe refractory thrombocytopenia (platelet count < 10 G/L); profound thrombocytopenia, a platelet count of between 10 and 20 G/L; and AIHA, autoimmune hemolytic anemia.
At the time when a score of 5 was attributed, the 12 remaining patients (46%) had SRT in the absence of any other documented autoimmune manifestation (see Table 2 for details). It is noteworthy that in 7 of these 12 patients, SRT remained the only manifestation of severe WAS for the first 2 years of life. SRT was associated with another manifestation (vasculitis) in 1 patient (P9). Overall, antiplatelet antibodies were found in 10 of the 17 tested cases of SRT or another type of thrombocytopenia. However, the limited feasibility and reliability of autoantibody screening in infants with profound or severe thrombocytopenia made it difficult to determine the autoimmune origin of these manifestations. Strikingly, patients who were first scored as a 5 before the age of 9 months (n = 16) differed significantly from those who were first scored as a 5 after that age in terms of the occurrence of SRT (P = .041), but not in terms of AIHA (n = 4, P = .234) or vasculitis (n = 1, P = .055; Table 3). Patients who were first scored as a 5 before the age of 9 months also differed significantly from those scored as a 5 after the age of 2 (see the supplemental materials, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
WAS mutations, WASp expression, and a summary of clinical manifestations in 26 patients with WAS and assignment of a score of 5 in the first 2 years of life
| Patient no. . | Mutation description . | WAS gene mutation . | WASp expression . | Age at onset of score 5, mo . | Thrombo-cytopenia . | Hemorrhages . | AIHA . | Vasculitis . | Tumor . | HSCT . | Overall outcome (cause of death), age at last follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ND | ND | 12.7 | Severe refractory | Intraoral; recurrent epistaxis; knee joint; brain | Yes | No | No | No HSCT | Died, age 4 y (brain hemorrhage) | |
| 2 | p.Y107S | Missense | ND | 6.2 | Profound | Severe otorrhagia; massive epistaxis | Yes | No | No | Haplo | A/W, age 28 y |
| 3 | c.1001delG | Nonsense | ND | 9.2 | Profound | Severe hematotympanum | Yes | No | No | Haplo | Died at M+3 (hemorrhagy) |
| 4 | E452 fs X494 | Nonsense | ND | 5.8 | Mild | No | Yes | No | No | 2 HSCT (haplo then MSD) | Alive, age 24 y |
| 5 | p.F114I | Missense | ND | 22.3 | Profound | Brain; gut | No | Yes | No | URD | Alive, age 22 y |
| 6 | ND | ND | 6.1 | Severe refractory | No | Yes | No | No | Haplo | Died, at M+4 (TRM, GvHD, Infections) | |
| 7 | p.R34X | Nonsense | ND | 11.2 | Mild | No | No | Yes | No | Haplo | Alive, age 21 y |
| 8 | c.1001delG | Nonsense | ND | 10.2 | Mild | Intraoral | Yes | No | No | Haplo | Died, M+1 (Viral pneumonitis) |
| 9 | p.W97X | Nonsense | ND | 14.2 | Severe refractory | No | Yes | Yes | No | URD | A/W, age 14 y |
| 10 | p.C73Y | Missense | ND | 10.3 | Profound | No | Yes | No | No | MSD (UCB) | A/W, age 13 y |
| 11 | Del Exons 11–12 | Deletion | ND | 3.1 | Severe refractory | Lung | Yes | No | No | No HSCT | Died, age 4 mo (fulminant sepsis, lung hemorrhage) |
| 12 | p.Asp132_Glu133del | Missense | Absent | 14.2 | Mild | Gut; Retina | Yes | No | Yes | mMRD 9.5/10 | A/W, age 11 y, thrombocytopenia resolved after splenectomy at M+4 |
| 13 | c.273 + 1G > A | Splice site | ND | 2.9 | Profound | Brain | Yes | No | No | URD | A/W, age 10 y |
| 14 | Del Exons 1–2 | Deletion | ND | 4.7 | Severe refractory | Intraoral; brain | Yes | No | No | No HSCT | Died, age 15 mo (brain hemorrhage during conditioning regimen) |
| 15 | Del Exons 1–7 | Deletion | Absent | 3.1 | Profound | Gut | No | Yes | No | 2 HSCT (haplo then URD) | Died, 3 y after HSCT (Enterobacter sepsis after abdominal surgery) |
| 16 | c.360 + 1G > T | Splice site | ND | 5.1 | Severe refractory | No | No | No | No | Haplo | A/W, age 7 y |
| 17 | p.Gly70_Ala71del | Deletion | Absent | 22.6 | Profound | Brain | Yes | Yes | No | Haplo | Top-up at M+4; A/W, age 6 y |
| 18 | c.1429_1433del | Nonsense | Absent | 9.5 | Mild | No | No | Yes | No | URD | A/W, age 6 y |
| 19 | p.N224G | Missense | Absent | 6.4 | Profound | No | Yes | No | No | Haplo | A/W, age 6 y |
| 20 | Insertion 4bp | Insertion | ND | 2.0 | Severe refractory | No | No | No | No | MRD | Died, 3.5 y after HSCT (sepsis) |
| 21 | c.1086delA | Nonsense | Absent | 4.2 | Severe refractory | No | Yes | No | No | Haplo | Top-up, M+4; A/W, age 5 y |
| 22 | p.R41X | Nonsense | Absent | 5.1 | Severe refractory | No | No | No | No | URD (UCB) | A/W, age 3 y |
| 23 | c.941delC | Nonsense | Absent | 3.1 | Severe refractory | No | No | No | No | Haplo | A/W, age 3 y |
| 24 | p.L435X | Nonsense | Absent | 9.1 | Severe refractory | Intraoral; macroscopic hematuria | No | No | No | URD | A/W, age 3 y |
| 25 | c.525delA | Nonsense | Absent | 6.2 | Severe refractory | No | No | No | No | URD | A/W, age 2 y |
| 26 | c.360 + 1G > A | Splice site | ND | 2.9 | Severe refractory | Brain | No | No | No | No HSCT | Died, age 6 mo (brain hemorrhage) |
| Patient no. . | Mutation description . | WAS gene mutation . | WASp expression . | Age at onset of score 5, mo . | Thrombo-cytopenia . | Hemorrhages . | AIHA . | Vasculitis . | Tumor . | HSCT . | Overall outcome (cause of death), age at last follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ND | ND | 12.7 | Severe refractory | Intraoral; recurrent epistaxis; knee joint; brain | Yes | No | No | No HSCT | Died, age 4 y (brain hemorrhage) | |
| 2 | p.Y107S | Missense | ND | 6.2 | Profound | Severe otorrhagia; massive epistaxis | Yes | No | No | Haplo | A/W, age 28 y |
| 3 | c.1001delG | Nonsense | ND | 9.2 | Profound | Severe hematotympanum | Yes | No | No | Haplo | Died at M+3 (hemorrhagy) |
| 4 | E452 fs X494 | Nonsense | ND | 5.8 | Mild | No | Yes | No | No | 2 HSCT (haplo then MSD) | Alive, age 24 y |
| 5 | p.F114I | Missense | ND | 22.3 | Profound | Brain; gut | No | Yes | No | URD | Alive, age 22 y |
| 6 | ND | ND | 6.1 | Severe refractory | No | Yes | No | No | Haplo | Died, at M+4 (TRM, GvHD, Infections) | |
| 7 | p.R34X | Nonsense | ND | 11.2 | Mild | No | No | Yes | No | Haplo | Alive, age 21 y |
| 8 | c.1001delG | Nonsense | ND | 10.2 | Mild | Intraoral | Yes | No | No | Haplo | Died, M+1 (Viral pneumonitis) |
| 9 | p.W97X | Nonsense | ND | 14.2 | Severe refractory | No | Yes | Yes | No | URD | A/W, age 14 y |
| 10 | p.C73Y | Missense | ND | 10.3 | Profound | No | Yes | No | No | MSD (UCB) | A/W, age 13 y |
| 11 | Del Exons 11–12 | Deletion | ND | 3.1 | Severe refractory | Lung | Yes | No | No | No HSCT | Died, age 4 mo (fulminant sepsis, lung hemorrhage) |
| 12 | p.Asp132_Glu133del | Missense | Absent | 14.2 | Mild | Gut; Retina | Yes | No | Yes | mMRD 9.5/10 | A/W, age 11 y, thrombocytopenia resolved after splenectomy at M+4 |
| 13 | c.273 + 1G > A | Splice site | ND | 2.9 | Profound | Brain | Yes | No | No | URD | A/W, age 10 y |
| 14 | Del Exons 1–2 | Deletion | ND | 4.7 | Severe refractory | Intraoral; brain | Yes | No | No | No HSCT | Died, age 15 mo (brain hemorrhage during conditioning regimen) |
| 15 | Del Exons 1–7 | Deletion | Absent | 3.1 | Profound | Gut | No | Yes | No | 2 HSCT (haplo then URD) | Died, 3 y after HSCT (Enterobacter sepsis after abdominal surgery) |
| 16 | c.360 + 1G > T | Splice site | ND | 5.1 | Severe refractory | No | No | No | No | Haplo | A/W, age 7 y |
| 17 | p.Gly70_Ala71del | Deletion | Absent | 22.6 | Profound | Brain | Yes | Yes | No | Haplo | Top-up at M+4; A/W, age 6 y |
| 18 | c.1429_1433del | Nonsense | Absent | 9.5 | Mild | No | No | Yes | No | URD | A/W, age 6 y |
| 19 | p.N224G | Missense | Absent | 6.4 | Profound | No | Yes | No | No | Haplo | A/W, age 6 y |
| 20 | Insertion 4bp | Insertion | ND | 2.0 | Severe refractory | No | No | No | No | MRD | Died, 3.5 y after HSCT (sepsis) |
| 21 | c.1086delA | Nonsense | Absent | 4.2 | Severe refractory | No | Yes | No | No | Haplo | Top-up, M+4; A/W, age 5 y |
| 22 | p.R41X | Nonsense | Absent | 5.1 | Severe refractory | No | No | No | No | URD (UCB) | A/W, age 3 y |
| 23 | c.941delC | Nonsense | Absent | 3.1 | Severe refractory | No | No | No | No | Haplo | A/W, age 3 y |
| 24 | p.L435X | Nonsense | Absent | 9.1 | Severe refractory | Intraoral; macroscopic hematuria | No | No | No | URD | A/W, age 3 y |
| 25 | c.525delA | Nonsense | Absent | 6.2 | Severe refractory | No | No | No | No | URD | A/W, age 2 y |
| 26 | c.360 + 1G > A | Splice site | ND | 2.9 | Severe refractory | Brain | No | No | No | No HSCT | Died, age 6 mo (brain hemorrhage) |
A/W indicates alive and well; Haplo, haploidentical donor (parent); mMRD, partially mismatched related donor; MRD, matched related donor; MSD, matched sibling donor; ND, not determined; TRM, transplantation-related mortality; UCB, umbilical cord blood; and URD, unrelated donor.
Occurrence of severe WAS complications as a function of assignment of a score of 5 before or after the age of 9 months
| Age at score 5 . | n . | SRT . | AIHA . | Vasculitis . | Alive at last follow-up . |
|---|---|---|---|---|---|
| < 9 mo | 16 | 11 | 4 | 1 | 10 |
| > 9 mo | 10 | 2 | 5 | 4 | 7 |
| Age at score 5 . | n . | SRT . | AIHA . | Vasculitis . | Alive at last follow-up . |
|---|---|---|---|---|---|
| < 9 mo | 16 | 11 | 4 | 1 | 10 |
| > 9 mo | 10 | 2 | 5 | 4 | 7 |
AIHA indicates autoimmune hemolytic anemia; f/u, follow-up; mo, months; and SRT, severe refractory thrombocytopenia (platelet count < 10 G/L) only.
Severe hemorrhage
Thirteen of the patients scored as a 5 before the age of 2 had experienced at least 1 severe hemorrhagic episode at some point between WAS onset and HSCT or death (n = 18 for events before the age of 2 years and n = 3 for events after that age). Five of these patients had SRT, 6 had profound thrombocytopenia, and 2 had mild (> 20 G/L) thrombocytopenia. A lethal, spontaneous brain hemorrhage before the age of 2 occurred in 2 patients (P14 and P26, at 15 and 6 months of age, respectively). These patients' severe thrombocytopenia had not responded to several different therapeutic approaches (a combination of IVIg, corticosteroids [CS], platelet transfusions, and the anti-CD20 monoclonal antibody rituximab; Mabthera; Roche) in P14 and a combination of IVIg, CS, platelet transfusions, and a short course of romiplostim (Nplate; Amgen) in P26. One patient (P1) with a history of intraoral, nasal, and joint hemorrhages died of a spontaneous brain hemorrhage at the age of 49 months. Three other patients suffered a brain hemorrhage before the age of 2 (after mild head trauma in 2 cases). A combination of massive pulmonary hemorrhage, AIHA, and bacterial sepsis led to the death of P11 at the age of 4 months. In that patient, neither thrombocytopenia nor AIHA responded to combination therapy with IVIg, high-dose CS, platelet transfusions, and high-dose cyclophosphamide. Severe digestive tract bleeding occurred in 3 patients (P5, P12, and P15), all of whom were successfully treated with a combination of platelet and packed RBC transfusions, IVIg, and CS. There were 4 significant hemorrhagic events affecting the ear-nose tract (2 cases of massive epistaxis, 1 of severe otorrhagia, and 1 of severe hematotympanum) in 3 patients (P1, P2, and P3), all of whom were treated with platelet transfusions, IVIg, and CS. One patient (P12) experienced intraocular bleeding and detachment of the retina at the age of 19 months (treated with platelet transfusions, IVIg, and CS). It is noteworthy that SRT did not respond to immunosuppression and remained a risk until HSCT was performed in 10 of the 13 surviving patients. All deaths from hemorrhage (n = 4) occurred before HSCT (see “Overall outcome and HSCT”).
AIHA
Nine patients had AIHA at diagnosis (supplemental Table 1) and all had a positive direct erythrocyte Coombs test and antiplatelet antibody assays. Antineutrophil antibodies were found in 3 of the 9 patients. Six other patients developed AIHA after diagnosis of WAS, making 15 patients in all (58%). Treatments were based on IV CS alone or in combination with rituximab, cyclosporine A, or azathioprine. Two patients with very severe, life-threatening AIHA were treated with a combination of high-dose CS, high-dose cyclophosphamide, and plasmapheresis (see supplemental Table 1 for details). Four of these patients underwent splenectomy, which led to a PR in 3 patients and failed to produce any response in the fourth. Two patients died before HSCT due to pulmonary and brain hemorrhages. Ultimately, 13 of the patients underwent HSCT (see “Overall outcome and HSCT”); 8 of the latter (62%) were in CR (n = 2) or PR (n = 6) from AIHA at the time of HSCT.
Vasculitis
Vasculitis occurred in 6 patients at a median age of 12.5 months (range, 2-22; Table 4). The main sites affected by severe visceral vasculitis were the brain, gut, and lungs (as shown by imaging or histology). Two patients (P9 and P15) had vasculitis at 2 sites and 1 patient had vasculitis at 3 sites (P5). Two patients (P17 and P18) experienced systemic vasculitis with intense fever, asthenia, diffuse pain, widespread joint inflammation, and swelling and a major inflammatory syndrome. All patients were treated with frontline IV CS, with a CR observed in 4 cases (66.7%). One patient relapsed 10 months later, but responded again to higher doses of CS. Another 2 patients subsequently experienced an episode of AIHA; 1 completely responded to IV CS and the other partially responded to CS and rituximab. In the other 2 cases, a combination of CS and high-dose IVIg or CS and cyclophosphamide led to a CR. All patients were in full remission from vasculitis when they underwent HSCT (supplemental Table 2).
Vasculitis in patients with severe WAS in the first 2 years of life
| Patient . | Manifestation(s) . | Age, mo . | Therapy . |
|---|---|---|---|
| P5 | Joints, skin, gut | 22 | IV CS → CR → relapse → CR → HSCT |
| P7 | Brain | 11 | IV CS → PR → CS + Cy → CR → HSCT |
| P9 | Joints, skin | 14 | IV CS → CR → AIHA → HSCT |
| P15 | Lungs, skin | 2 | IV CS → CR → HSCT |
| P17 | Joints, skin, high fever | 17 | IV CS → CR → AIHA → HSCT |
| P18 | Joints, skin, high fever | 9 | IV CS → PR → CS + IVIgs → CR → HSCT |
| Patient . | Manifestation(s) . | Age, mo . | Therapy . |
|---|---|---|---|
| P5 | Joints, skin, gut | 22 | IV CS → CR → relapse → CR → HSCT |
| P7 | Brain | 11 | IV CS → PR → CS + Cy → CR → HSCT |
| P9 | Joints, skin | 14 | IV CS → CR → AIHA → HSCT |
| P15 | Lungs, skin | 2 | IV CS → CR → HSCT |
| P17 | Joints, skin, high fever | 17 | IV CS → CR → AIHA → HSCT |
| P18 | Joints, skin, high fever | 9 | IV CS → PR → CS + IVIgs → CR → HSCT |
Cy indicates cyclophosphamide.
Tumors
One patient (P12) had 2 different malignant complications (human herpesvirus 8–associated Kaposi sarcoma and an intracerebral, EBV-related, lymphoproliferative disorder) that were diagnosed at 19 and 23 months of age, respectively (reported in Picard et al22 ; Table 2). He underwent HSCT with his 9.5/10 HLA-matched father (mismatch of a subtype of HLA-A on allelic HLA typing) after treatment with paclitaxel for Kaposi sarcoma and rituximab (combined with intrathecal infusions of rituximab and methotrexate because of intracranial hypertension) for the B-cell lymphoproliferative disorder.
Infectious complications
Major infections occurred in 12 patients but were not inaugural. The median age at the time of the first major infection was 5 months (range, 2-21). Nine patients had 14 episodes of severe life-threatening infections caused variously by bacteria (n = 9), viruses (n = 2), and fungi (n = 3). One patient experienced fungal sepsis (Candida) and bacterial enteritis (Salmonella) 3 months after splenectomy. One patient died of pneumococcal sepsis and massive lung hemorrhage before HSCT. Overall, 24 of the 26 patients had some form of infection.
WAS gene mutations, WASp expression, and immunologic phenotype
Mutations in the WAS gene were found in 24 of the 26 patients (92%) assigned a score of 5 before the age of 2 (Table 2). There were 3 splice site mutations, 5 missense mutations, 10 nonsense mutations (5 of which were small deletions), 5 deletions (3 of which were large deletions), and 1 insertion. These mutations were clearly predictive of the absence of WASp expression in 19 cases. The absence of WASp expression in lymphocytes was confirmed in all 10 studied patients. It is noteworthy that these 26 patients had 6 affected relatives (brothers, n = 4; maternal uncles, n = 2). The fact that all had died from WAS early in life (from either a severe infection or vasculitis) suggested the concordant expression of WAS disease within kindreds (supplemental Figure 1). The immunologic characteristics (total lymphocyte counts, T-, B- and NK-cell subset distributions and Ig plasma levels) of the 26 patients assigned an Ochs score of 5 before the age of 2 did not differ significantly from those of patients assigned a score of 4 or less at that age (data not shown).
Overall outcome and HSCT
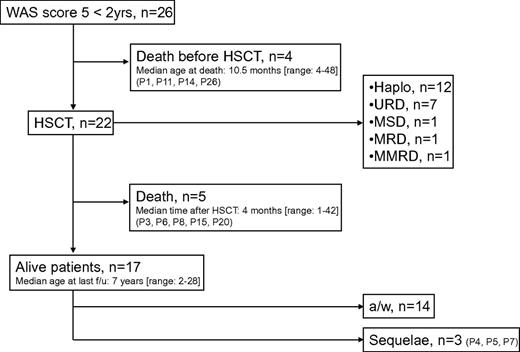
Four children died from one of the WAS-related complications at a median age of 10.5 months (range, 4-49) before allogeneic HSCT could be attempted. Seventeen patients (65%) are alive, with a median age at last follow-up of 7 years (see Table 2). Twenty-two patients received transplantation at a median age of 15.5 months (range, 5-32; Figure 2 and supplemental Table 2). The median interval between the assignment of a score of 5 and HSCT was 5.5 months (range, 2-22). The median follow-up time after HSCT was 6 years (range, 1.5-26). At the time when the HSCT conditioning regimen was initiated, 6 patients had a CR, 5 had a PR, and 11 had not responded to treatment for WAS manifestations. Twelve patients received a haploidentical transplantation from a parent, 7 were transplanted with cells from an unrelated donor (a matched donor in 4 patients and a single HLA-mismatched donor in 3 patients), 1 had a matched sibling donor, 1 had a matched related donor (a parent), and 1 had a partially matched (9.5 of 10) related donor (the father). Two patients underwent a second HSCT (with a matched sibling donor and a matched unrelated donor, respectively), after rejection of a haploidentical transplant. Two patients received a CD34+ cell top-up after a haploidentical HSCT, with a favorable outcome. Overall, 17 patients (77%) are currently alive after HSCT, with a median age at last follow-up of 7 years. Autoimmunity transiently recurred in 6 patients and required (successful) emergency splenectomy in 1 patient. The causes of death after HSCT are shown in supplemental Table 2. It is noteworthy that (1) 3 of the patients who died after HSCT had undergone a splenectomy before HSCT and (2) the other 2 were receiving immunosuppressive therapy for chronic graft-versus-host disease. The sequelae were bronchiectasis and chronic obstructive pulmonary disease in P4, partial epilepsy in P5, and spastic diplegia and unilateral blindness vasculitis in P7 (supplemental Table 2). There was no statistically significant association between outcome and donor type or pre-HSCT status. Similarly, the patients' pre-HSCT status was not significantly correlated with the nature of the HSCT (ie, haploidentical HSCT vs HSCT with other types of donors). The occurrence of autoimmune manifestations before HSCT was also not correlated with outcome (data not shown).
The clinical course of patients with WAS and the attribution of a score of 5 before the age of 2. a/w indicates alive and well; f/u, follow-up; Haplo, haploidentical donor; MRD, matched related donor; mMRD, mismatched related donor; MSD, matched sibling donor; and URD, unrelated donor.
The clinical course of patients with WAS and the attribution of a score of 5 before the age of 2. a/w indicates alive and well; f/u, follow-up; Haplo, haploidentical donor; MRD, matched related donor; mMRD, mismatched related donor; MSD, matched sibling donor; and URD, unrelated donor.
Interestingly, age at the time of assignment of a score of 5 appeared to affect the overall prognosis. Indeed, 7 of the 16 patients having been scored as a 5 before the age of 9 months died (including 4 deaths before HSCT could be attempted), whereas only 2 of the 10 patients assigned a score of 5 after the age of 9 months died (P = .3). Overall, SRT could be a risk factor for a poor prognosis, because 6 of the 12 patients with this condition died (including the 4 deaths before HSCT could be attempted) compared with 3 of 14 of the patients without SRT (P = .22). However, full assessment of the significance of these trends would require larger cohorts of patients.
Discussion
In the present study, we have shown that infants accounted for most of the patients with the most severe WAS phenotype (an Ochs score of 5 with autoimmunity, inflammation, SRT, and/or tumors) in the French national registry of PID. Our calculation of the cumulative risk of being assigned a score of 5 in the first 2 years of life identified a specific subset of WAS patients with a potentially poor prognosis. Autoimmune and/or cancerous complications in the course of WAS are relatively frequent,14,15 and even patients diagnosed with XLT or a milder form of WAS may be assigned a score of 5 at some point in their life.16 We found many different mutations in our cohort of young patients with a severe WAS phenotype. A history of WAS in 4 unrelated kindreds suggested concordance within families. Imai et al showed that there is a genotype/phenotype correlation in Japanese patients and that WASp expression is a long-term prognostic factor.7,13 In our series, none of the tested patients had detectable amounts of WASp and most of the identified mutations were predictive of a complete lack of WASp. However, the complete absence of WASp expression is not always associated with the most severe WAS phenotypes.7
Life-threatening SRT appears to be a fairly specific entity in infants with WAS. The disease mechanism and course are not clear, because SRT initially appeared as an isolated manifestation in these patients. The lack of efficacy of platelet transfusion indicates that thrombocytopenia is peripheral and suggests an autoimmune etiology (although the latter was impossible to document in all cases). This setting is a strong risk factor for poor survival, because therapeutic approaches other than HSCT are poorly effective. Our findings suggest that in this specific context, HSCT should be performed as early as possible in life. The new thrombopoietic agents used to treat immune thrombocytopenic purpura such as romiplostim (Nplate) and eltrombopag (Revolade) might be of value in preventing life-threatening bleeding while patients wait for HSCT.23
A significant percentage of patients with WAS (24%-72%)7,14,15,24 are at risk of autoimmune and inflammatory complications. AIHA is frequent and may be associated with other types of cytopenia (sometimes leading to autoimmune pancytopenia). In our series, treatments for AIHA were variously associated with CR, PR, or no response. However, relapses were frequent and prompted the use of long-term immunosuppression (thus increasing morbidity). Vasculitis is another known complication of WAS and was relatively frequent in patients before the age of 2 years. Milder forms of vasculitis (ie, skin and/or joint inflammation) are frequent in WAS, but the condition can also affect the lungs, brain, and gut.
HSCT is indicated in severe WAS and produces relatively good results.25,26 The present series showed that this is also the case for the most severe form of WAS (characterized by the assignment of an Ochs score of 5 before the age of 2), because 65.4% of the patients survived. However, some issues concerning transplantation outcome remain, because mixed chimerism can lead to post-HSCT autoimmunity.8,26,27 However, in general, HSCT is associated with a good outcome. so these infants should be transplanted as soon as possible with the best available donor (including haploidentical donor).26 Gene therapy for WAS patients lacking a suitable donor may also deliver further substantial improvements and may constitute a cure for patients with a severe WAS phenotype.11 Our study has some limitations: it was a retrospective study and there was incomplete information on some key biomarkers such as WASp expression. Therefore, there is a need for further large, prospective, collaborative studies that might also help to address additional points (eg, the significance of the lack of WASp expression in predicting severe evolution of patients who will eventually develop a score of 5 and correlation with outcome).
In conclusion, we report herein that a severe WAS phenotype (with an Ochs score of 5) occurs significantly more frequently in infants than in older patients with XLT/WAS. We therefore propose a slightly revised version of the Ochs score, including the definition of a distinct WAS subgroup with respect to SRT status and the assignment of a score of 5 under the age of 2 years and independent of the previous score of 5. HSCT should be performed as early as possible in this subgroup (Table 5). The early-onset phenotype was associated with significantly higher morbidity and mortality rates (especially concerning SRT). Early diagnosis of these complications should prompt early, potentially curative HSCT. A systematic study of WASp expression early in life in newly diagnosed patients may help to determine whether this parameter is of value for predicting severity and indicating early HSCT.
Definition of a distinct WAS subgroup with respect to SRT status and assignment of an Ochs score of 5 under the age of 2 years. In this subgroup, HSCT should be performed as early as possible.
| Disease phenotype . | XLT . | Classical WAS phenotype . | Severe WAS phenotype . | Early-onset severe WAS . | ||
|---|---|---|---|---|---|---|
| Severity score | 1 | 2 | 3 | 4 | 5 | EOS |
| Onset before the age of 2 y | −/+ | −/+ | −/+ | −/+ | − | + |
| Thrombocytopenia | + | + | + | + | + | + |
| Microthrombocytes | + | + | + | + | + | + |
| Eczema | − | −/+ | + | +/++ | +/++ | +/++ |
| Immunodeficiency | − | −/+ | + | + | + | + |
| Infections | − | −/+ | + | +/++ | +/++ | + |
| Autoimmunity and/or vasculitis and/or neoplasia | − | − | − | − | + | +/++ |
| Severe refractory thrombocytopenia | − | − | − | − | − | +* |
| Disease phenotype . | XLT . | Classical WAS phenotype . | Severe WAS phenotype . | Early-onset severe WAS . | ||
|---|---|---|---|---|---|---|
| Severity score | 1 | 2 | 3 | 4 | 5 | EOS |
| Onset before the age of 2 y | −/+ | −/+ | −/+ | −/+ | − | + |
| Thrombocytopenia | + | + | + | + | + | + |
| Microthrombocytes | + | + | + | + | + | + |
| Eczema | − | −/+ | + | +/++ | +/++ | +/++ |
| Immunodeficiency | − | −/+ | + | + | + | + |
| Infections | − | −/+ | + | +/++ | +/++ | + |
| Autoimmunity and/or vasculitis and/or neoplasia | − | − | − | − | + | +/++ |
| Severe refractory thrombocytopenia | − | − | − | − | − | +* |
EOS indicates early-onset, severe WAS phenotype.
Observed frequently, but not in all patients.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alexis Proust from the Hematology Laboratory at Bicêtre University Hospital (Paris, France) for performing the genetic tests, and the hospital's physicians and nursing staff and the patients and their families.
This study was funded by the French Ministry of Health and received additional support from the French Association of Patients with PIDs (IRIS). The CEREDIH received educational grants from LFB, Baxter Biosciences, CSL Behring, Octapharma, and Pfizer. The CEREDIH would like to thank the staff of the European Society for Immunodeficiencies online database.
Authorship
Contribution: N.M. collected the data; N.M., I.P., and A.F. designed the study and wrote the manuscript; N.M., C.M., J.-P.J., and A.F. performed the statistical analyses; C.B.-N. and G.d.S.-B. performed the genetic analysis; N.M., I.P., D.M., B.N., C.P., M.C.-C., S.B., and A.F. provided clinical care of patients and contributed to data analysis; and C.P. performed the WASp expression assays.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Professor Alain Fischer, Unité d'Immunologie et Hématologie Pédiatrique and Inserm, U768, Hôpital Universitaire Necker-Enfants Malades, 149 rue de Sèvres, 75015 Paris, France; e-mail: alain.fischer@nck.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal