Abstract
Chronic lymphocytic leukemia (CLL) cells proliferate in pseudofollicles within the lymphatic tissues, where signals from the microenvironment and BCR signaling drive the expansion of the CLL clone. Mobilization of tissue-resident cells into the blood removes CLL cells from this nurturing milieu and sensitizes them to cytotoxic drugs. This concept recently gained momentum after the clinical activity of kinase inhibitors that target BCR signaling (spleen tyrosine kinase, Bruton tyrosine kinase, PI3Kδ inhibitors) was established. Besides antiproliferative activity, these drugs cause CLL cell redistribution with rapid lymph node shrinkage, along with a transient surge in lymphocytosis, before inducing objective remissions. Inactivation of critical CLL homing mechanism (chemokine receptors, adhesion molecules), thwarting tissue retention and recirculation into the tissues, appears to be the basis for this striking clinical activity. This effect of BCR-signaling inhibitors resembles redistribution of CLL cells after glucocorticoids, described as early as in the 1940s. As such, we are witnessing a renaissance of the concept of leukemia cell redistribution in modern CLL therapy. Here, we review the molecular basis of CLL cell trafficking, homing, and redistribution and similarities between old and new drugs affecting these processes. In addition, we outline how these discoveries are changing our understanding of CLL biology and therapy.
The microenvironment in CLL
Circulating chronic lymphocytic leukemia (CLL) cells are nondividing resting B cells, but a significant fractions of tissue CLL cells proliferate in distinct microanatomical sites called proliferation centers or pseudofollicles,1,2 accounting for a daily birth rate of 1%-2% of the entire CLL clone.3 For survival and expansion, CLL cells rely on external signals from the microenvironment and normally undergo spontaneous apoptosis in tissue culture unless they are cocultured with stromal cells.2 In the lymphatic tissues, CLL cells interact with various stromal cells, such as CD68+ nurselike cells (NLC),4-6 smooth muscle actin-positive mesenchymal stromal cells,7 and CD4+ T cells.8,9
By inference from in vitro studies, we assume that stromal cells provide growth and survival signals to the CLL cells that are largely contact-dependent and can cooperate with intrinsic oncogenic lesions.2,10,11 For example, interactions within the lymphatic tissue microenvironment result in BCR activation in the CLL cells,11 and activation of this signaling cascade is favored by presence of unmutated IGHV genes and ZAP70 expression.12 Although the affinity of CLL cells for stromal cells has long been recognized, the cross-talk between stroma and CLL cells only recently has been explored in a more systematic fashion.11,13,14 We currently know that chemokine receptors and adhesion molecules are critical for the homing and retention of CLL cells in tissue compartments (bone marrow, secondary lymphatic tissues).15 Gene expression profiling (GEP) revealed BCR and NFκB pathway activation in CLL cells by the CLL microenvironment, as determined by in vitro models13 and comparative GEP of CLL cells isolated from lymph nodes.11 These GEP studies identified the secondary lymphatic tissues as critical site for CLL disease progression on the basis of up-regulation of BCR and NFκB gene signatures, phosphorylation of spleen tyrosine kinase (SYK) and IκBα, and greater CLL cell proliferation within these tissues.11 The central role of BCR signaling in CLL pathogenesis is corroborated by the activity of BCR signaling inhibitors in vitro,16-19 in a mouse model of CLL,18 and most importantly, in CLL patients treated with these novel agents.20-22 Although these kinase inhibitors preferentially target kinases in BCR signaling cascade (SYK, Bruton tyrosine kinase [BTK], PI3Kδ) and hence are referred to as “BCR signaling inhibitors,” off-target inhibition of other kinases is a characteristic feature of these agents,23,24 and such off-target activities may play a greater role than currently appreciated.
Interestingly, among the different B-cell malignancies, CLL is the most responsive disease to BCR signaling inhibitors, suggesting a particular microenvironment dependence in CLL. Despite the central role of BCR signaling in the dialogue between CLL cells and their milieu, which reflects the key role of BCR signaling in normal B-cell survival and function, other interactions are recognized and are likely of major importance. CLL cells, for example, secrete chemokines (CCL3, CCL22),8,13 which can attract accessory cells, such as T cells and monocytes. This finding suggests that CLL cells are not simply seed in a supportive soil, the microenvironment, but instead are actively involved in a complex cross-talk that establishes and maintains the characteristic microenvironment of proliferation centers.15 B-cell homing and positioning within the lymphatic tissues, BCR signaling, and activation via costimulatory signals such as CD40 ligand and BAFF (ie, B cell–activating factor of the tumor necrosis factor [TNF] family) and APRIL (a prolifer ation-inducing ligand) are prerequisites for normal B-cell expansion in the germinal center.25 CLL cells use these pathways in a similar fashion, indicating that CLL cells retain the capacity to respond to key programs of normal B-cell expansion.
Trafficking of normal lymphocytes and CLL cells
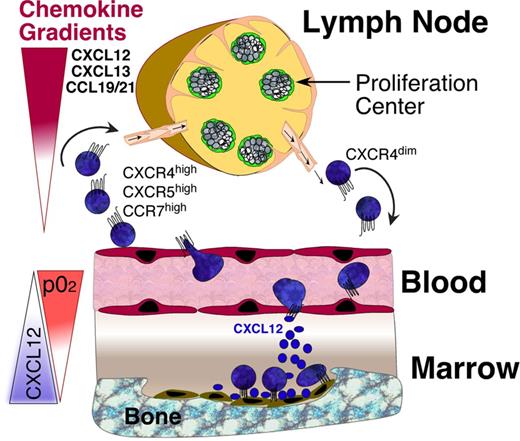
Lymphocyte trafficking between blood and secondary lymphoid tissues is organized by tissue-specific expression of chemokines and ligand- and activation-regulated expression of chemokine receptors on lymphocytes, which cooperate with adhesion molecules and their ligands.26 Lymphocytes in the blood interact with vascular endothelium via selectins and integrins in a process called rolling. Chemokines displayed on the luminal surface of the endothelium activate chemokine receptors on rolling lymphocytes, which in turn triggers integrin activation.27 This causes arrest, firm adhesion, and transendothelial migration into the tissues, where stromal cells organize the localization and retention of the lymphocytes via chemokine gradients (Figure 1).28 This process, often referred to as “homing,” is part of immune surveillance and function of the immune system. Specific to B cells is their localization in germinal centers during adaptive immune responses, where somatic hypermutation and clonal selection occurs within distinct microanatomical regions called dark zone (DZ) and light zone (LZ). Ag-primed centroblasts proliferate in the DZ and subsequently migrate toward the LZ, where they are selected on the basis of antigen affinity.29 B-cell positioning in these areas is coordinated by the chemokines CXCL12 and CXL13, which are expressed by stromal cells,30 and CXCR4 and CXCR5, which are differentially expressed by the B cells. Centroblasts express high levels of CXCR431 and migrate toward CXCL12, which is more abundant in the DZ. In turn, CXCL13 directs CXCR4−/CXCR5+ centrocytes to the LZ, where greater levels of CXCL13 are present.32 Interestingly, CXCR4+ centroblasts can be distinguished from CXCR4− centrocytes solely on the basis of CXCR4 expression levels.31
CLL cell trafficking and tissue homing. CLL cells circulate in the peripheral blood, where they become attracted into the lymph nodes and bone marrow by chemokine gradients established by tissue stromal cells. Critical chemokines for lymph node homing are CXCL12, CXCL13, and CCL19/21, which bind to CXCR4, CXCR5, and CCR7 chemokine receptors on CLL cells, respectively. The CXCR4-CXCL12 axis is the predominant factor for marrow homing, and CXCL12 tissue expression is regulated by oxygen tension, as indicated by the triangles. Expression levels of CXCR4 on blood CLL cells can be used to distinguish CLL cells that are on their way into the tissues (CXCR4 high) versus CLL cells that recently have exited the tissues (CXCR4 dim). CLL adhesion molecules (integrins, selectins, CD44) co-operate with chemokine receptors during tissue homing. Pharmacologic inhibition of these homing mechanisms interferes with 2 distinct events: first, it leads to exit of tissue CLL cells into the blood, causing an increase in lymphocytosis. Second, this also causes inhibition of recirculation of blood CLL cells into the tissues.
CLL cell trafficking and tissue homing. CLL cells circulate in the peripheral blood, where they become attracted into the lymph nodes and bone marrow by chemokine gradients established by tissue stromal cells. Critical chemokines for lymph node homing are CXCL12, CXCL13, and CCL19/21, which bind to CXCR4, CXCR5, and CCR7 chemokine receptors on CLL cells, respectively. The CXCR4-CXCL12 axis is the predominant factor for marrow homing, and CXCL12 tissue expression is regulated by oxygen tension, as indicated by the triangles. Expression levels of CXCR4 on blood CLL cells can be used to distinguish CLL cells that are on their way into the tissues (CXCR4 high) versus CLL cells that recently have exited the tissues (CXCR4 dim). CLL adhesion molecules (integrins, selectins, CD44) co-operate with chemokine receptors during tissue homing. Pharmacologic inhibition of these homing mechanisms interferes with 2 distinct events: first, it leads to exit of tissue CLL cells into the blood, causing an increase in lymphocytosis. Second, this also causes inhibition of recirculation of blood CLL cells into the tissues.
Solid evidence exists that many of the physiologic mechanisms of tissue-specific territoriality also are functional in neoplastic lymphocytes, such as CLL cells. CXCR4 chemokine receptors are expressed at high levels on blood CLL cells,33 mediating CLL cell chemotaxis, migration across vascular endothelium, actin polymerization, and migration beneath and underneath bone marrow stromal cells in response to CXCL12 gradients.33 CXCL12 down-regulates CXCR4 on CLL cells via receptor endocytosis,33 which can be used to distinguish tissue-derived from blood CLL cells, ie, CXCR4dim CLL cells from CXCL12-abundant tissues (bone marrow, lymph nodes) versus CXCR4high CLL cells from blood. Consequently, proliferating, tissue-derived CLL cells displayed lower levels of CXCR4 than nonproliferating cells.34 In vivo deuterium (2H) labeling of CLL cells revealed that patients with greater CXCR4 expression on blood CLL cells had delayed appearance of newly produced, CD38+ cells in the blood, an increased risk for lymphoid organ infiltration, and poor outcomes.35 These data suggest that greater CXCR4 expression on CLL cells favors prolonged tissue retention and proliferation. These 2H studies also revealed intraclonal heterogeneity of CXCR4 expression, with an enrichment of CLL cells expressing lower CXCR4 surface levels in the CD38+/CD5bright fraction, along with increased 2H incorporation.35 These in vivo data indicate that lower blood CXCR4 surface levels label a fraction of CLL cells that has recently exited the tissues into the blood (Figure 1).
Interference with lymphocyte homing and redistribution
Effects of glucocorticoids on trafficking of normal lymphocytes
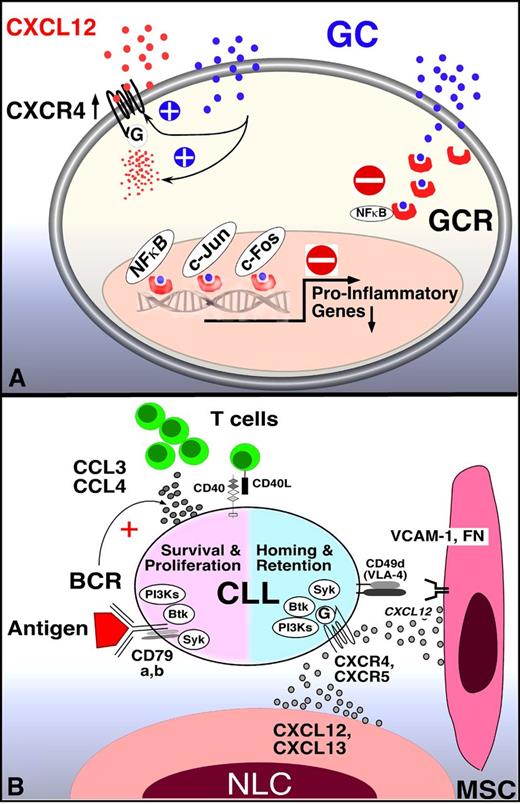
Despite the widespread use of glucocorticoids (GCs) in autoimmune and inflammatory diseases, the precise mechanisms how GCs modulate the immune system and affect B-cell malignancies such as CLL remains ambiguous.36 Generally, GCs act via intracellular GC receptors (GCR), which inhibit expression of NF-κB– and activator protein-1 transcription factors–regulated proinflammatory genes by interference with transcription factor activity (Figure 2A).37 Besides these transcriptional effects, GCs also directly interfere with signaling of various surface receptors, including the T-cell antigen receptor,38 and downstream kinases such as ZAP70, Lck, and Fyn kinases.39 It was early recognized that in healthy subjects the administration of GCs has profound effects on leukocyte subsets in the peripheral blood (PB), causing lymphocytopenia,40 monocytopenia, and eosinopenia, along with neutrophilia, which peaks at approximately 4-6 hours after the administration of GC.41 The transient lymphocytopenia results from the egress of lymphocytes from the blood to tissue sites, particularly the bone marrow,42 rather than GC-induced cell death.
Mechanism of GCs and BCR signaling inhibitors in CLL. (A) GCs inhibit transcription-dependent and -independent lymphocyte activation. Related to lymphocyte redistribution, GCs can up-regulate CXCR4 expression and signaling in normal T cells, thereby enhancing T-cell homing to the marrow. The mechanism of lymphocyte redistribution in CLL is currently unknown, but likely also is attributable to interference with homing mechanism. Besides effects on lymphocyte migration and homing, GCs bind to GCR in the cytosol, displacing heat-shock protein 90. GC-GCR complexes move into the nucleus, where they interfere with transcription. They also initiate transcription and translation of proteins, for example, of inhibitor of NF-κB (IκB). IκB then sequesters NF-κB. In addition, GC-GCR complexes can directly interact with NF-κB to suppress cytokine production. How these GCs mechanisms apply to CLL survival and proliferation is currently unknown. (B) Molecular interactions between CLL and stromal cells in the marrow and/or lymphoid tissue microenvironments and how these relate to BCR signaling and BCR-associated kinases (SYK, BTK, PI3K, modified after Figure 2 in Burger et al2 ). BCR-associated kinases can influence CLL cell survival and proliferation (left) and CLL cell homing and retention in the tissues (right). Contact between CLL cells and NLC or mesenchymal stromal cells (MSC) is established and maintained by chemokine receptors and adhesion molecules expressed on CLL cells. NLCs express the chemokines CXCL12 and CXCL13, whereas MSCs predominantly express CXCL12. NLCs and MSCs attract CLL cells via the G-protein–coupled chemokine receptors CXCR4 and CXCR5, which are expressed at high levels on CLL cells. Integrins, particularly VLA-4 integrins (CD49d), expressed on the surface of CLL cells co-operate with chemokine receptors in establishing cell-cell adhesion through respective ligands on the stromal cells (VCAM-1 and fibronectin/FN). SYK, BTK, and PI3Ks are involved in chemokine receptor and adhesion molecule signaling in normal B cells89 and CLL cells.18 The clinical responses to small molecule antagonists to each of these kinases are characterized by “mobilization” of tissue-resident CLL cells into the blood, which indicates an important role of these kinases for CLL tissue homing and retention, as indicated in the diagram. Self and/or environmental antigens are considered a key factor in activation and expansion of the CLL clone. The nature and source of antigens and its mode of presentation to CLL cells are largely unknown. Stimulation of the BCR complex (BCR and CD79a,b) induces downstream signaling by recruitment and activation of SYK, BTK, and PI3Ks. Finally, BCR activation causes CLL cells to secrete high levels of the chemokines CCL3 and CCL4, which are potent T-cell attractants.
Mechanism of GCs and BCR signaling inhibitors in CLL. (A) GCs inhibit transcription-dependent and -independent lymphocyte activation. Related to lymphocyte redistribution, GCs can up-regulate CXCR4 expression and signaling in normal T cells, thereby enhancing T-cell homing to the marrow. The mechanism of lymphocyte redistribution in CLL is currently unknown, but likely also is attributable to interference with homing mechanism. Besides effects on lymphocyte migration and homing, GCs bind to GCR in the cytosol, displacing heat-shock protein 90. GC-GCR complexes move into the nucleus, where they interfere with transcription. They also initiate transcription and translation of proteins, for example, of inhibitor of NF-κB (IκB). IκB then sequesters NF-κB. In addition, GC-GCR complexes can directly interact with NF-κB to suppress cytokine production. How these GCs mechanisms apply to CLL survival and proliferation is currently unknown. (B) Molecular interactions between CLL and stromal cells in the marrow and/or lymphoid tissue microenvironments and how these relate to BCR signaling and BCR-associated kinases (SYK, BTK, PI3K, modified after Figure 2 in Burger et al2 ). BCR-associated kinases can influence CLL cell survival and proliferation (left) and CLL cell homing and retention in the tissues (right). Contact between CLL cells and NLC or mesenchymal stromal cells (MSC) is established and maintained by chemokine receptors and adhesion molecules expressed on CLL cells. NLCs express the chemokines CXCL12 and CXCL13, whereas MSCs predominantly express CXCL12. NLCs and MSCs attract CLL cells via the G-protein–coupled chemokine receptors CXCR4 and CXCR5, which are expressed at high levels on CLL cells. Integrins, particularly VLA-4 integrins (CD49d), expressed on the surface of CLL cells co-operate with chemokine receptors in establishing cell-cell adhesion through respective ligands on the stromal cells (VCAM-1 and fibronectin/FN). SYK, BTK, and PI3Ks are involved in chemokine receptor and adhesion molecule signaling in normal B cells89 and CLL cells.18 The clinical responses to small molecule antagonists to each of these kinases are characterized by “mobilization” of tissue-resident CLL cells into the blood, which indicates an important role of these kinases for CLL tissue homing and retention, as indicated in the diagram. Self and/or environmental antigens are considered a key factor in activation and expansion of the CLL clone. The nature and source of antigens and its mode of presentation to CLL cells are largely unknown. Stimulation of the BCR complex (BCR and CD79a,b) induces downstream signaling by recruitment and activation of SYK, BTK, and PI3Ks. Finally, BCR activation causes CLL cells to secrete high levels of the chemokines CCL3 and CCL4, which are potent T-cell attractants.
Sequential in vivo analyses demonstrated that blood B and T cells follow the same GC-regulated circadian rhythm, resulting in lymphocytopenia when GC levels are high. In mice, a nocturnal animal active at nighttime, the greatest levels of B and T cells are found during the daytime, which is opposite to humans, where the greatest levels of blood lymphocytes are found around midnight,43,44 demonstrating an inverse correlation between plasma GC levels and blood lymphocyte counts. Another interesting observation regarding GC-induced lymphocyte redistribution is that it affects only lymphocytes capable of trafficking between blood and the tissues, the so-called “recirculating lymphocyte pool.” A different, distinct lymphocyte population incapable of migrating from the intravascular space (“nonrecirculating lymphocytes”) is insensitive to GC-induced redistribution.45 Recently, CXCR4 was identified as the central element of GC-mediated lymphocyte redistribution. Dimitrov et al demonstrated that circadian decreases in peripheral T cells are the result of GC-induced rising in CXCR4 expression in naive T cells, promoting increased homing of the naive T-cell subset to the marrow.46 In addition, GCs also enhance CXCR4 signaling via Lck activation in resting but not in activated T cells, resulting in greater CXCR4 responsiveness to CXCL12.47
Compared with T cells, there is considerably less information available regarding effects of GC on B-cell function and signaling. B-cell redistribution from blood to the tissues was demonstrated after GC administration, although B cells were less sensitive than T cells.45 GCs inhibit B-cell activation and proliferation in response to anti-μ or Staphylococcus aureus, as well as early steps of B-cell development.48 On the other hand, GCs promote generation of antibody secreting–plasma cells and secretion of immunoglobulins.49 During maturation, B cells become progressively more resistant to the inhibitory actions of GC. Surprisingly, there are no data available about specific effects of GC on BCR signaling. Unfortunately, no conclusions can be drawn about the mechanism underlying CLL cell redistribution seen after GC administration, given the fact that in CLL, as discussed in more detail in the next paragraph, GCs cause lymphocytosis, rather than lymphocytopenia. To elucidate the mechanism of GC-induced lymphocytosis, the effects of GCs on chemokine receptor and adhesion molecule expression and function in CLL should be explored.
GC in CLL: lymphocyte redistribution and transient clinical responses
In the 1940s, GCs were introduced as first systemic therapy for patients with CLL. Because of profound inhibition of lymphoid tissue growth by pituitary adrenotropic hormone in animal models,40 in 1943 Rosenthal et al treated 2 CLL patients with crude pituitary adrenotropic hormone extract without achieving any response.50 Subsequently, in 1949, Pearson et al from the Sloan-Kettering Institute reported a small series of patients with lymphoid malignancies, including 4 CLL patients treated with adrenocorticotropic hormone or cortisone acetate. They described an abrupt increase in blood lymphocyte counts up to more than 1 000 000/μL in CLL patients, along with rapid reduction in spleen and lymph node sizes.51 Once GC therapy was stopped, lymphocytes counts decreased to lower than pretreatment levels. This response pattern was subsequently confirmed by several other groups50,52-56 who used prednisone, typically at doses ranging from 0.5 to 1 mg/kg (Figure 3B). Because of the lymphotoxic effects of GC in animal models,40 investigators were puzzled by the steroid-induced lymphocytosis.53 In 1951, Rosenthal et al discussed the possibility of CLL cell redistribution after GC administration: “There is furthermore the possibility that the reduction in swelling of the lymphoid masses in the chronic cases might result in an increased delivery of small lymphocytes into the blood thus accounting for the initial leukocytosis and lymphocytosis.”50 Galton argued, “Since the cells present in the blood are of the same type as those present before the administration of the steroid, the rise is less likely to be because of increased production than to a redistribution of lymphocytes between the tissues and the blood” (David Galton, Doctoral Thesis, University of Cambridge, United Kingdom, 1963 page 117). Along the same lines, Shaw et al commented: “The marked elevation of the absolute lymphocyte count despite regression, often complete, of organ infiltration has been previously observed and remains unexplained. In patients without hematologic disorders corticosteroid administration produces a lymphopenia as well as decrease in lymphatic tissue. In acute lymphatic leukemia marked reduction of circulating and tissue leukemic cells is the usual result of corticosteroid therapy. The increase in WBC and decrease in organ size were correlated in that the maximum changes and regression toward pretreatment values tended to be concurrent. However, the magnitude of the changes, that is, the correlation of change in organ size and change in the WBC was not good. Calculation based on leukocrits, estimated blood volume, and estimated volume of leukemic cells in the spleens suggested that the increase in white blood count could not account for the decrease in organ size. Thus, though lymphocyte redistribution undoubtedly occurs, it cannot account completely for the decrease in organ size. Thus, some degree of lympholysis must also occur.”55 Because of the transient nature of responses and side effects of long-term administration, particularly infections, GCs have not become part of standard therapy for CLL.
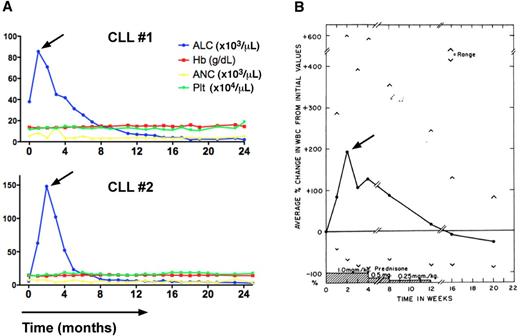
Transient lymphocytosis in CLL patients after treatment with the BTK inhibitor ibrutinib or GCs. (A) Trended ALC, hemoglobin levels (Hb), absolute neutrophil counts (ANC), and platelet counts (Plt) in 2 CLL patients (CLL #1, CLL #2) during continuous therapy with the BTK inhibitor ibrutinib at a dose of 420 MG daily. The horizontal axis shows the time of treatment. Please note the early, transient lymphocytosis, which peaked during the first weeks of therapy, then resolved, and both patients continue on therapy at 24+ months. (B) Effect of prednisone therapy on white cell counts in CLL patients (adapted from Shaw et al55 ) is shown. In 16 of 18 patients a rapid increase in the total white cell count occurred during prednisone administration. This increase in the white count was noted early, the maximum occurring at 2 weeks, and could almost entirely be accounted for by an increase in lymphocytes. Subsequently the white cell count gradually decreased, reaching pretreatment values at the end of prednisone therapy and 2 months later were significantly below pretreatment values.
Transient lymphocytosis in CLL patients after treatment with the BTK inhibitor ibrutinib or GCs. (A) Trended ALC, hemoglobin levels (Hb), absolute neutrophil counts (ANC), and platelet counts (Plt) in 2 CLL patients (CLL #1, CLL #2) during continuous therapy with the BTK inhibitor ibrutinib at a dose of 420 MG daily. The horizontal axis shows the time of treatment. Please note the early, transient lymphocytosis, which peaked during the first weeks of therapy, then resolved, and both patients continue on therapy at 24+ months. (B) Effect of prednisone therapy on white cell counts in CLL patients (adapted from Shaw et al55 ) is shown. In 16 of 18 patients a rapid increase in the total white cell count occurred during prednisone administration. This increase in the white count was noted early, the maximum occurring at 2 weeks, and could almost entirely be accounted for by an increase in lymphocytes. Subsequently the white cell count gradually decreased, reaching pretreatment values at the end of prednisone therapy and 2 months later were significantly below pretreatment values.
Current role of GC in CLL therapy
In contrast to B-cell lymphomas and B-cell acute lymphoblastic leukemia, GCs at standard doses have not a consolidated role in the armamentarium for CLL treatment. In a classic randomized study in which prednisone was used as a control arm, the efficacy of this agent was only 11% (all partial remissions).57 Subsequently, British Medical Council I and II trials suggested that prednisone added little if any value to chlorambucil therapy,58 a notion that was confirmed in a meta-analysis of randomized trials (CLL Trialist).59 In the purine analogs era, no differences in the outcome of cohorts of patients treated with fludarabine alone versus fludarabine plus prednisone were found.60,61
In contrast to the modest therapeutic effect of GCs given at standard doses, high-dose GCs (high-dose methylprednisolone [HDMP]) have shown activity in refractory CLL (reviewed in Smolej62 ), reigniting the interest in GCs, particularly in combination with monoclonal antibodies, in CLL treatment. Despite relatively minor effects on hematopoiesis compared with the myelosuppression after chemoimmunotherapy, HDMP combinations are immunosuppressive, and patients typically receive anti-infective prophylaxis during and after such therapies to reduce the incidence of infections. Interestingly, in contrast to lower-doses of prednisone therapy, lymphocytosis because of CLL cell redistribution has not been described for HDMP. This may be, at least in parts, because of the combined administration with anti–B-cell antibodies (rituximab), which may mask the lymphocytosis. Alternatively, the GC-induced redistribution may not occur at high doses of GC, and only analyses of the underlying mechanism and single-agent studies with HDMP might help to address this interesting discrepancy between low- and high-dose GC.
Other drugs causing CLL redistribution
CXCR4 antagonists
On the basis of the importance of CXCR4 for CLL cell migration, stromal cell adhesion, and survival,33,63,64 the therapeutic potential of CXCR4 (plerixafor, T140 analogs, and others) and CXCL12 antagonists (NOX-A12) is explored in CLL. CXCR4 antagonists inhibit CLL cell activation by CXCL12, and reverse, at least in parts, stromal cell–mediated drug resistance (“chemo-sensitization”) in in vitro models.65,66 A major concern associated with the chemosensitization approach is the fact that CXCR4 antagonists also mobilize normal HSCs, and hence would exposes HSC to drugs outside of their protective niches, which may result in increased toxicity, particularly if cytotoxic drugs were chosen as combination partner. However, recent data from patients with relapsed acute myelogenous leukemia, combining plerixafor with chemotherapy, demonstrated safety and lack of increased myelotoxicity,67 and therefore abated this concern.
The currently available clinical data about a CXCR4 antagonist in CLL are from a phase 1 clinical trial in relapsed CLL in which patients were treated with a combination of rituximab and plerixafor. The aim of this proof-of-principle trial was to determine safety and feasibility of CLL cell mobilization with the use of plerixafor. The investigators reported about a plerixafor dose-dependent mobilization of CLL cells from the tissues to the blood. For example, on day 8, there was a median 3.8-fold increase in PB CLL cells (range, 1.2- to 15.0-fold), indicating CLL cell mobilization.68 However, because patients were pretreated with 3 doses of rituximab before receiving their first dose of plerixafor, these data likely underestimate the extent of plerixafor-induced CLL cell mobilization because a fraction of mobilized CLL cells are likely captured by circulating rituximab and hence are not counted. More trials of CXCR4 antagonists in CLL are warranted to better define effects of CXCR4 antagonists on CLL cell redistribution, and, more importantly, on outcome when combined with traditional anti-CLL therapy.
7-hydroxystaurosporine (UCN-01) and rapamycin (sirolimus)
UCN-01 is a nonspecific protein kinase inhibitor that blocks several signaling pathways, including the 3-phosphoinositide-dependent protein kinase-1 that displayed preclinical activity in CLL. In a phase 1 clinical trial in relapsed lymphoma patients, the investigators noted a brief, transient lymphocytosis in 15 of 18 patients, which peaked 1 day after 3 days of continuous infusion of UCN-01.69 Rapamycin (sirolimus), an inhibitor of the mammalian target of rapamycin signaling molecule, also caused lymphocytosis with a median 4.8-fold increase in the ALC compared with baseline, as reported by Zent and colleagues.70 These report indicate that various signaling inhibitors can interfere with homing receptor signaling, resulting in this off target lymphocytosis effect.
Inhibitors of BCR-associated kinases (SYK, BTK, PI3Kδ)
BCR activation triggers a cascade of downstream signaling events, which begins with phosphorylation of immunoreceptor tyrosine-based activation motifs in the cytoplasmatic tails of Ig-α and -β. Subsequently, SYK is recruited and activated in BCR microclusters, followed by activation of BTK and PI3K. SYK, BTK, and PI3Ks then trigger downstream signaling, including AKT, extracellular signal-related kinase 1/2, myeloid cell leukemia-1, and MYC activation.71 B cells from ZAP70+12 and unmutated CLL patients72,73 are more responsive to BCR stimulation and other microenvironmental signals,74 suggesting that patients with these high-risk disease features may particularly benefit from treatments targeting BCR-associated kinases. The most striking clinical observation common to inhibitors of SYK,20 BTK,22 and PI3Kδ19,21 is the rapid resolution of lymphadenopathy and splenomegaly within the first few weeks of therapy, which is accompanied by a transient increase in blood lymphocyte/CLL cell counts, suggesting CLL cell redistribution from the tissues into the PB. At least in the ibrutinib experience, the lymphocytosis appears to persist longer in previously treated CLL patients (n = 27) where it can last for several months,22,75 compared with previously untreated CLL patients (n = 26).75
SYK, and its inhibitor, fostamatinib (R788)
SYK, a member of the SYK/ZAP70 family of nonreceptor kinases, is an upstream BCR-signaling molecule. In SYK-deficient mice, B-cell development is blocked at the stage of pro-B to pre-B-cell transition.76,77 SYK also regulates survival and maintenance of mature normal and malignant B cells76,78 and modulates leukocyte adhesion and chemotaxis.79,80 Fostamatinib (FosD, also called R788), the clinically used oral formulation, is a prodrug converted in vivo into the bioactive form R406.81 R406 is not a highly selective SYK inhibitor, given that it also has activity against Flt3, Jak, and Lck.81 After encouraging results in a phase 1/2 study in patients with relapsed B cell lymphomas,20 FosD was primarily developed for the treatment of rheumatoid arthritis.82 In the lymphoma trial, the greatest response rates were seen in CLL patients, where the objective response rate was 55%.20 The investigators reported a transient lymphocytosis and concurrent resolution of lymphadenopathy, but details about the degree and duration of lymphocytosis were not specified. One patient with CLL actually discontinued therapy because of lymphocytosis before the redistribution phenomenon was recognized.20 More specific SYK inhibitors are active in preclinical models of CLL83 and diffuse large B-cell lymphoma,84 and we expected that SYK inhibitors will reappear on the clinical stage in selected B-cell malignancies in the near future.
BTK and its inhibitor, Ibrutinib
BTK is a nonreceptor tyrosine kinase of the Tec kinase family and plays a central role in BCR signaling. BTK is primarily expressed in hematopoietic cells, particularly in B cells.85 BTK mutations are the genetic basis for X-linked agammaglobulinemia,86,87 a primary immunodeficiency characterized by low serum immunoglobulin levels and lack of peripheral B cells. After BCR triggering, BTK is activated by Lyn and SYK, causing the activation of transcription factors necessary for B-cell proliferation and differentiation.88 Besides its role in BCR signaling, BTK also is involved in signaling of receptors related to B-cell migration and adhesion, such as the CXCR4 and CXCR5 chemokine receptors and adhesion molecules (integrins, see Figure 2B).89-91 PCI-32 765, recently named ibrutinib, is a BTK inhibitor that binds specifically and irreversibly to a cysteine residue in the BTK kinase domain and thereby blocks BTK activation and enzymatic activity.23 Ibrutinib displays encouraging clinical activity in patients with B-cell malignancies, particularly in patients with CLL and mantle cell lymphoma.22 Herman et al recently reported that ibrutinib can antagonize CLL cell survival after stimulation with various factors (CD40L, BAFF, IL-6, IL-4, TNF-α, fibronectin, stromal cell contact), as well as CpG-induced CLL cell proliferation.17 Ponader et al demonstrated that BCR- and NLC-derived survival signals in CLL cells were inhibited by ibrutinib. Interestingly, the secretion of the BCR activation-dependent chemokines CCL3 and CCL4 by CLL cells was down-regulated both in vitro and in vivo in plasma from CLL patients receiving therapy with ibrutinib,18 similar to effects of the PI3Kδ inhibitor GS-1101 on these chemokines,19 indicating that CCL3 and CCL4 plasma levels are robust biomarkers for kinase inhibitors targeting the BCR signaling axis. In an adoptive transfer TCL1 mouse model of CLL, we demonstrated inhibition of CLL progression and redistribution of CLL cells into the blood.18 De Rooij et al recently reported that ibrutinib inhibits BCR-controlled signaling and integrin α(4)β(1)–mediated adhesion to fibronectin and VCAM-1 in CLL cells.92 They also confirmed that ibrutinib inhibits CLL cell migration toward CXCL12, CXCL13, and CCL19. Clinically, the ibrutinib-induced lymphocytosis is variable among patients and directly related to the presence of the drug: when ibrutinib was given in an intermittent fashion with a monthly 7-days-off-drug period, a saw-toothed pattern of absolute lymphocyte counts (ALC) was noticed, where ALC rapidly decreased during the off-drug period and then increased again once ibrutinib was restarted.22
The PI3Kδ inhibitor GS-1101
PI3Ks integrate and transmit signals from different surface molecules, such as the BCR,93 chemokine receptors, and adhesion molecules, thereby regulating important cellular functions, including cell growth, survival, and migration.94 There are 4 PI3K isoforms designated PI3Kα, β, γ, and δ. PI3Kα and β isoforms are ubiquitously expressed, PI3Kγ isoform has a particular role in T-cell activation, and PI3Kδ expression is largely restricted to hematopoietic cells. PI3Kδ plays a critical role in B-cell homeostasis and function,95 based on studies with gene deleted mice that harbor reduced numbers of B1 and marginal zone B cells, display reduced immunoglobulin levels and poor responses to immunization, as well as defective BCR and CD40 signaling.95-97 In CLL cells, PI3K are constitutively activated,98 presumably by growth and survival signals from the microenvironment, such as adhesion to bone marrow stromal cells,99 CXCR4 activation,33 and BCR activation.100 GS-1101 (previously called CAL-101) is a potent and highly selective PI3Kδ inhibitor and the first PI3Kδ inhibitor in clinical use.24 GS-1101 promotes apoptosis in B-cell lines and primary cells from patients with different B-cell malignancies, including CLL,19,101 mantle cell lymphoma and multiple myeloma.24,102 GS-1101 also inhibits constitutive and CD40-, TNF-α–, fibronectin-, and BCR-derived PI3K signaling, leading to suppression of Akt activation.24,101,102
These studies suggested that disruption of survival signals could be a critical mechanism for the clinical activity of GS-1101. However, in CLL patients on GS-1101 treatment the initial CLL cell redistribution is the first and most striking early finding,21 which is not explained by inhibition of prosurvival signaling. We recently reported that GS-1101 inhibits CLL cell chemotaxis toward CXCL12 and CXCL13 and migration beneath stromal cells (pseudoemperipolesis).19 These in vitro results are corroborated by clinical data showing marked reductions in circulating CCL3, CCL4, and CXCL13 levels, and increasing lymphocytosis during GS-1101 treatment.19 Thus, GS-1101 displays a dual mechanism of action, directly decreasing cell survival while reducing interactions that retain CLL cells in the tissue microenvironments (Figure 2B). The similarities in clinical responses of CLL patients to treatment with a SYK-, BTK-, or PI3Kδ inhibitor suggest overlapping functions of these kinases in BCR signaling, CLL cell migration, and homing.
Conclusion and perspectives
After introduction of the monoclonal antibodies into CLL therapy in the 1990s, kinase inhibitors targeting BCR signaling are now emerging as the next breakthrough in targeted therapy of CLL patients. Data from early-stage clinical trials with the SYK inhibitor FosD,20 the BTK inhibitor ibrutinib,22 and the PI3Kδ inhibitor GS-110121 demonstrate that patients with CLL are particularly sensitive to inhibitors of BCR-associated kinases. Clinical responses are characterized by an early redistribution of tissue-resident CLL cells into the blood, resulting in rapid resolution of lymphadenopathy and organomegaly, along with a surge in lymphocytosis during the first weeks of therapy (Figure 3A, Furman et al,21 and Advanti et al22 ). Later, often after months, the growth- and survival-inhibitory activities of these agents become more apparent and resulted in the normalization of lymphocyte counts and remissions in a majority of patients.20,22 The lymphocytosis is variable among patients and directly related to the continuous presence of the drug: when ibrutinib was given in an intermittent fashion with a 7-days-off-drug period, a sawtooth pattern of ALC was noticed, where ALC rapidly dropped during the off-drug period, and then increased again, once ibrutinib was restarted (Figure A3 in Advanti et al22 ).
Considering the total disease burden, the lymphocytosis induced by BCR signaling inhibitors generally appears to be smaller than what would be expected from the total amount of tissue CLL cells, particularly in bulky disease patients. These findings suggest that BCR-targeting inhibitors also have important immediate effects on CLL cell disease burden by inhibiting proliferation and inducing apoptosis, which apparently differs among patients and probably reflects variable clonal dependence on BCR signaling. The prominent antiproliferative activity of ibrutinib in the TCL1 mouse model, where leukemia cell redistribution was only a minor, transient finding,18 supports the hypothesis that this class of agents has dual activity, directly blocking BCR-related survival and proliferation signals (see Figure 2B), resulting in CLL cell growth inhibition and apoptosis. Tissue redistribution, on the other hand, is the clinically more apparent activity of these agents during early therapy in most patients. The relative contribution of each mechanism to responses in individual patients can substantially differ, likely because there is overlap between both activities. More detailed analyses of responses, for example, in CLL subgroups that are more or less BCR dependent, may help to clarify the gray zone of CLL cell growth and survival inhibition versus redistribution as the mechanism of action of this class of drugs. Because of the early lymphocytosis, BCR kinase inhibitors currently are combined with antibodies such as rituximab and ofatumumab to shorten the duration of lymphocytosis. Alternatively, combinations of these novel agents with standard chemoimmunotherapy regimen are also explored and likely will result in greater rates of complete remissions.
Despite many unresolved questions, it is apparent that lymphocyte redistribution has come full circle in CLL therapy, from corticosteroids to BCR signaling inhibitors. With our improved knowledge about molecular drivers in the CLL microenvironment and mechanism of leukemia cell trafficking and tissue homing, we can now explain at least some of the molecular mechanism underlying CLL cell redistribution. In contrast to GCs, BCR signaling inhibitors are well tolerated and active for longer periods of time, although the numbers of treated patients and the follow-up are limited.20,22 However, the discussed similarities in activities of GC and BCR signaling inhibitors and the recent positive data of HDMP combinations in CLL also indicate that GC therapy in CLL needs to be revisited. The appreciation of the different disease compartments (blood vs tissues) and interference with these compartments via redistribution has touched critical elements of CLL disease biology and demonstrates that deeper insight into disease biology, along with innovative drug development, can change treatment paradigms. In CLL, where chemoimmunotherapy is accepted standard therapy for younger patients, kinase inhibitors targeting BCR signaling already are valuable clinical trial alternatives for relapsed patients or those who harbor the high-risk 17p deletion. Larger, phase 3 trials with ibrutinib and GS-1101 are in progress and will determine how successful these new drugs compare with established therapies.
Acknowledgments
This work was supported by CLL Global Research Foundation grants (to J.A.B. and E.M.), a Cancer Prevention and Research Institute of Texas (CPRIT) grant (to J.A.B.), and Instituto Carlos III FISS PI080304 and Generalitat de Catalunya 2009SGR1008 grants (to E.M).
Authorship
Contribution: J.A.B. and E.M. wrote the paper; and J.A.B. designed the figures.
Conflict-of-interest disclosure: J.A.B. has received research funding from Gilead, Pharmacyclics, and Genzyme. The remaining author declares no competing financial interests.
Correspondence: Jan A. Burger, MD, PhD, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, PO Box 301402, Houston, TX 77230-1402; e-mail: jaburger@mdanderson.org.