Key Points
FGF2 is able to directly interact with LYVE-1 and glycosylation of LYVE-1 is important for the interaction with FGF2.
LYVE-1 inhibits FGF2-dependent lymphangiogenesis and FGF2 modulates LYVE-1's endogenous expression and reverses the effect of TNFβ.
Abstract
LYVE-1 (lymphatic vessel endothelial hyaluronan receptor-1) is a homolog of the hyaluronan receptor CD44, and one of the most widely used markers of lymphatic endothelial cells in normal and tumor tissues. However, the physiologic role of LYVE-1 in the lymphatic system still remains unclear. It is well established that fibroblast growth factor 2 (FGF2) induces lymphangiogenesis. Based on the known interaction between FGF2 and CD44 and based on the structural similarity of CD44 and LYVE-1, we investigated whether FGF2 might interact with LYVE-1. We found that FGF2 is able to bind LYVE-1 using AlphaScreen, or after surface-immobilization or in solution. FGF2 binds to LYVE-1 with a higher affinity than any other known LYVE-1–binding molecules, such as hyaluronan or PDGF-BB. Glycosylation of LYVE-1 is important for FGF2 binding. Furthermore, FGF2 interacts with LYVE-1 when overexpressed in CHO cells. Soluble LYVE-1 and knockdown of LYVE-1 in lymphatic endothelial cells impaired FGF2 signaling and functions. In addition, FGF2 but not VEGF-C-induced in vivo lymphangiogenesis, was also inhibited. Conversely, FGF2 also modulates LYVE-1 expression in cells and ex vivo. Thus, our data demonstrate a functional relationship to the interaction between FGF2 and LYVE-1.
Introduction
The lymphatic system is important to maintain fluid homeostasis by collecting fluid that leaks from capillary blood vessels and returning it to the blood circulation.1 Perturbations in the development, maintenance and function of the lymphatic system can lead to a variety of pathologic lymphatic disorders including lymphedema, inflammation, and tumor metastasis.2 The understanding of the molecular and cellular regulation of lymphangiogenesis has greatly advanced in recent years with the identification of the lymphangiogenic vascular endothelial growth factors VEGF-C and VEGF-D and their lymphatic vessel-specific VEGF receptor-3.3 The repertoire of lymphangiogenic factors increased when it became apparent that other growth factor molecules or angiogenic factors were also regulating lymphangiogenesis.4-6 As such, it has been recognized that FGF2 induces lymphangiogenesis by both direct and indirect mechanisms; it binds lymphatic endothelial cells (LECs) and stimulates their proliferation and migration in vitro7,8 ; in addition, recent findings suggested that FGF2 reciprocally interacts with VEGF-C leading to additive lymphangiogenic activity and metastasis.9,10
LYVE-1 (lymphatic vessel endothelial hyaluronan receptor-1), a homolog of hyaluronan receptor CD44, is expressed predominantly on lymphatic endothelium.11 It is equally exposed to both the luminal and subluminal face of lymphatic vessel.12 LYVE-1 cDNA encodes a 322-residue type-I integral membrane glycoprotein with a 21-residue transmembrane domain and a 63-residue cytoplasmic region. Similar to CD44, LYVE-1 has a single hyaluronan-binding domain at the N-terminus followed by a juxtamembrane region that is predicted to be heavily glycosylated.12 LYVE-1 is also expressed in discrete populations of activated tissue macrophages and in the sinusoidal endothelium of the liver and the spleen, as well as in cancer tissues.11,13-15
LYVE-1 has been proposed to participate in binding and internalization of hyaluronan (HA), a substrate for migrating lymphocytes that facilitates their transport to lymph nodes.16 However, recent studies suggest that HA homeostasis is unperturbed in LYVE-1−/− mice and that lymphatic adhesion/transmigration may be also mediated by other molecules than LYVE-1.17
In parallel, CRSBP-1, a membrane glycoprotein that can mediate cell surface retention of platelet-derived growth factor-BB (PDGF-BB), was identified and characterized.18 CRSBP-1 has been found to be identical to LYVE-1.19 The biologic properties of CRSBP-1 have been studied mainly using cultured fibroblasts. CRSBP-1 mediates the cell-surface retention of the simian sarcoma virus oncogene v-sis gene product (PDGF-BB) and insulin-like growth factor-binding protein-3 (IGFBP-3) in the simian sarcoma virus-transformed fibroblasts and in HI299 cells co-expressing CRSBP-1 and IGFBP-3.19 However, the precise role of CRSBP-1/LYVE-1 remains unknown. It has been reported that CRSBP-1–null mice are overtly normal and fertile but exhibit identifiable morphologic and functional alterations of lymphatic capillary vessels in only few tissues including the liver and the intestine.20 Furthermore, PDGF-BB and HA enhance interstitial-lymphatic flow in wild-type (WT) mice but not in CRSBP-1–null animals.20 In conflict with these results, LYVE-1–deficient mice were reported to display a normal lymphatic vasculature and secondary lymphoid tissue with normal unperturbed HA homeostasis.17 Similarly, recent studies demonstrate the lack of a lymphatic vessel phenotype in LYVE-1/CD44 double-knockout mice under normal conditions.21 As a consequence, the functional role of LYVE-1 protein is not firmly established and remains controversial.
As mentioned, CD44 is the closest homolog of LYVE-1. CD44 belongs to a family of cell surface receptors expressed on a wide variety of cell types, including leukocytes, endothelial cells, and smooth muscle cells but is absent on lymphatic vasculature.22 It was shown that CD44 regulates the biologic activity of several growth factors, such as FGF2, PDGF-BB, and hepatocyte growth factor.23 Indeed CD44 cooperates with these growth factors and promotes their signaling by protecting them from degradation.23 It was demonstrated that CD44 knockout mice exhibit severely impaired arteriogenesis accompanied by strong reduced expression of FGF2 and PDGF-BB in collateral vessels.24 Based on the structural similarity between CD44 and LYVE-1, we reasoned that FGF2 and LYVE-1 might also functionally interact in lymphatic vessels. This interaction may be of importance for the FGF-related effects in the lymphatic vasculature. In this report, we provide evidence for both physical and functional interaction of FGF2 and LYVE-1 in vitro and in vivo.
Methods
Cell lines
The following cell lines were used: human dermal LECs (Promocell), human dermal blood endothelial cells (BECs, Promocell), human umbilical vein endothelial cells (HUVECs, Lonza), CHO, and CHO FGFR3 cells (kindly provided by M. Presta, University of Brescia, Italy). Animal experiments have been conducted according to EU rules and have been approved by the institutional animal care and use committee.
AlphaScreen assay
Amplified luminescence proximity homogeneous assays (AlphaScreen) was carried out with FGF2-N-GST, LYVE-1 N-His, and His-tagged hIRE1cyto according to the manufacture's indications (PerkinElmer).
125I-FGF2 binding assay
Ten μg of recombinant FGF2 was labeled with 125I-Na (1mCi) using Iodogen precoated tubes (Pierce Biotechnology) according to the manufacturer‘s indications and as previously described.25
siRNA knockdown experiments
LECs were plated at a density of 8 × 104 cells per well in 6-well plates. Small interfering RNA (siRNA) against human LYVE-1, ON-TARGETplus SMARTpool, ON-TARGETplus siRNA-human XLKD1 n°J-020129, and nontargeting siRNA pool were from Dharmacon. Transfection was performed using lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions with a final siRNA concentration of 10 to 83.5nM for 48 to 96 hours. siRNA-transfected cells were then used for RNA and protein extraction, tubulogenesis, or proliferation assays.
Cross-linking of FGF2 and LYVE-1
The amine-specific homobifunctional cross-linker bis(sulfosuccinimidyl) suberate (BS3; Pierce Biotechnology) was used to cross-link FGF2 and LYVE-1 according to the protocol modified from Perollet et al.26
Tubulogenesis of LYVE-1 siRNA-treated LECs
Tubulogenesis of LYVE-1 siRNA LECs in the presence or absence of 50 ng/mL FGF2 was performed using IBIDI microslides (IBIDI) coated with 7 μg/μL reduced matrigel. After 12 to 24 hours of incubation, 4 digitized pictures were made per well and the images were processed with the Wimasis.com platform to determine the total branching points and the number of loops.
Cell proliferation, migration, and invasion assays
LEC proliferation, migration, and invasion assays were carried out using IncuCyte (Essen Bioscience).
Solid-phase ligand binding assay
Solid-phase ligand binding assay was performed as previously described with minor modifications.27
Statistics
Data are presented as mean ± SD. Statistical analyses were performed using the unpaired Student t test.
The following information can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article): details on cell lines, plasmid constructions and recombinant protein production, and quantitative real-time PCR (qPCR); details on the AlphaScreen assay, apoptosis, and immunoprecipitation; details of FGF2 binding experiments and cross-linking, cell signaling, immunofluorescence analyses, deglycosylation, and quantitative cell attachment assay; and details on the Incucyte proliferation assay, migration and invasion assays, and animals studies.
Results
Identification and characterization of the interaction between FGF2 and LYVE-1
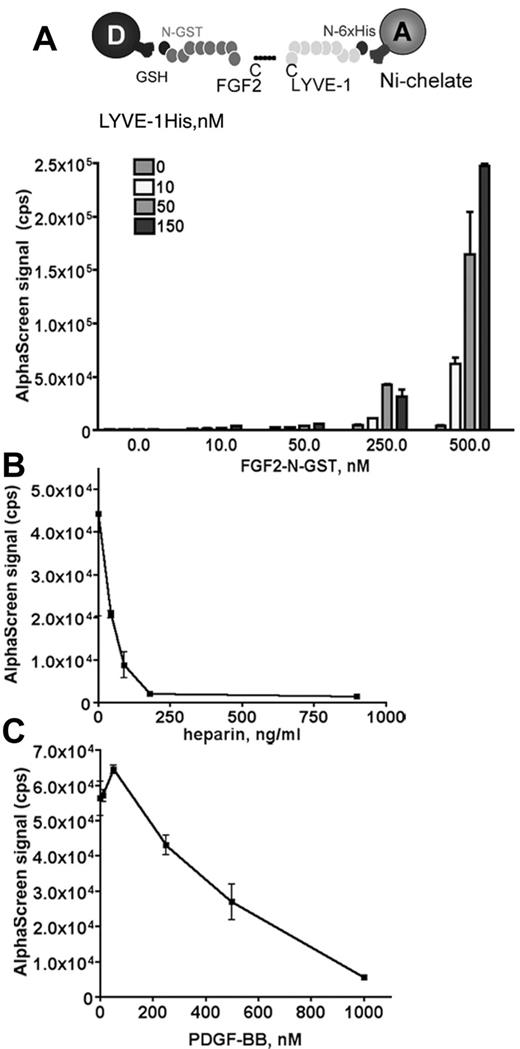
We used an amplified luminescence proximity homogeneous assay, AlphaScreen to detect and measure the interaction between FGF2 and LYVE-1.28 To configure an AlphaScreen assay to monitor the interaction between FGF2 and LYVE-1, recombinant tagged proteins for FGF2 and LYVE-1 were used. FGF2 fused to GST was expressed in Escherichia coli, purified using glutathione-sepharose affinity chromatography. The purity (supplemental Figure 1A) and the biologic activity of FGF2 to stimulate endothelial cell proliferation were verified (supplemental Figure 1B). In addition, a commercial preparation of soluble extracellular part of LYVE-1 was used. In our assay, GST tagged FGF2 was bound to AlphaScreen glutathione donor beads and 6xHis tagged LYVE-1 to Nickel Chelate acceptor beads. The AlphaScreen assay was first carried out using increasing amounts of FGF2 (10-500nM) and LYVE-1 (10-150nM; Figure 1A). A maximal AlphaScreen signal reflecting the direct interaction between FGF2 and LYVE-1 was reached at concentrations of 500nM FGF2 and 150nM LYVE-1. This suggests a FGF2/LYVE-1 interaction of 3:1. We next performed competition assays in the presence of increasing concentrations of PDGF-BB or heparin. Both molecules competed with the FGF2/LYVE-1 interaction with IC50 27.2 ng/mL for heparin and 360nM for PDGF-BB (Figure 1B-C). We also carried out a control assay for the interaction of LYVE-1 with FGF2 and used the unfolded response protein IRE1 instead of LYVE-1 in the AlphaScreen (supplemental Figure 2). In this assay, FGF2 was not able to interact with IRE1.
Analysis of FGF2/LYVE-1 interaction by AlphaScreen-based technology. (A) Detection of FGF2/LYVE-1 interaction. Top panel: Assay design of the AlphaScreen experiment. GST tagged FGF2 (FGF2-N-GST) was bound to AlphaScreen glutathione donor beads and 6xHis-tagged LYVE-1 (LYVE-1 N-6xHis) to AlphaScreen Ni chelate acceptor beads. Bottom panel: Direct interaction between FGF2 and LYVE-1. The recombinant proteins of indicated concentrations were incubated with donor and acceptor beads for 1 hour at room temperature before signal measurement. (B-C) AlphaScreen-based competition assays. The interaction between 500nM FGF2-N-GST and 50nM LYVE-1 N-6xHis was competed in the presence of increasing concentrations of heparin (B) and PDGF-BB (C). IC50 were determined with equation 1-site competition (GraphPad Prism Version 5). (A-C) Results are representative experiments from 3 independent experiments. Results are mean values ± SD (n = 3).
Analysis of FGF2/LYVE-1 interaction by AlphaScreen-based technology. (A) Detection of FGF2/LYVE-1 interaction. Top panel: Assay design of the AlphaScreen experiment. GST tagged FGF2 (FGF2-N-GST) was bound to AlphaScreen glutathione donor beads and 6xHis-tagged LYVE-1 (LYVE-1 N-6xHis) to AlphaScreen Ni chelate acceptor beads. Bottom panel: Direct interaction between FGF2 and LYVE-1. The recombinant proteins of indicated concentrations were incubated with donor and acceptor beads for 1 hour at room temperature before signal measurement. (B-C) AlphaScreen-based competition assays. The interaction between 500nM FGF2-N-GST and 50nM LYVE-1 N-6xHis was competed in the presence of increasing concentrations of heparin (B) and PDGF-BB (C). IC50 were determined with equation 1-site competition (GraphPad Prism Version 5). (A-C) Results are representative experiments from 3 independent experiments. Results are mean values ± SD (n = 3).
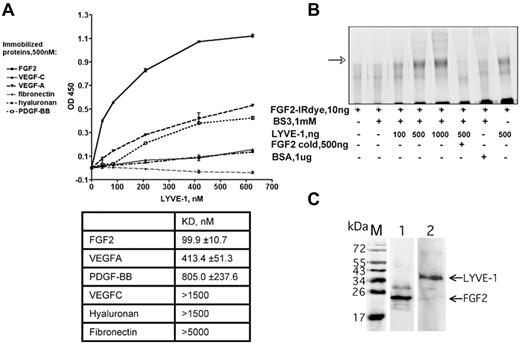
In addition to the AlphaScreen proximity assay, we further analyzed the FGF2/LYVE-1 interaction by other assays. As shown in Figure 2A, LYVE-1 interacted with immobilized FGF2 in a solid-phase ligand-binding assay.27 The dissociation constant is lower than that for VEGFA, PDGF-BB, VEGFC, fibronectin, and HA. This indicates that LYVE-1 interacted with FGF2 with a higher affinity than with the other potential binding molecules tested. We finally performed an interaction assay to document the FGF2/LYVE-1 interaction in solution. To this end, 10 ng of labeled FGF2-IRDye800CW were incubated with increasing amounts of LYVE-1 (100-1000 ng/well, molar ratio of FGF2/LYVE-1 1/4.4-44) in the absence or presence of unlabeled FGF2 and cross-linked in solution using BS3 (Figure 2B). Cross-linked FGF2/LYVE-1 oligomers were detected in the presence of increasing concentrations of LYVE-1 (Figure 2B lanes 3-5).
Detection of FGF2/LYVE-1 interaction using other assays. (A) Interaction of LYVE-1 with various ligands detected by a solid-phase ligand-binding assay. LYVE-1 was added to wells coated with different recombinant proteins as indicated, and incubated at 2 hours at 37°C. Anti–LYVE-1, secondary peroxidase-conjugated anti–goat antibodies, and TMB substrate were used to detect bound LYVE-1. Representative experiment was done in duplicate. Error bars represent the mean ± SD (n = 4). Values of dissociation constants (KD) are presented in Table in the bottom. (B) Cross-linking of FGF2 to LYVE-1 in solution. FGF2 labeled with near-infrared fluorescent IRDye800CW (LI-COR Biosciences) was incubated with increasing concentrations of LYVE-1 and reacted for 30 minutes with the cross-linker BS3. Bovine serum albumin (BSA) and unlabeled FGF2 were used as negative controls. Cross-linking samples were analyzed by 10% SDS-PAGE under reducing conditions. The IR signal was visualized using Odyssey infrared imaging system (LI-COR Biosciences). The arrow shows cross-linked FGF2/LYVE-1 oligomers. (C) Co-immunoprecipitation of FGF2/LYVE-1 complexes in solution. Premixed FGF2-N-His/LYVE-1 (500/150nM) was incubated either with mix of 2.6 μg anti–LYVE-1 antibody and 50 μL Dynabeads protein G beads and revealed with rabbit anti-FGF2 antibody and secondary donkey anti–rabbit IRDye800CW conjugated antibody (lane 1) or with mix of 3.3 μg anti-FGF2 antibody and 50 μL Dynabeads protein G beads and revealed with mouse anti–LYVE-1 antibody and secondary donkey anti–mouse IRDye800CW conjugated antibody (lane 2). M, protein molecular weight markers.
Detection of FGF2/LYVE-1 interaction using other assays. (A) Interaction of LYVE-1 with various ligands detected by a solid-phase ligand-binding assay. LYVE-1 was added to wells coated with different recombinant proteins as indicated, and incubated at 2 hours at 37°C. Anti–LYVE-1, secondary peroxidase-conjugated anti–goat antibodies, and TMB substrate were used to detect bound LYVE-1. Representative experiment was done in duplicate. Error bars represent the mean ± SD (n = 4). Values of dissociation constants (KD) are presented in Table in the bottom. (B) Cross-linking of FGF2 to LYVE-1 in solution. FGF2 labeled with near-infrared fluorescent IRDye800CW (LI-COR Biosciences) was incubated with increasing concentrations of LYVE-1 and reacted for 30 minutes with the cross-linker BS3. Bovine serum albumin (BSA) and unlabeled FGF2 were used as negative controls. Cross-linking samples were analyzed by 10% SDS-PAGE under reducing conditions. The IR signal was visualized using Odyssey infrared imaging system (LI-COR Biosciences). The arrow shows cross-linked FGF2/LYVE-1 oligomers. (C) Co-immunoprecipitation of FGF2/LYVE-1 complexes in solution. Premixed FGF2-N-His/LYVE-1 (500/150nM) was incubated either with mix of 2.6 μg anti–LYVE-1 antibody and 50 μL Dynabeads protein G beads and revealed with rabbit anti-FGF2 antibody and secondary donkey anti–rabbit IRDye800CW conjugated antibody (lane 1) or with mix of 3.3 μg anti-FGF2 antibody and 50 μL Dynabeads protein G beads and revealed with mouse anti–LYVE-1 antibody and secondary donkey anti–mouse IRDye800CW conjugated antibody (lane 2). M, protein molecular weight markers.
We then performed co-immunoprecipitation experiments using both anti-FGF2 and anti–LYVE-1 antibodies (Figure 2C). These experiments revealed that FGF2/LYVE-1 complexes could be co-immunoprecipitated using either by anti-FGF2 or anti–LYVE-1 antibodies. Taken together, these data demonstrate that FGF2 directly interacts with LYVE-1 in vitro.
We next investigated whether glycosylation is important for the FGF2/LYVE-1 interaction. Deglycosylation with PGNaseF led to a drastic decrease in the AlphaScreen signal (supplemental Figure 3). This indicates that glycosylation is important for the FGF2/LYVE-1 interaction.
Role of the FGF2/LYVE-1 interaction in LECs
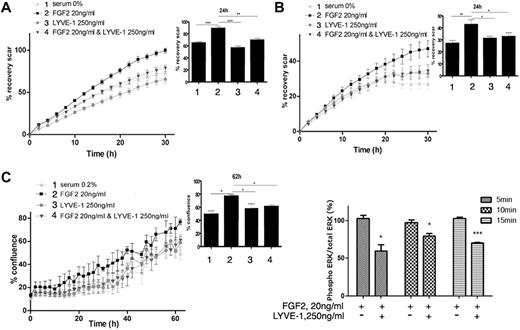
We first examined whether the soluble extracellular part of LYVE-1 (sLYVE-1) inhibits FGF2-mediated effects in LECs. To this end, we conducted both migration and invasion assays using IncuCyte technology. As shown in Figure 3A and B, sLYVE-1 (250 ng/mL) inhibited FGF2-induced migration and invasion in a time-dependent manner. Moreover, VEGF-C induced migration was not inhibited by sLYVE-1 (supplemental Figure 4A). In addition, we also observed LYVE-1–dependent inhibition of FGF2-induced proliferation in the IncuCyte assay but not of apoptosis (Figure 3C, supplemental Figure 4B).
Biologic function of the FGF2/LYVE-1 interaction in the endothelial cells. FGF2 stimulated migration (A), invasion (B), and proliferation (C) of LECs in the presence of soluble LYVE-1 (250 ng/mL) and/or FGF2 (20 ng/mL). Cells were incubated at 37°C 5% CO2 in the IncuCyte live-cell imaging system as described in “Methods.” The time course of cell migration or invasion was quantified by dynamic imaging (pictures at 2-hour time intervals) as percentage of scar recovery (cells migrated/invaded into the wound). The proliferation was quantified by dynamic imaging using percentage of confluence at 2 hour time intervals. In the insets, diagrams of 1 time point are represented. Experiment was performed 3 times and 1 representative experiment is shown (n = 4 for proliferation; n = 6 for migration and invasion; *P < .05,** < .01,***P < .001; NS, nonsignificant versus control cells stimulated by FGF2 in the absence of LYVE-1). (D) Effect of soluble LYVE-1 on ERK phosphorylation using AlphaScreen SureFire phosphorylation assay. LECs were stimulated with 20 ng/mL FGF2 in the presence or absence of 250 ng/mL LYVE-1 for 5, 10, and 15 minutes. Phosphorylated ERK1/2 in cell lysates was quantified in an AlphaScreen SureFire p-ERK1/2 (Thr202/Tyr204) Assay (PerkinElmer) according to the manufacturer's instructions. Results are representative experiments from 3 independent experiments. Results are mean values ± SD (n = 3).
Biologic function of the FGF2/LYVE-1 interaction in the endothelial cells. FGF2 stimulated migration (A), invasion (B), and proliferation (C) of LECs in the presence of soluble LYVE-1 (250 ng/mL) and/or FGF2 (20 ng/mL). Cells were incubated at 37°C 5% CO2 in the IncuCyte live-cell imaging system as described in “Methods.” The time course of cell migration or invasion was quantified by dynamic imaging (pictures at 2-hour time intervals) as percentage of scar recovery (cells migrated/invaded into the wound). The proliferation was quantified by dynamic imaging using percentage of confluence at 2 hour time intervals. In the insets, diagrams of 1 time point are represented. Experiment was performed 3 times and 1 representative experiment is shown (n = 4 for proliferation; n = 6 for migration and invasion; *P < .05,** < .01,***P < .001; NS, nonsignificant versus control cells stimulated by FGF2 in the absence of LYVE-1). (D) Effect of soluble LYVE-1 on ERK phosphorylation using AlphaScreen SureFire phosphorylation assay. LECs were stimulated with 20 ng/mL FGF2 in the presence or absence of 250 ng/mL LYVE-1 for 5, 10, and 15 minutes. Phosphorylated ERK1/2 in cell lysates was quantified in an AlphaScreen SureFire p-ERK1/2 (Thr202/Tyr204) Assay (PerkinElmer) according to the manufacturer's instructions. Results are representative experiments from 3 independent experiments. Results are mean values ± SD (n = 3).
It is well known that FGF2 induces the phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) by ERK kinase.29 We observed a significant inhibition of ERK phosphorylation by LYVE-1 in LEC (Figure 3D). Thus, these results indicate that sLYVE-1 acts on endothelial cell migration, invasion and on cell proliferation but not on apoptosis. This is in agreement with the inhibition of ERK phosphorylation.
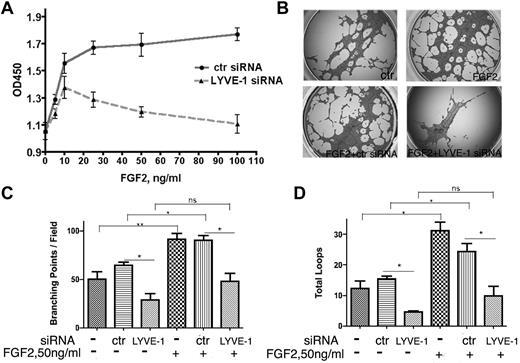
In a second approach, we investigated whether the down-regulation of membrane-bound LYVE-1 in LECs altered FGF2 activity. Knockdown of LYVE-1 in LECs was achieved using a pool of 4 LYVE-1 siRNAs or a selected LYVE-1 siRNA and resulted in the inhibition of LYVE-1 mRNA and protein (supplemental Figure 5A). We observed an inhibition of FGF2-stimulated LEC proliferation after knockdown of membrane LYVE-1 (Figure 4A). As a control, we attenuated LYVE-1 expression in VEGF-stimulated LECs. No significant inhibition of LEC proliferation was seen under this condition (supplemental Figure 5B). To ascertain the specificity of LYVE-1 siRNA for lymphatic cells, FGF2-stimulated blood vascular endothelial cells (BECs) were incubated with LYVE-1 siRNA. No effect of LYVE-1 siRNA was observed under these conditions (supplemental Figure 5C). This indicated the specificity of LYVE-1 siRNA for LECs that express LYVE-1. Finally, we observed an inhibition of FGF2-induced tubulogenesis when LYVE-1 was silenced (Figure 4B-D). These data demonstrate that attenuation of LYVE-1 expression results in an inhibition of biologic processes stimulated by FGF2.
Effect of LYVE-1 knockdown in LEC on FGF2 activity. (A) FGF2 concentration-dependent proliferation of LEC with down-regulated LYVE-1. LECs were transfected for 72 hours with LYVE-1 siRNA or ctr siRNA. Cell proliferation was determined by cell proliferation reagent WST-1. (B-D) Effect of LYVE-1 down-regulation on endothelial tube formation of LECs. LECs were transfected for 24 hours with LYVE-1 siRNA or ctr siRNA and then basal and FGF2-induced capillary tube formation of LECs were performed as indicated in “Methods.” Images of tubulogenesis processed by the Wimasis.com platform. (B) Diagrams of total branching points (C) and number of loops (D). Values are the means ± SD (n = 3).
Effect of LYVE-1 knockdown in LEC on FGF2 activity. (A) FGF2 concentration-dependent proliferation of LEC with down-regulated LYVE-1. LECs were transfected for 72 hours with LYVE-1 siRNA or ctr siRNA. Cell proliferation was determined by cell proliferation reagent WST-1. (B-D) Effect of LYVE-1 down-regulation on endothelial tube formation of LECs. LECs were transfected for 24 hours with LYVE-1 siRNA or ctr siRNA and then basal and FGF2-induced capillary tube formation of LECs were performed as indicated in “Methods.” Images of tubulogenesis processed by the Wimasis.com platform. (B) Diagrams of total branching points (C) and number of loops (D). Values are the means ± SD (n = 3).
Cell-based characterization of the FGF2-LYVE-1 interaction
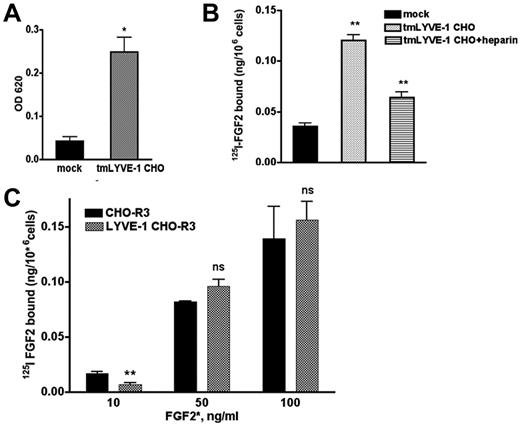
We next examined whether the interaction of FGF2 and LYVE-1 occurs in cell culture. To this end, we cloned the full-length transmembrane LYVE-1 (tmLYVE-1) cDNA in the pcDNA3.1 vector, and stably transfected CHO cells that lack endogenous LYVE-1. After selection, several clones were obtained and analyzed for LYVE-1 mRNA expression using qPCR and for cell-surface localization of LYVE-1 using immunofluorescence with an antibody specific for human LYVE-1 (supplemental Figure 6A-B). Quantitative cell attachment assay demonstrated that the LYVE-1–expressing cells were able to bind to immobilized HA 2-fold more than mock-transfected cells lacking LYVE-1 (Figure 5A). We then tested whether LYVE-1 expressed on the cell surface of CHO cells could have an effect on FGF2 binding to low or high-affinity binding sites and carried out binding experiments with radiolabeled 125I-FGF2 to CHO cells stably transfected with tmLYVE-1. As seen in Figure 5B, CHO cells transfected with full-length LYVE-1 showed an increased 125I-FGF2 binding to low affinity sites. Moreover, incubation of LYVE-1–transfected CHO cells in the presence of heparin resulted in a reduction of 125I-FGF2 binding to low-affinity heparan sulfate proteoglycans (HSPGs; Figure 5B).
Effect of exogenously expressed cell-surface tmLYVE-1 on 125I-FGF2 binding. (A) Quantitative cell attachment assay of tmLYVE-1 CHO cells. TmLYVE-1 CHO and mock-transfected CHO cells were plated onto microtiter wells coated with HA and cell attachment was analyzed as described in supplemental Methods. The experiment was repeated 2 times in triplicate, values are mean ± SD. (B) 125I-FGF2 binding to low-affinity sites in the tmLYVE-1 CHO clone. TmLYVE-1 CHO and mock-transfected cells were incubated with 10 ng/mL 125I-FGF2 in the presence or absence of 100 ng/mL heparin. After 2 hours of incubation at 4°C, the radioactivity associated with HSPGs was measured as indicated in “Methods.” Data are expressed as a specific activity (125I-FGF2 ng/106 cells). The binding of the figure depicts representative experiment done in duplicates (datapoints as mean ± SD, n = 3). (C) 125I-FGF2 binding to high-affinity sites in tmLYVE-1 CHO-R3. TmLYVE-1 CHO-R3 and mock-transfected cells were incubated with increased concentrations of 125I-FGF2. After 2 hours of incubation at 4°C, the radioactivity associated with FGFR was measured as indicated in “Methods.” Data are expressed as a specific activity (125I-FGF2 ng/106 cells). The binding of the figure depicts representative experiments done in duplicates (datapoints as mean ± SD, n = 3).
Effect of exogenously expressed cell-surface tmLYVE-1 on 125I-FGF2 binding. (A) Quantitative cell attachment assay of tmLYVE-1 CHO cells. TmLYVE-1 CHO and mock-transfected CHO cells were plated onto microtiter wells coated with HA and cell attachment was analyzed as described in supplemental Methods. The experiment was repeated 2 times in triplicate, values are mean ± SD. (B) 125I-FGF2 binding to low-affinity sites in the tmLYVE-1 CHO clone. TmLYVE-1 CHO and mock-transfected cells were incubated with 10 ng/mL 125I-FGF2 in the presence or absence of 100 ng/mL heparin. After 2 hours of incubation at 4°C, the radioactivity associated with HSPGs was measured as indicated in “Methods.” Data are expressed as a specific activity (125I-FGF2 ng/106 cells). The binding of the figure depicts representative experiment done in duplicates (datapoints as mean ± SD, n = 3). (C) 125I-FGF2 binding to high-affinity sites in tmLYVE-1 CHO-R3. TmLYVE-1 CHO-R3 and mock-transfected cells were incubated with increased concentrations of 125I-FGF2. After 2 hours of incubation at 4°C, the radioactivity associated with FGFR was measured as indicated in “Methods.” Data are expressed as a specific activity (125I-FGF2 ng/106 cells). The binding of the figure depicts representative experiments done in duplicates (datapoints as mean ± SD, n = 3).
To assess whether LYVE-1 could affect FGF2-binding to high-affinity tyrosine kinase FGFRs in CHO cells, we generated double transfected FGFR3-LYVE-1 CHO cells, using CHO cells stably transfected with FGFR3,30 because FGFR3 is present on the cell surface of lymphatic endothelial cells.31 Double-transfected clones were analyzed for LYVE-1 expression by qPCR and immunofluorescence (supplemental Figure 6C-D). In contrast to FGF2 binding to low-affinity sites, LYVE-1 had no effect on 125I-FGF2 binding to high-affinity sites (Figure 5C).
Effect of LYVE-1 on FGF2-induced lymphangiogenesis in vivo
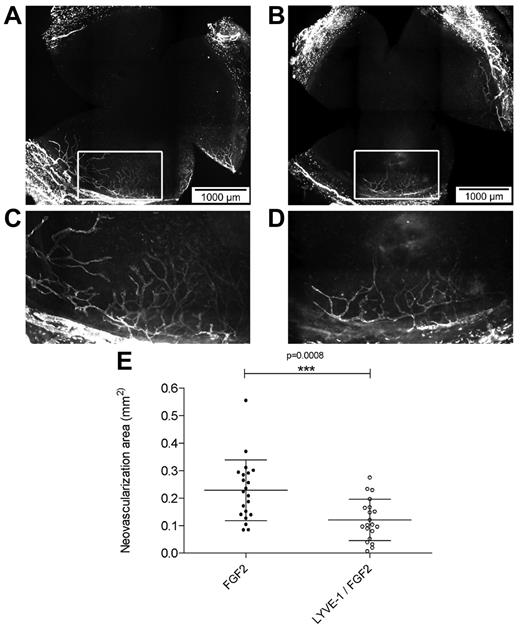
To analyze the effect of LYVE-1 on lymphangiogenesis in vivo we applied a corneal lymphangiogenesis assay to quantitatively study the formation of new lymphatic vessels.32 It has been shown that FGF2 implanted in the mouse cornea stimulated lymphangiogenesis.7,33 To analyze the effect of LYVE-1 on FGF2-induced lymphangiogenesis, pellets containing FGF2 alone or a combination of FGF2/LYVE-1 were implanted in corneas of BALB/c mice (Figure 6A-E). Implantation of pellets containing FGF2 alone resulted in the outgrowth of lymphatic vessels with a mean neovascularization area of 0.23 ± 0.11 mm2. On the contrary, in corneas with pellets containing FGF2/LYVE-1, the neovascularization area decreased around 48% (mean vascularization area of 0.12 ± 0.07 mm2, P = .0008). This clearly indicates that LYVE-1 also inhibits FGF2 activity on lymphangiogenesis in vivo.
Effect of LYVE-1 on FGF2-induced lymphangiogenesis in vivo. Corneal lymphangiogenesis after implantation of pellets containing FGF-2 alone or FGF-2 and LYVE-1. (A) Corneal whole mount with LYVE-1+ lymphatic vessels induced by 80 ng FGF-2. (B) Corneal whole mount with LYVE-1+ lymphatic vessels induced by a combination of 80 ng FGF2 and 1 μg LYVE-1. (C-D) Details of panels A and B, respectively. (E) Quantitative analysis of corneal lymphangiogenesis induced by FGF2 or FGF2/LYVE-1 containing pellets (nFGF2 = 21, nFGF2/LYVE-1 = 20; P = .0008). Data represent mean ± SD.
Effect of LYVE-1 on FGF2-induced lymphangiogenesis in vivo. Corneal lymphangiogenesis after implantation of pellets containing FGF-2 alone or FGF-2 and LYVE-1. (A) Corneal whole mount with LYVE-1+ lymphatic vessels induced by 80 ng FGF-2. (B) Corneal whole mount with LYVE-1+ lymphatic vessels induced by a combination of 80 ng FGF2 and 1 μg LYVE-1. (C-D) Details of panels A and B, respectively. (E) Quantitative analysis of corneal lymphangiogenesis induced by FGF2 or FGF2/LYVE-1 containing pellets (nFGF2 = 21, nFGF2/LYVE-1 = 20; P = .0008). Data represent mean ± SD.
To ascertain the specificity, the effect of LYVE-1 on VEGF-C–induced corneal lymphangiogenesis was investigated. VEGF-C–induced lymphangiogenesis and VEGF-C/LYVE-1–induced lymphangiogenesis did not differ significantly in the micropocket assay (supplemental Figure 7A-E). This indicates that LYVE-1 does not inhibit the lymphangiogenic effect of VEGF-C in vivo.
FGF2-dependent regulation of LYVE-1 expression
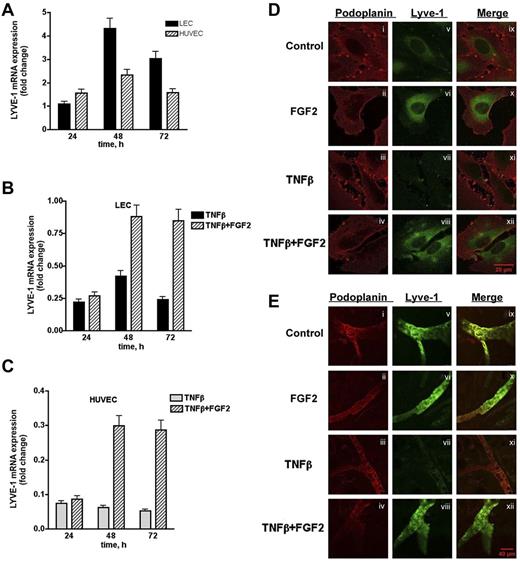
To further characterize the interaction between FGF2 and LYVE-1 in lymphatic cells, we investigated whether FGF2 has an effect on LYVE-1 expression in LEC. Indeed, FGF2-stimulated LECs and HUVECs exhibited a maximum in LYVE-1 expression at 48 hours (Figure 7A). We next investigated whether FGF2 could reverse the TNFβ-dependent down-regulation of LYVE-1.34 LECs and HUVECs were stimulated with FGF2 for 24 to 72 hours in the presence of TNFβ. As expected, FGF2 was able to reverse the TNFβ-dependent down-regulation of LYVE-1 in both cell types (Figure 7B-C). These results were confirmed by immunofluorescence labeling of LEC using anti–LYVE-1 antibodies. As seen in Figure 7D (right panel), FGF2 increases the fluorescent signal for LYVE-1, whereas TNFβ significantly diminishes it. In the presence of FGF2, immunoreactivity for LYVE-1 is restored. We then examined whether FGF-2 is able to counteract the effect of TNFβ on LYVE-1 expression in an ex vivo assay (Figure 7E). Ear explants from mice were exposed for 48 hours to TNFβ (200 ng/mL) in the presence or absence of FGF2 (200 ng/mL). TNFβ clearly decreases the immunofluorescence signal for LYVE-1. This is no more observed when FGF2 is added together with TNFβ. Taken together, these results indicate that FGF2-positive regulates LYVE-1 expression in LECs and HUVECs, and is able to counteract the inhibitory effect of TNFβ on LYVE-1 expression in vitro and ex vivo.
Effect of FGF2 on endogenous LYVE-1 expression in endothelial cells. (A) Endogenous LYVE-1 expression in FGF2-stimulated endothelial cells. LECs and HUVECs were stimulated with 10 ng/mL FGF2 in a time-dependent manner 24, 48, and 72 hours. Endogenous LYVE-1 was measured by qPCR. Results are expressed in fold-change of LYVE-1 mRNA in FGF2-stimulated cells versus nonstimulated cells on each time-point using the GAPDH reference gene. Reversion of the TNFβ-dependent down-regulation of LYVE-1 by FGF2 in LECs (B) and HUVECs (C). LECs and HUVECs were stimulated with 10 ng/mL FGF2 for 24, 48, 72 hours, and 10 ng/mL TNFβ was added to the culture medium. Endogenous LYVE-1 was measured by qPCR as described. Values obtained by qPCR are the means ± SD (n = 3). (D) Immunofluorescence of LECs incubated with TNFβ in the absence or presence of FGF2. Cells were incubated for 48 hours with TNFβ (20 ng/mL) in the absence or presence of FGF2 (20 ng/mL). Immunofluorescence was performed using anti-podoplanin (positive control for lymphatic cells, i-iv) or anti–LYVE-1 (v-viii) antibodies. (ix-xii) Represent the merge of both labeling. (E) Effect of FGF2 on the down-regulation of LYVE-1 in an ex vivo explant culture assay. Explants from male BALB/c mice were cultured in a humidified atmosphere at 37°C in 5% CO2 in the presence of recombinant human 200 ng/mL TNFβ and/or FGF2 at 200 ng/mL for 48 hours. Immunostaining using anti-podoplanin or anti–LYVE-1 antibodies were carried out as indicated in “Methods.” (i-iv) Podoplanin staining; (v-viii) LYVE-1 staining; and (ix-xii) merge.
Effect of FGF2 on endogenous LYVE-1 expression in endothelial cells. (A) Endogenous LYVE-1 expression in FGF2-stimulated endothelial cells. LECs and HUVECs were stimulated with 10 ng/mL FGF2 in a time-dependent manner 24, 48, and 72 hours. Endogenous LYVE-1 was measured by qPCR. Results are expressed in fold-change of LYVE-1 mRNA in FGF2-stimulated cells versus nonstimulated cells on each time-point using the GAPDH reference gene. Reversion of the TNFβ-dependent down-regulation of LYVE-1 by FGF2 in LECs (B) and HUVECs (C). LECs and HUVECs were stimulated with 10 ng/mL FGF2 for 24, 48, 72 hours, and 10 ng/mL TNFβ was added to the culture medium. Endogenous LYVE-1 was measured by qPCR as described. Values obtained by qPCR are the means ± SD (n = 3). (D) Immunofluorescence of LECs incubated with TNFβ in the absence or presence of FGF2. Cells were incubated for 48 hours with TNFβ (20 ng/mL) in the absence or presence of FGF2 (20 ng/mL). Immunofluorescence was performed using anti-podoplanin (positive control for lymphatic cells, i-iv) or anti–LYVE-1 (v-viii) antibodies. (ix-xii) Represent the merge of both labeling. (E) Effect of FGF2 on the down-regulation of LYVE-1 in an ex vivo explant culture assay. Explants from male BALB/c mice were cultured in a humidified atmosphere at 37°C in 5% CO2 in the presence of recombinant human 200 ng/mL TNFβ and/or FGF2 at 200 ng/mL for 48 hours. Immunostaining using anti-podoplanin or anti–LYVE-1 antibodies were carried out as indicated in “Methods.” (i-iv) Podoplanin staining; (v-viii) LYVE-1 staining; and (ix-xii) merge.
We then investigated whether the expression of endogenous LYVE-1 is down-regulated in FGF2 knockout mice. Accumulating evidences indicate that LYVE-1 expression is not restricted to the lymphatic endothelium, but it is also expressed in the normal liver and spleen sinusoids, heart myocytes and lung macrophages.11,13,14,35 We isolated total RNA from liver, lung, spleen, and heart of WT male and female mice and examined the levels of LYVE-1 expression by qPCR (supplemental Figure 8A). We found abundant LYVE-1 expression in WT tissue of lung, heart, and liver, and low LYVE-1 levels in spleen. Furthermore, we observed that the levels of endogenous LYVE-1 were not identical in male and female mice, especially in liver and spleen. Therefore we analyzed LYVE-1 expression levels in male and female FGF2 knockout mice separately, normalizing them to LYVE-1 level in WT tissues (n = 5). We found that LYVE-1 expression levels were decreased only in the liver and spleen of male mice, whereas female showed no difference in LYVE-1 expression in these organs (supplemental Figure 8B). Thus, FGF2 may positively regulate LYVE-1 expression in vivo in a tissue-specific manner. The data indicate that FGF2 is critical for LYVE-1 expression in liver and spleen sinusoids.
Discussion
LYVE-1 is a lymphatic endothelial cell marker that is also expressed in some other cell types including activated tissue macrophages, the sinusoidal endothelium of liver and spleen, heart myocytes as well as some tumors.11,13-15,35 However, its function is still not clearly elucidated. In this article, we report that a member of the FGF family, FGF2 can interact with LYVE-1 and that this interaction impacts some of the FGF2-mediated effects in the lymphatic endothelium. Furthermore, FGF2 can modulate LYVE-1 endogenous expression and reverse the effect of inflammatory cytokines.
We found that LYVE-1 binds FGF2 using AlphaScreen technology. These results were reinforced using solid phase binding assays with surface immobilized FGF2 and injected LYVE-1. Under these conditions, a significant binding was observed. The affinity as reflected by the calculated KD was of 100nM and was 4- to 10-fold lower than those obtained for other LYVE-1 binding molecules tested including VEGFA, PDGF-BB, VEGFC, fibronectin, and HA. This indicates a better affinity of LYVE-1 for FGF2 than for other binding molecules. We were also able to demonstrate that the FGF2/LYVE-1 complex occurred in solution using chemical cross-linking or co-immunoprecipitation experiments. Another observation of note is the fact that heparin and PDGF-BB were able to compete with the FGF2/LYVE-1 interaction. These findings indicate the existence of a probable overlapping binding site in LYVE-1 or FGF2 that also binds to heparin and PDGF-BB. Indeed, PDGF-BB has been reported to be an important growth factor contributing to lymphatic metastasis36 and bind LYVE-118 and our observations are in line with these data. These data suggest that LYVE-1 may be involved in the modulation of VEGF-C–independent lymphangiogenesis by directly interacting with critical lymphangiogenesis factors, such as FGF2 or PDGF-BB. Moreover, it has also been reported that PDGF-BB directly interacts with FGF237 and thus PDGF-BB may also compete for LYVE-1 binding on FGF2. Heparin is known to be implicated in the dimerization of FGF2 and to modulate the interaction of FGF2 with FGF receptors38 and thus might compete for LYVE-1 binding on FGF2. However, the inverse may also be true.
In addition, we demonstrated that glycosylation of LYVE-1 is important for the interaction with FGF2. This may explain the completion of LYVE-1 by heparin, because the heparin-binding domain of FGF2 may be involved in the FGF2/LYVE-1 interaction. The exact nature of the interacting domains for FGF2 or LYVE-1 is not known at the present moment.
We next investigated whether LYVE-1 was able to interact with FGF2 when overexpressed at the cell surface. We clearly demonstrated a significant increase of FGF2 binding in CHO cells that overexpressed LYVE-1. This binding could also be competed with heparin, thus confirming the binding data obtained in vitro. When binding data were analyzed by distinguishing high affinity binding from low affinity binding, it appeared that only low affinity binding but not high affinity binding of FGF2 was increased. High affinity binding was tested specifically for FGFR3 because FGFR3 is overexpressed in lymphatic endothelial cells.31 Because LYVE-1 overexpression only modulates low-affinity binding to HSPGs but not high affinity binding, one must explain how LYVE-1 is implicated in the regulation of FGF2 activity. A substantial body of literature suggests that full activity of FGFs requires not only receptor interactions but also their internalization,39 and that the latter may proceed via HSPG-dependent and independent pathways.25,40,41 In this regard, LYVE-1 may participate in FGF2 internalization and subsequent FGF2 signaling inside of cell. LYVE-1 may also cooperate with some other not yet identified binding molecule that is important for FGF activity. For instance, transmembrane proteoglycan syndecan-4 may be a potential candidate. Indeed, it has been demonstrated that syndecan-4 is directly involved in FGF2 internalization in endocytic vesicles.42 Regarding these observations we can therefore speculate that LYVE-1 may help FGF2 in proteoglycan's binding such as syndecan-4. Our data are in agreement with this hypothesis because LYVE-1 increased low affinity binding of FGF2 in LYVE-1–transfected CHO cells, which is competed by heparin. Further studies are however needed to clarify whether LYVE-1 is involved in syndecan-4–dependent FGF2 interactions.
We next sought to investigate whether LYVE-1 was able to alter some of FGF2 functions, such as migration, invasion, and proliferation. We observed that FGF2-stimulated migration, invasion, and proliferation were inhibited in the presence of LYVE-1. We showed that FGF2-induced signaling was impaired by LYVE-1. These experiments were made using both, LYVE-1 and siRNA for LYVE-1. Most importantly, sLYVE-1 is able to inhibit FGF2-induced lymphangiogenesis in vivo in a mouse corneal lymphangiogenesis assay. However, we were unsuccessful in knocking-down LYVE-1 expression in the in vivo rat cornea assay using specific and validated siRNA for LYVE-1. Thus, the effect on FGF-2–induced lymphangiogenesis in vivo could not be investigated for membrane-bound LYVE-1.
Taken together, our results demonstrate that LYVE-1 is able to interfere with LEC function in vitro and in vivo. In agreement with our results it has been recently shown that LYVE-1 is involved in the disruption of VE-cadherin–mediated intercellular adhesion and opening of intercellular junctions in LEC monolayers.43 Furthermore, it has been demonstrated that the inhibition of FGF signaling is important for vascular integrity.44 Indeed, blockade of FGF activity in vitro and in vivo results in dissociation of the VE-cadherin/p120-catenin complex and disassembly of adherent and tight junctions, which progress to loss of endothelial cells and disintegration of the vasculature. Thus, our results together with these observations point to an important interaction between FGFs and LYVE-1 in the lymphatic endothelium.
We also demonstrated that FGF2 not only interacts with LYVE-1 but can also induce its expression in LECs. More importantly, FGF2 is able to counteract the down-regulation of LYVE-1 by TNFβ in LECs. This is based on in vitro and ex vivo experimental evidences. It was previously reported that LYVE-1 expression is down-regulated by TNFβ through internalization and degradation of receptors in lysosomes, coupled with a shutdown in gene expression.34 This is quite remarkable and may be of importance in chronic inflammation where TNF is abundantly produced. In some circumstances, FGF may be released by stroma cells and reverse the effect of TNF. In addition, it has been reported that FGF2 mediates a protective effect against TNFα-mediated apoptosis in HUVEC.45 Recently, the presence of LYVE-1–negative gaps in the corneal lymphatic endothelium has been observed.46 The number of gaps in FGF2, but not VEGFA implanted corneas was significantly lower than in untreated corneas, which indicates a potential stimulatory effect of FGF2 on LYVE-1 expression. Another hypothesis is that in some tumors, FGF2 overexpression may influence the lymphatic endothelial cell phenotype. It has been shown that PROX1, the homeobox-containing transcription factor expressed by LECs, is able to up-regulate FGFR3 receptor in LECs.8 Thus it is possible that FGF2 blocks external cues that negatively act on the lymphatic phenotype.
To reinforce the contention that FGF2 positively regulates LYVE-1 expression, we analyzed whether FGF2 loss in FGF2−/− mice led to a decrease of LYVE-1 expression in some of their organs. Indeed, we show a decrease in LYVE-1 expression in the liver and spleen of FGF2−/− mice. These organs are responsible for the uptake and degradation of high molecular weight HA.11
Thus, FGF2 and LYVE-1 are possibly integrated in a regulatory loop, in which FGF2 activates LYVE-1 expression, which in turn, limits FGF2 activity by directly interacting with the growth factor.
In conclusion, we show for the first time that LYVE-1 is able to interact with one of the fibroblast growth factor members; namely FGF2. This interaction appears stronger than any other known LYVE-1 binding molecules. Furthermore, FGF2/LYVE-1 interactions may be of importance for some of the FGF2 related effects in the vasculature and for in vivo lymphangiogenesis. Finally, FGF2 prevents the down-regulation of LYVE-1–induced by proinflammatory cytokines, such as TNFβ.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank A. Dubrac and H. Prats (Inserm, Toulouse) for providing tissues of FGF2−/− mouse. CHO-FGFR3 cells were kindly provided by Dr M. Presta (University of Brescia, Italy).
This work was supported by grants from the Agence National de la Recherche (ANR), The Institut National du Cancer (INCA), and the Conseil Régional d'Aquitaine to A.B., INCA, Association de Recherche contre le Cancer, Inserm (Avenir) to E.C., and German Research Council DFG to C.C. (SFB 643/B-10 and Cu 47/4-1).
Authorship
Contribution: N.P., G.M., and B.R. performed research; N.P., S.T., and E.C. designed and performed AlphaScreen experiments; N.P., G.M., B.R., C.C., and A.B. analyzed results; and N.P. and A.B designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for N.P. is Department of Health Sciences, San Paolo Hospital, University of Milan, Milan, Italy.
Correspondence: Andreas Bikfalvi, Inserm U1029, University Bordeaux I, Avenue des Facultés, 33405 Talence, France; e-mail: a.bikfalvi@angio.u-bordeaux1.fr.