In this issue of Blood, Schmidt et al report a lipid nanoparticle (LNP)–based pharmacologic treatment to induce liver-specific Tmprss6 silencing and modulate hepcidin, the main regulator of iron homeostasis, in murine models of primary and secondary iron overload.1
In genetic hemochromatosis and in β-thalassemia, iron overload is the primary cause of liver cirrhosis, diabetes, heart failure, and other clinical complications, including liver cancer. In both conditions iron overload is due to inappropriately low levels of the iron regulatory hormone hepcidin, which controls iron absorption from the diet and iron release from macrophages through the degradation of the sole cell iron exporter ferroportin.2
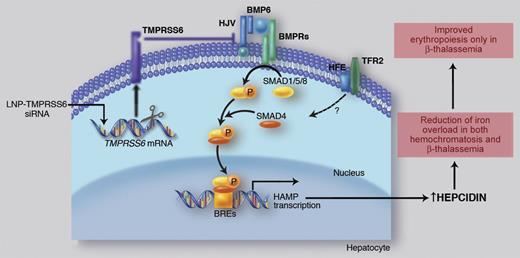
Liver iron transcriptionally activates BMP6, which recruits BMP receptors (BMPRs) and HJV for SMAD1/5/8 pathway activation. The SMAD complex translocates to the nucleus to bind the BMP Responsive Elements (BREs) in hepcidin promoter. Binding of HFE to TfR2 positively modulates hepcidin expression through a still unclear, likely SMAD-related, mechanism. TMPRSS6 inhibits hepcidin through the cleavage of HJV that reduces the BMP-SMAD pathway signaling. Ineffective erythropoiesis down-regulates hepcidin expression through activation of hepcidin inhibitors (iron deficiency and hypoxia and/or TMPRSS6, not shown). High hepcidin/low iron improves ineffective erythropoiesis, likely decreasing iron supply to single erythroid cells. Professional illustration by Marie Dauenheimer.
Liver iron transcriptionally activates BMP6, which recruits BMP receptors (BMPRs) and HJV for SMAD1/5/8 pathway activation. The SMAD complex translocates to the nucleus to bind the BMP Responsive Elements (BREs) in hepcidin promoter. Binding of HFE to TfR2 positively modulates hepcidin expression through a still unclear, likely SMAD-related, mechanism. TMPRSS6 inhibits hepcidin through the cleavage of HJV that reduces the BMP-SMAD pathway signaling. Ineffective erythropoiesis down-regulates hepcidin expression through activation of hepcidin inhibitors (iron deficiency and hypoxia and/or TMPRSS6, not shown). High hepcidin/low iron improves ineffective erythropoiesis, likely decreasing iron supply to single erythroid cells. Professional illustration by Marie Dauenheimer.
Hepcidin is up-regulated by iron through Bone Morphogenetic Protein (BMP) 6, which activates the BMP-Son of Mothers Against Decapentaplegic (SMAD) signaling pathway upon interaction with BMP receptors and their co-receptor hemojuvelin (HJV). This pathway can be modulated by the hemochromatosis gene HFE through a still unclear mechanism (see figure).2 Hepcidin inhibition occurs in iron deficiency, hypoxia, and erythropoiesis expansion to meet the increased iron requests. TMPRSS6 is the only hepcidin inhibitor whose role has been clearly demonstrated in vivo. TMPRSS6 encodes for the hepatocyte-specific type II transmembrane serine protease, matriptase-2, that plays an essential role in erythropoiesis because its inactivation causes iron refractory iron deficiency anemia (IRIDA), characterized by inappropriately high levels of hepcidin both in mice3 and in humans.4 In vitro, TMPRSS6 cleaves HJV,5 thus decreasing the BMP-SMAD signaling and inhibiting hepcidin expression (see figure).
Manipulation of ferroportin levels by biomimetic minihepcidins6 and manipulation of endogenous hepcidin expression7,8 are promising approaches to control excess iron deposition.
Schmidt et al report that down-regulation of Tmprss6 by LNP-delivered siRNA efficiently increases hepcidin levels, thus decreasing transferrin saturation and body iron in animal models of iron overload.1 Using both Hfe−/− mice, a model of the most common form of hemochromatosis, and Hbbth3/+ mice, which develop a form of thalassemia closely resembling the human β-thalassemia intermedia, the authors show an efficient increase in hepcidin levels after Tmprss6 silencing, with a reduction of iron overload in both models and an improvement of erythropoiesis in β-thalassemia mice.
LNP-based treatment, already used in clinics,1 is particularly attractive to manage iron overload because the liver, which is central to the regulation of iron homeostasis, is a direct target of LNP due to its high vascularization, permeable endothelium, and high numbers of lipid particle receptors.
Both short- and long-term treatment with LNP-Tmprss6 siRNA efficiently target Tmprss6 and although the transcriptional effect is transient, the resulting hepcidin increase and transferrin saturation decrease are prolonged.
It was previously demonstrated that genetic loss of Tmprss6 reduces systemic iron overload in Hfe−/− mice by increasing hepcidin through activation of the BMP-SMAD signaling.7 Accordingly, Tmprss6 silencing in Hfe null adult mice modulates systemic iron, reducing liver iron concentration, serum iron, and transferrin saturation. However, as already reported in the double Hfe-Tmprss6 KO mice,7 the long-term (6 weeks) treatment with Tmprss6 siRNA had a cumulative effect on erythropoiesis causing a mild degree of hypochromic microcytic iron deficiency anemia.
Even more striking are the results of Tmprss6 silencing in β-thalassemia mice. β-thalassemias are severe recessive disorders characterized by defective globin chain synthesis, microcytic anemia, and secondary iron overload. In the transfusion-independent β-thalassemia intermedia, anemia results from both ineffective erythropoiesis and decreased red blood cell (RBC) survival. Iron overload is caused by increased iron absorption because ineffective erythropoiesis suppresses hepcidin production. Homozygous inactivation of Tmprss6 in thalassemic mice increases hepcidin and ameliorates iron overload, but surprisingly improves ineffective erythropoiesis.8 These results provided a proof of principle for the therapeutic approach proposed by Schmidt et al. Accordingly, pharmacologic activation of hepcidin, achieved by Tmprss6 silencing in Hbbth3/+ adult mice, not only corrects iron overload but also ameliorates anemia, as demonstrated by the increased hemoglobin levels, RBC count, and RBC survival, and by the decreased spleen size and serum erythropoietin. Trying to explain the mechanism of improved erythropoiesis, Schmidt et al demonstrate that the amount of membrane associated α globin is decreased in the double mutant mice compared with the thalassemic mice relieving the erythroid damage and death, likely as a result of low iron availability for single erythroid cell.
The effects of Tmprss6 inactivation strengthen and extend previous findings on the beneficial role of limiting iron supply in thalassemia by increasing circulating transferrin9 or directly enhancing hepcidin levels by transplantation of β-thalassemia bone marrow in Hamp transgenic mice.10 However, despite the advantage obtained in β-thalassemia, the LNP-Tmprss6 siRNA treatment impaired erythropoiesis, thus causing hypochromic microcytic iron deficiency anemia in hemochromatosis mice, as shown in Hfe−/− mice with genetic inactivation of Tmprss6.7
Tmprss6 appears to be a key molecule in the negative regulation of hepcidin, attenuating the BMP6 signaling effect in mice; as such, it could become an important therapeutic target also in humans. In the perspective of applying a similar therapeutic approach in patients, the challenge will be to develop protocols that precisely titrate hepcidin expression to avoid the unwanted side-effect of iron deficiency, as observed in human and murine models with chronic hepcidin activation.3,4
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal