Abstract
Multiple myeloma (MM) is a heterogeneous disease with certain genetic features [eg, t(4;14), del17p] associated with worse outcome. The introduction of thalidomide, lenalidomide, and bortezomib has dramatically improved the outlook for patients with MM, but their relative benefit (or harm) for different genetic patient subgroups remains unclear. Unfortunately, the small number of patients in each subgroup frequently limits the analysis of high-risk patients enrolled in clinical trials. Strategies that result in survival of high-risk genetic subgroups approximating that of patients lacking high-risk features are said to overcome the poor prognostic impact of these high-risk features. This outcome has been difficult to achieve, and studies in this regard have so far been limited by inadequate sample size. In contrast, strategies that compare the survival of high-risk genetic subgroups randomized to different treatment arms can identify approaches that improve survival. This type of analysis is clinically useful, even if the absolute gains do not improve outcomes to levels seen in patients without high-risk cytogenetics. Reviewing available data in high-risk MM from this perspective, it appears that bortezomib has frequently been associated with improved survival, whereas thalidomide maintenance has sometimes been associated with a shorter survival.
Introduction
In the last 10 years, the overall survival (OS) of multiple myeloma (MM) has improved considerably.1 Best estimates indicate that patients who are transplant eligible have a 5-year survival rate of > 70% with modern therapy.2,3 The 5-year survival rate of elderly transplant-ineligible patients is ∼ 50%.4 These are impressive gains, but the improvements have not been uniform, and prognosis continues to vary considerably based on a variety of prognostic factors (Table 1).5 Importantly, the survival of a subgroup of patients with certain cytogenetic abnormalities (collectively referred to as high-risk MM) has remained poor (median survival of 2-3 years) despite aggressive therapy incorporating almost every available drug and treatment modality.2,6-9 We need to identify strategies to overcome high-risk prognostic factors and improve survival in this patient population. As shown in Table 1, the therapeutic strategy needed to overcome high-risk prognostic factors and improve survival will necessarily be different based on the mechanism by which a given prognostic characteristic produces its adverse impact.
Prognostic factors and risk-stratification in myeloma
| Prognostic determinant . | Standard-risk . | High-risk . | Therapeutic implication . |
|---|---|---|---|
| Host factors | ECOG performance status 0-2 | ECOG performance status 3-4 | High-risk patients typically require a decrease in treatment intensity |
| Normal renal function | Renal failure (serum creatinine ≥ 2.0) | ||
| Advanced age | |||
| Tumor burden | Durie-Salmon stage I, II | Durie-Salmon stage III | Limited; some stage I patients require no therapy (smoldering myeloma, and some require radiation only (if solitary bone lesion) |
| Tumor biology (disease aggressiveness) | Hyperdiploidy | t(4;14)* | Treatment of high-risk patients remains unsatisfactory, but bortezomib appears to overcome some high-risk features (t4;14) |
| t(11;14) | t(14;16) | ||
| t(6;14) | t(14;20) | ||
| del17p | |||
| High LDH | |||
| High plasma cell proliferative rate | |||
| High-risk signature on GEP |
| Prognostic determinant . | Standard-risk . | High-risk . | Therapeutic implication . |
|---|---|---|---|
| Host factors | ECOG performance status 0-2 | ECOG performance status 3-4 | High-risk patients typically require a decrease in treatment intensity |
| Normal renal function | Renal failure (serum creatinine ≥ 2.0) | ||
| Advanced age | |||
| Tumor burden | Durie-Salmon stage I, II | Durie-Salmon stage III | Limited; some stage I patients require no therapy (smoldering myeloma, and some require radiation only (if solitary bone lesion) |
| Tumor biology (disease aggressiveness) | Hyperdiploidy | t(4;14)* | Treatment of high-risk patients remains unsatisfactory, but bortezomib appears to overcome some high-risk features (t4;14) |
| t(11;14) | t(14;16) | ||
| t(6;14) | t(14;20) | ||
| del17p | |||
| High LDH | |||
| High plasma cell proliferative rate | |||
| High-risk signature on GEP |
Modified from Rajkumar et al5 with permission.
ECOG indicates Eastern Cooperative Oncology Group; and LDH, lactate dehydrogenase.
t(4;14) is considered “intermediate-risk” based on improved results seen now with bortezomib-based initial therapy.
Genetic markers with prognostic significance
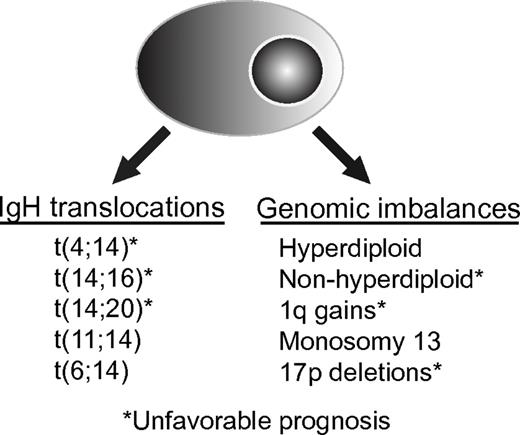
As in other hematologic malignancies, cytogenetics is one of the most important prognostic factors for MM (Figure 1). The advent of high-throughput methodologies for genomic analysis has greatly increased the sensitivity of available technologies for investigating genetic abnormalities. Whole-genome techniques, such as comparative genomic hybridization, mapping arrays based on single nucleotide polymorphisms, and gene expression profiling (GEP), have been added to traditional classic karyotyping and molecular cytogenetics based on fluorescence approaches for patient tumor cell characterization. Nevertheless, many of these approaches require sophisticated equipment and complex bioinformatic analyses, which has hindered their implementation in routine clinical practice. Although promising, most studies incorporating these approaches are small, and new molecular markers still need broader validation.
Genetic classifications of MM. The most commonly recognized high-risk genetic features are t(4;14) and del17p detected by FISH on either CD138-selected BM cells, or with the identification of clonally restricted plasma cells staining for cytoplasmic light chain immunoglobulin
Genetic classifications of MM. The most commonly recognized high-risk genetic features are t(4;14) and del17p detected by FISH on either CD138-selected BM cells, or with the identification of clonally restricted plasma cells staining for cytoplasmic light chain immunoglobulin
Chromosomal abnormalities
Nowadays, cytogenetic evaluation is mandatory in all patients with newly diagnosed MM and should always include interphase FISH in purified plasma cells or in combination with immunofluorescent detection of light-chain–restricted plasma cells (cIg-FISH).10,11 Cytogenetic abnormalities in MM can be classified into 2 main groups: translocations involving the IGH locus and genomic imbalances. Patients can have one or more of these abnormalities, and in general, over time, there is accumulation of new cytogenetic abnormalities. Some cytogenetic abnormalities have no adverse prognostic impact (eg, trisomies of odd numbered chromosomes), whereas others are unequivocally associated with poor outcomes (17p deletion).
IGH translocations.
IGH translocations are detectable in ∼ 40% of patients. There is a notable diversity of chromosomal partners involved in IGH translocations. The most frequently involved loci are 11q13 (CCND1) in 15%, 4p16 (FGFR3/MMSET) in 15% and 16q23 (MAF) in 5% of cases. Other recurrent loci, including 6p21 (CCND3) and 20q11 (MAFB), occur less frequently.12 Several groups have demonstrated that t(4;14)(p16;q32) is associated with poor survival.6,13-15 Patients with t(4;14) treated with both conventional or intensive chemotherapy display shorter event-free survival and OS.13,14,16,17 However, recent analysis support the impression that patients with t(4;14) make up a heterogeneous group. Thus, the French group has detected a subgroup of these patients (∼ 45%) with both low β2-microglobulin and high hemoglobin levels at diagnosis who experience prolonged survival after tandem transplant, and benefits from high-dose therapy.18 This heterogeneity has been also reported by the Arkansas group using their 70 gene-expression model that enables a clear separation of 2 groups of t(4;14) patients with significantly different OS.19
The association of t(14;16)(q32;q23) with prognosis is not definitive because it has a low frequency. According to Mayo Clinic data, t(14;16) was linked to poor outcome in the context of conventional chemotherapy.13 These results were confirmed by the association of MAF up-regulation with impaired prognosis by the UAMS group.2 However, the IFM group did not confirm the poor prognostic value of t(14;16) in patients receiving a tandem-autologous transplantation approach, whereas a recent study from the MRC showed a significantly shorter survival among patients with t(14;16) treated with autologous transplant.20,21
In contrast to t(4;14) and t(14;16), the presence of t(11;14)(q13;q32) has been shown to have either a favorable or no significant impact on prognosis.6,14,15,22 Using a risk model based on gene expression profiling, patients with t(11;14) can be further categorized into 2 subsets. The first, associated with CD20 expression, is characterized by slow onset of complete remission, but a significantly longer duration of complete remission. In contrast, the second subset of t(11;14) lacks CD20 expression and is associated with a more rapid onset and higher rate of complete remission, but a short duration.2
Genomic imbalances
MM is characterized by the frequent occurrence of chromosomal imbalances, including gains and losses that lead to the classification into hyperdiploid (characterized by trisomies) and nonhyperdiploid subgroups. The most common gains are those of 1q and of chromosomes 3, 5, 7, 9, 11, 15, 19, and 21, whereas the most common monosomy is of chromosome 13. Trisomies are typically associated with a better prognosis.23 Trisomies can be further stratified, using array comparative genomic hybridization, into 2 subclasses, with a survival disadvantage among patients with 1q gain and chromosome 13 loss, compared with chromosome 11 gain and absence of 1q gain or chromosome 13 loss. However, these observations need confirmation as the sample size was small and follow-up relatively short.24 A recent study showed that the presence of trisomies in patients with high-risk cytogenetic abnormalities can almost fully abrogate the adverse prognostic impact of the additional abnormalities on MM survival.25 Several studies have shown that 1q gain is a significant and independent poor prognostic factor, although there are also series that have failed to confirm that.26-29
Monosomy 13/13q deletions (present in ∼ 50% of cases) have been associated with short survival in almost all large series of patients treated with both conventional and high-dose therapy.24,30,31 However, this adverse prognostic implication seems to stem primarily from its close association with other high-risk genetic features, such as t(4;14), which harbor monosomy 13 in 80% of cases. Therefore, deletion of RB1 on 13q on its own is not a negative prognostic factor.6,15 Although deletion of 17p, which includes the TP53 locus, is less frequent in MM than in many other malignancies (∼ 10% of cases), it remains a strong poor prognostic factor in several different treatment contexts.6,13,14,32,33 Extramedullary disease, which is commonly related to more aggressive disease, has a higher frequency of 17p deletion.34,35
Cytogenetic risk-stratification
One of the aims of cytogenetic risk-stratification is to define a high-risk group that should be managed differently from standard-risk patients. Multiple classification systems have been proposed, and these continue to evolve. According to the International Myeloma Working Group, the term “high-risk MM” should include those patients with at least one of the following features: deletion of 17p, t(4;14) or t(14;16), detected by FISH analysis.11 Hypodiploidy defined by karyotyping and t(14;20) are also considered by the Mayo Clinic as high-risk factors.36 Recently, a prognostic model in MM based on the most frequently detected genetic lesions has been proposed. Accordingly, 3 genetic risk groups were defined: a favorable risk group with no adverse FISH lesions; an intermediate group with one adverse genetic lesion -t(4;14), t(14;16), t(14;20), 17p deletion, or 1q gain; and a high-risk group with more than one adverse genetic lesions.21 An updated risk-stratification model that takes into account the recent data suggesting that patients with t(4;14) have improved outcome in certain situations is shown in Table 2. Risk scores based on gene expression profiling have been recently reported as powerful prognostic predictors.19,37 They synthesize the biologic impact of multiple abnormalities, including chromosomal aberrations and point mutations or other changes on multiple important cellular pathways impacting on proliferation, differentiation, apoptosis, and other less-defined features in a single signature of myeloma cell characteristics.38 The validation of these signatures in different series and therapeutic contexts may one day yield a more refined and reproducible risk stratification.37
Cytogenetic risk-stratification of myeloma
| Standard-risk . | Intermediate-risk . | High-risk . |
|---|---|---|
| Trisomies* | t(4;14) | 17p deletion |
| t(11;14) | t(14;16) | |
| t(6;14) | t(14;20) |
| Standard-risk . | Intermediate-risk . | High-risk . |
|---|---|---|
| Trisomies* | t(4;14) | 17p deletion |
| t(11;14) | t(14;16) | |
| t(6;14) | t(14;20) |
Modified from Rajkumar et al5 with permission.
Presence of trisomies abrogates the poor prognostic impact of high-risk disease.
Outcome of high-risk cytogenetics
On analyzing the role of novel drugs in patients with high-risk cytogenetic characteristics, it is important to differentiate 2 concepts: “to overcome adverse prognosis” and “to improve outcome.” The first implies that with the use of specific treatments, the survival of high-risk patients becomes similar to standard risk patients. To improve outcome, on the other hand, means that a particular new treatment strategy is able to improve the outcome in these patients compared with standard treatments in the same high-risk patient subgroup. The former has the advantage that it can be evaluated in single arm studies, and the larger size of the standard-risk group can help the analysis to achieve statistical significance. Unfortunately, the failure to show a difference between the high-risk and standard-risk groups may simply be the result of small sample sizes and short follow-up. Furthermore, when faced with a patient with a given adverse prognostic feature, this analysis is not particularly helpful. The physician cannot choose which adverse prognostic features a patient has, but can choose which treatment to give the patient. It is far more important to the physician designing a treatment plan for an individual high-risk patient to know how the patient is likely to fare with different treatments than how the patient would fare with a different set of prognostic factors. This information can be obtained from randomized controlled clinical trials, specifically looking at outcomes for patients with high-risk genetic features. A limitation of this analysis is that high-risk groups tend to be small in both arms of most randomized trials, reducing the power of the analysis. It is important to note in these 2 types of analysis that in the former (overcoming), a lack of statistical power favors the novel therapy, whereas in the latter (improving) it favors the standard therapy. We will review both types of analyses in MM patients with high-risk genetics, placing the greatest emphasis on analyses that report therapies that improve outcome.
One problem that is difficult to solve is that the definition of “high risk” varies considerably across studies, and details on individual cytogenetic factors are often not available. Nevertheless, we find that the t(4;14) subset of MM is sufficiently unique, and dramatic improvements in outcome have been seen in this particular subset of patients with certain therapeutic strategies. In some models, t(4;14) is now no longer considered “high-risk” as a result of these new data (Table 2). Thus, we will consider this cytogenetic entity separately from other high-risk cytogenetic factors.
t(4;14) MM
The IFM group retrospectively analyzed the prognostic value of cytogenetic abnormalities in a large series of newly diagnosed MM patients treated with VAD (vincristine, adriamycin and dexamethasone) plus tandem autologous stem cell transplantation (ASCT) according to IFM99 protocols6 and found a significantly shorter median OS (41 vs 65 months) for patients with t(4;14) compared those without. They confirmed these findings in the IFM 2005 trial, comparing bortezomib plus dexamethasone to VAD as induction therapy, followed by ASCT.39 This study also showed that a short course of bortezomib-based induction and one/2 ASCT improved survival for t(4;14) compared with VAD followed by ASCT but was insufficient to overcome the poor prognostic impact of t(4;14).39 Thus the 4-year OS of patients with t(4;14) treated with bortezomib was shorter (63%) compared with patients without t(4;14) (79%), but longer than for t(4;14) patients treated with VAD (32%).
Three recent studies show that it may be possible to almost completely overcome the poor prognostic impact of t(4;14) using an approach that combines bortezomib-based therapy for 2-3 years in conjunction with tandem ASCT (Tables 3 and 4). A large randomized trial conducted by the HOVON and German MM study groups compared the efficacy of PAD (bortezomib, adriamycin and dexamethasone) versus VAD, both followed by ASCT and maintenance therapy.7,8 After induction, patients received either single (HOVON) or double (German) ASCT based on the study group. After ASCT, patients on the PAD arm received bortezomib maintenance, whereas in the VAD arm, they received thalidomide maintenance. The 3-year survival rate in 24 patients with t(4;14) treated with PAD was 66% compared with 82% for patients without t(4;14) (P = .2), whereas in 21 patients with t(4;14) treated with VAD, the survival rate was only 44% (P = .37). These data, although not reaching statistical significance, give some support to the concept that PAD with bortezomib maintenance does not completely overcome the adverse prognosis of t(4;14).7 Nevertheless, in the German subgroup (which included tandem ASCT), the 3-year OS for patients with and without t(4;14) was very similar in the PAD treatment arm (78% vs 87%), suggesting that a strategy of bortezomib-based induction, tandem ASCT, and bortezomib maintenance may overcome the adverse prognostic effect of t(4;14).7
Survival of MM patients with compared with those without high-risk genetic features
| Genetics . | Np/Na . | End point . | Therapy . | Present . | Absent . | Comment . |
|---|---|---|---|---|---|---|
| Conventional therapy | ||||||
| t(4;14) | 42/290 | 3-y OS | VBMCP | 24% | 64% | E948613 |
| 100/616 | 3-y OS | VAD + ASCT × 2 | 55% | 80% | IFM-996 | |
| 98/414 | 3-y OS | VAD + ASCT × 1/2 | 40% | 72% | IFM-200539 | |
| del17p | 37/308 | 3-y OS | VBMCP | 32% | 68% | E948613 |
| 58/474 | 3-y OS | VAD + ASCT × 2 | 50% | 78% | IFM-996 | |
| 119/393 | 3-y OS | VAD + ASCT × 1 | 49% | 82% | IFM-200539 | |
| Unfavorable FISH | 141/166 | 3-y OS | CVAD + ASCT × 1 | 58% | 81% | MRC IX intensive51 |
| 90/125 | 3-y OS | MP | 26% | 48% | MRC IX nonintensive9 | |
| 98/129 | 3-y OS | Placebo maintenance | 69% | 72% | MRC IX maintenance54 | |
| 18/111 | 3-y OS | VBMCP/VBAD + Bz × 2 + ASCT × 1 | 48% | 84% | GEM2005 < 6550 | |
| Thalidomide | ||||||
| t(4;14) | 57/181 | 3-y PFS | TD + ASCT × 2 + TD | 20% | 48% | GIMEMA43 |
| 26/156 | 3-y OS | VAD + ASCT × 1 + Thal maintenance | 44% | 79% | HOVON/GMMG7 | |
| del17p | 21/161 | 3-y OS | VAD + ASCT × 1 + Thal maintenance | 17% | 79% | HOVON/GMMG7 |
| Unfavorable FISH | 43/302 | 5-y OS | Thal induction, consolid, maintenance | 56% | 72% | Total Therapy 241 |
| 152/167 | 3-y OS | CTD + ASCT × 1 | 59% | 82% | MRC IX intensive51 | |
| 96/129 | 3-y OS | CTDa | 58% | 78% | MRC IX nonintensive9 | |
| 99/126 | 3-y OS | Thalidomide maintenance | 45% | 76% | MRC-IX maintenance54 | |
| 17/110 | 3-y OS | TD + ASCT × 1 | 56% | 86% | GEM2005 < 6550 | |
| Lenalidomide | ||||||
| t(4;14) | 28/102 | Median OS | LD in RRMM | 18 m | 23 m | MM-01645 |
| 26/158 | Median OS | LD in RRMM | 9 m | 15 m | IFM46 | |
| 152/355 | Median PFS | Lenalidomide maintenance | 27 m | 42 m | IFM-200547 | |
| del17p | 12/118 | Median OS | LD in RRMM | 4 m | 23 m | MM-01645 |
| 6.6% | Median PFS | Lenalidomide maintenance | 29 m | 42 m | IFM 200547 | |
| Unfavorable FISH | 16/84 | 3-y OS | LD | 77% | 86% | Mayo55 |
| 21/105 | 2-y OS | LD | 76% | 91% | E4A0356 | |
| Bortezomib | ||||||
| t(4;14) | 106/401 | 4-y OS | BzD + ASCT × 1 | 63% | 85% | IFM-200539 |
| 53/183 | 3-y PFS | BzTD + ASCT × 2 + BzTD | 65% | 61% | GIMEMA43 | |
| 24/148 | 3-y OS | BzAD + ASCT × 1 + Bz | 66% | 82% | HOVON/GMMG7 | |
| del17p | 54/453 | 4-y OS | BzD + ASCT × 1 | 50% | 79% | IFM-200539 |
| 16/156 | 3-y OS | BzAD + ASCT × 1 + Bz | 69% | 82% | HOVON/GMMG7 | |
| Unfavorable FISH | 18/112 | 3-y OS | BzTD + ASCT × 1 | 60% | 88% | GEM2005 < 6550 |
| 44/188 | 3-y OS | BzMP/BzTP then BzT/BzP | 55% | 73% | GEM2005 > 6558 | |
| 28/140 | 3-y OS | BzMP | 56% | 71% | VISTA57 |
| Genetics . | Np/Na . | End point . | Therapy . | Present . | Absent . | Comment . |
|---|---|---|---|---|---|---|
| Conventional therapy | ||||||
| t(4;14) | 42/290 | 3-y OS | VBMCP | 24% | 64% | E948613 |
| 100/616 | 3-y OS | VAD + ASCT × 2 | 55% | 80% | IFM-996 | |
| 98/414 | 3-y OS | VAD + ASCT × 1/2 | 40% | 72% | IFM-200539 | |
| del17p | 37/308 | 3-y OS | VBMCP | 32% | 68% | E948613 |
| 58/474 | 3-y OS | VAD + ASCT × 2 | 50% | 78% | IFM-996 | |
| 119/393 | 3-y OS | VAD + ASCT × 1 | 49% | 82% | IFM-200539 | |
| Unfavorable FISH | 141/166 | 3-y OS | CVAD + ASCT × 1 | 58% | 81% | MRC IX intensive51 |
| 90/125 | 3-y OS | MP | 26% | 48% | MRC IX nonintensive9 | |
| 98/129 | 3-y OS | Placebo maintenance | 69% | 72% | MRC IX maintenance54 | |
| 18/111 | 3-y OS | VBMCP/VBAD + Bz × 2 + ASCT × 1 | 48% | 84% | GEM2005 < 6550 | |
| Thalidomide | ||||||
| t(4;14) | 57/181 | 3-y PFS | TD + ASCT × 2 + TD | 20% | 48% | GIMEMA43 |
| 26/156 | 3-y OS | VAD + ASCT × 1 + Thal maintenance | 44% | 79% | HOVON/GMMG7 | |
| del17p | 21/161 | 3-y OS | VAD + ASCT × 1 + Thal maintenance | 17% | 79% | HOVON/GMMG7 |
| Unfavorable FISH | 43/302 | 5-y OS | Thal induction, consolid, maintenance | 56% | 72% | Total Therapy 241 |
| 152/167 | 3-y OS | CTD + ASCT × 1 | 59% | 82% | MRC IX intensive51 | |
| 96/129 | 3-y OS | CTDa | 58% | 78% | MRC IX nonintensive9 | |
| 99/126 | 3-y OS | Thalidomide maintenance | 45% | 76% | MRC-IX maintenance54 | |
| 17/110 | 3-y OS | TD + ASCT × 1 | 56% | 86% | GEM2005 < 6550 | |
| Lenalidomide | ||||||
| t(4;14) | 28/102 | Median OS | LD in RRMM | 18 m | 23 m | MM-01645 |
| 26/158 | Median OS | LD in RRMM | 9 m | 15 m | IFM46 | |
| 152/355 | Median PFS | Lenalidomide maintenance | 27 m | 42 m | IFM-200547 | |
| del17p | 12/118 | Median OS | LD in RRMM | 4 m | 23 m | MM-01645 |
| 6.6% | Median PFS | Lenalidomide maintenance | 29 m | 42 m | IFM 200547 | |
| Unfavorable FISH | 16/84 | 3-y OS | LD | 77% | 86% | Mayo55 |
| 21/105 | 2-y OS | LD | 76% | 91% | E4A0356 | |
| Bortezomib | ||||||
| t(4;14) | 106/401 | 4-y OS | BzD + ASCT × 1 | 63% | 85% | IFM-200539 |
| 53/183 | 3-y PFS | BzTD + ASCT × 2 + BzTD | 65% | 61% | GIMEMA43 | |
| 24/148 | 3-y OS | BzAD + ASCT × 1 + Bz | 66% | 82% | HOVON/GMMG7 | |
| del17p | 54/453 | 4-y OS | BzD + ASCT × 1 | 50% | 79% | IFM-200539 |
| 16/156 | 3-y OS | BzAD + ASCT × 1 + Bz | 69% | 82% | HOVON/GMMG7 | |
| Unfavorable FISH | 18/112 | 3-y OS | BzTD + ASCT × 1 | 60% | 88% | GEM2005 < 6550 |
| 44/188 | 3-y OS | BzMP/BzTP then BzT/BzP | 55% | 73% | GEM2005 > 6558 | |
| 28/140 | 3-y OS | BzMP | 56% | 71% | VISTA57 |
V indicates vincristine; A, adriamycin; D, dexamethasone; ASCT, autologous stem cell transplantation; Thal, thalidomide; Bz, bortezomib; M, melphalan; P, prednisone; B, BCNU; C, cyclophosphamide; L, lenalidomide; RRMM, relapsed refractory multiple myeloma; and /, sequental therapies.
Survival of high-risk genetic subgroups on randomized controlled clinical trials of untreated MM
| Genetics . | N1/N2 . | End point . | Arm 1 . | Arm 2 . | Arm 1, % . | Arm 2, % . | Comment . |
|---|---|---|---|---|---|---|---|
| t(4;14) | 26/24 | 3-y OS | V*-AD/ASCT/Thal* | Bz*-AD/ASCT/Bz* | 44 | 66 | HOVON/GMMG7 |
| 98/106 | 4-y OS | VA*-D | Bz*-D | 32 | 63* | IFM-200539 | |
| 21/23 | 2-y OS | Thal* | Placebo* | 67 | 87 | TT242 | |
| 21/29 | 2-y OS | Thal*-TT2 | Bz*-TT3 | 67 | 97* | TT2 vs TT342 | |
| del17p | 21/16 | 3-y OS | V*-AD/ASCT/Thal* | Bz*-AD/ASCT/Bz* | 17 | 69* | HOVON/GMMG7 |
| 119/54 | 4-y OS | VA*-D | Bz*-D | 36 | 50 | IFM-200539 | |
| Nonhyperdiploid | 92 | 3-y OS | Thal*-D-Bz | Mel*-P-Bz | 53 | 72* | PETHEMA4 |
| Unfavorable FISH | 152/141 | 3-y OS | Thal*-D-Cyclo | VA*-D-Cyclo | 58 | 56 | MRC IX intensive51 |
| 96/90 | 3-y OS | Thal*-D-Cyclo | Placebo*-P-Mel | 34 | 26 | MRC IX nonintensive9 | |
| 99/98 | 3-y OS | Thal* maintenance | Placebo* maintenance | 45 | 69* | MRC IX maintenance54 |
| Genetics . | N1/N2 . | End point . | Arm 1 . | Arm 2 . | Arm 1, % . | Arm 2, % . | Comment . |
|---|---|---|---|---|---|---|---|
| t(4;14) | 26/24 | 3-y OS | V*-AD/ASCT/Thal* | Bz*-AD/ASCT/Bz* | 44 | 66 | HOVON/GMMG7 |
| 98/106 | 4-y OS | VA*-D | Bz*-D | 32 | 63* | IFM-200539 | |
| 21/23 | 2-y OS | Thal* | Placebo* | 67 | 87 | TT242 | |
| 21/29 | 2-y OS | Thal*-TT2 | Bz*-TT3 | 67 | 97* | TT2 vs TT342 | |
| del17p | 21/16 | 3-y OS | V*-AD/ASCT/Thal* | Bz*-AD/ASCT/Bz* | 17 | 69* | HOVON/GMMG7 |
| 119/54 | 4-y OS | VA*-D | Bz*-D | 36 | 50 | IFM-200539 | |
| Nonhyperdiploid | 92 | 3-y OS | Thal*-D-Bz | Mel*-P-Bz | 53 | 72* | PETHEMA4 |
| Unfavorable FISH | 152/141 | 3-y OS | Thal*-D-Cyclo | VA*-D-Cyclo | 58 | 56 | MRC IX intensive51 |
| 96/90 | 3-y OS | Thal*-D-Cyclo | Placebo*-P-Mel | 34 | 26 | MRC IX nonintensive9 | |
| 99/98 | 3-y OS | Thal* maintenance | Placebo* maintenance | 45 | 69* | MRC IX maintenance54 |
V indicates vincristine; A, adriamycin; D, dexamethasone; ASCT, autologous stem cell transplantation; Thal, thalidomide; Bz, bortezomib; TT2, Total Therapy 2; TT3, Total Therapy 3; Mel, low-dose oral melphalan; P, prednisone; Cyclo, cyclophosphamide; and /, sequential therapies.
The drugs randomized in Arm 1 versus Arm 2 and the survival outcomes that are significantly superior.
Additional data supporting this treatment strategy come from analysis of the results of the Total Therapy 2 and Total Therapy 3 trials done at Arkansas. Prognosis of t(4;14) was inferior to standard-risk MM in Total Therapy 2, which had tandem ASCT and a randomization to thalidomide but lacked upfront bortezomib.40,41 The 2-year OS for the t(4;14) randomized to thalidomide was 67%, compared with 87% in those randomized to placebo (P = .80), and 97% in those treated in Total Therapy 3 (P = .01), in which patients received bortezomib-based induction, tandem ASCT, and bortezomib based maintenance therapy; however, these comparisons are difficult based on the nonconcurrent and nonrandomized nature of these sequential studies.2,42
The final piece of data comes from a randomized study by Cavo et al in which the poor prognosis associated with t(4;14) was overcome in the treatment arm that used bortezomib-based induction (VTD), tandem ASCT, and bortezomib based consolidation [3-year progression-free survival (PFS) 65% vs 61% for patients with and without t(4;14), respectively].43 In contrast to these positive results in tandem SCT studies, in the Spanish study (GEM05), which used VTD as induction but only one ASCT and no consolidation therapy, patients with t(4;14) showed significantly inferior survival compared with those without this abnormality.
There are limited data concerning the ability of other strategies to overcome the poor prognostic impact of t(4;14). There are no data that thalidomide can overcome the poor prognostic effect of t(4;14) on OS. The Australasian MM6 trial compared post-transplantation consolidation with thalidomide and prednisone versus prednisone alone in 243 patients. They found the addition of thalidomide significantly increased PFS (29 vs 17 months) in patients with t(4;14) detected by RT-PCR, but interestingly not in patients with elevated expression of FGFR3 (31 vs 29 months, P = .76), suggesting that additional information may be gained by studying FGFR3 expression.44 Similarly, there are limited data on lenalidomide and t(4;14). In a series of 130 relapsed/refractory patients treated with lenalidomide plus dexamethasone (MM016 trial), those with deletion (13q) or t(4;14) had a similar time to progression and OS as patients without these abnormalities.45 But this was a retrospective analysis of a group of patients with relapsed refractory MM, not newly diagnosed disease, and there was lumping of cytogenetic categories. Further, in contrast to these findings, Avet-Loiseau et al found that deletion (13q) and t(4;14) both had a significantly adverse effect in 207 relapse/refractory patients treated with the same scheme (lenalidomide + dexamethasone).46 Thus, in these patients, the OS was 9.4 months compared with 15.4 months (P < .005) for patients with and without these genetic changes, respectively. In the IFM study of maintenance lenalidomide versus no maintenance, a robust increase in PFS was observed with lenalidomide maintenance in the overall study population (median 42 vs 24 months, P < .0001) and in patients with del17p (median 29 vs 14 months, P < .02) but not in patients with t(4;14) (median 28 vs 24 months, P < .04; based on analysis presented at the annual meeting of the American Society of Hematology in 2010).47,48 In this setting, the prolongation of PFS with lenalidomide maintenance appears less than the benefit that can be obtained by treating at the time of relapse with lenalidomide plus dexamethasone rescue therapy. Therefore, in patients with t(4;14), lenalidomide maintenance should be used primarily in the context of clinical trials.
High-risk MM
In this section, we review all papers in which the information either does not separate between t(4;14) and del17p (grouping all together as high risk) or the information available is specifically focused on del17p.
Conventional therapy.
Thalidomide.
Current data indicate that thalidomide does not overcome the adverse prognosis of high-risk cytogenetics. Moreover, in some settings (maintenance therapy), patients with high-risk MM may actually do worse with thalidomide therapy than without. The pivotal initial study conducted by the Arkansas group in relapsed and/or refractory patients showed that patients with deletion 13q, detected by conventional cytogenetics, had short survival.49 The Spanish group (GEM 2005 < 65 years), using TD as induction before ASCT50 and the MRC IX Myeloma trial for patients receiving CTD or CTDa induction,9,51 both found significantly shorter OS for patients with compared with those without high-risk FISH (Table 3).
The use of thalidomide as maintenance does not seem to be of value in high-risk patients. Thus, the IFM group reported that, although thalidomide maintenance was of benefit for the overall population, patients with 13q deletion had short survival.52 The Austrian group evaluated the role of Thal-interferon versus interferon in elderly patients after induction with Thal-Dex or MP. Patients randomized to Thal-interferon with or without high-risk FISH had a median OS of 39 vs 72 months, respectively (P = .082).53 In the combined Hovon and German group study comparing the efficacy of PAD versus VAD, patients carrying del (17p) treated with VAD induction and thalidomide maintenance had 3-year OS of 19%.7,8 The MRC Myeloma IX trials found more resistant relapses after thalidomide maintenance, particularly in patients with del(17p)26 and the 3-year OS was considerably worse for patients with unfavorable FISH randomized to thalidomide as opposed to placebo maintenance (45% vs 69% P < .009).54 Remarkably, 44% of patients in this study had unfavorable FISH, defined as gain 1q, del1p32, del17p, t(4;14), t(14;16), and t(14;20), identifying a very large group of patients apparently harmed by thalidomide maintenance. Finally, the Arkansas group showed that, in the Total Therapy II program, which includes thalidomide throughout all treatment phases, the outcome of patients with high-risk GEP is significantly worse than that of standard risk patients (5-year OS 56% vs 72%).41
Regarding elderly patients, the efficacy of MPT or thalidomide-dexamethasone in high-risk patients has not been reported. The only available information corresponds to the CTDa combination, which does not overcome the effect of high risk CA (3-year OS 34% for high risk vs 58% for standard risk), nor is it significantly better than MP in high-risk CA (3-year OS 26%; Gareth Morgan, Institute for Cancer Research, written personal communication, September 6, 2011).9
Lenalidomide.
The efficacy of lenalidomide in high-risk patients has mainly been explored in the relapsed setting. Thus, Reece et al, studying a series of 130 relapsed/refractory patients treated with lenalidomide plus dexamethasone (MM016 trial), reported that the presence of deletion (17p13) was associated with very short time to progression (2.2 months) and OS (4.7 months).45
Kapoor et al evaluated outcomes after initial therapy with lenalidomide/dexamethasone in 100 newly diagnosed patients55 and found the time to progression was significantly shorter in the high-risk subgroup (18.5 vs 36.5 months). In the context of the phase 3 clinical trial E4A03 (lenalidomide plus either low or high doses of dexamethasone), in 21 of 126 patients classified as high-risk by FISH, the 2-year OS was 76% vs 91% (P = .004).56 As mentioned in relationship to t(4;14) MM above, Avet-Loiseau et al have investigated the role of lenalidomide maintenance in newly diagnosed patients with high-risk cytogenetics.47 The PFS of patients with del 17p (29 months) was significantly shorter compared with the PFS of the overall series of patients (42 months) maintained with lenalidomide. This indicates that lenalidomide maintenance does not overcome the adverse prognosis of del17p. However, it significantly improved the PFS compared with no maintenance (14 months). A recent update of this data reported that 52 patients with either t(4;14) or del17p were randomized to lenalidomide in this trial, whereas 29 were randomized to placebo. Although data were not split between patients with t(4;14) and del17p, a hazard ratio for progression or death of 0.58 in favor of lenalidomide maintenance (P = .39) was noted.48
Bortezomib.
In the IFM-2005-01 trial, bortezomib-based induction plus ASCT was unable to rescue patients with del(17p); the 4-year OS was 50% vs 79% in patients with and without del (17p), respectively. The Spanish Myeloma group has also tested the efficacy of VTD followed by single ASCT in the randomized GEM 2005 trial. The 3-year OS of patients with t(4;14), t(14;16) or deletion of 17p was significantly shorter to that of standard-risk patients 60% vs 88% (P = .01).50 As previously mentioned, the Hovon group has explored the efficacy of PAD versus VAD before single8 or double7 ASCT in patients with del17p.7 In this cohort, a major benefit for the bortezomib-based scheme was observed compared with VAD with thalidomide maintenance: 3-year OS 69% vs 17%. However, bortezomib does not completely overcome the adverse prognosis of del17p because in patients not having this abnormality the 3-years OS rate was higher (85%).
The Total Therapy 3 program conducted by the Arkansas group in which bortezomib is incorporated during induction, consolidation, and maintenance demonstrated a major advantage over TT2 in low-risk MM and in patients with t(4;14), but only a minor improvement was achieved in high-risk patients defined by GEP, or in high-risk patients with t(14;16) or t(14;20).2,40-42 The survival of patients with a high-risk gene expression profile was significantly shorter than that of standard-risk patients (2-year OS of 56% vs 88%,).2 A similar picture was observed with the TT4 program that includes VRD as consolidation: the 2-year OS was 51% vs 85% for patients classified as high versus standard risk according to GEP-70.2
In newly diagnosed elderly patients, the use of bortezomib plus melphalan and prednisone (VMP) in the VISTA trial appeared to overcome the adverse prognosis of high-risk cytogenetics, because comparison of high- and standard-risk cases had a similar 3-year OS (56% vs 71%).57 Nevertheless, it should be noted that the number of patients analyzed was relatively small, and results included patients with t(4;14) who are known to benefit from bortezomib. A subsequent study by the Spanish Myeloma group (GEM/Pethema) did not demonstrate such an effect. In this study, also in newly diagnosed elderly patients, 2 induction regimens, VMP and VTP (bortezomib, melphalan or thalidomide, prednisone), were followed by maintenance with bortezomib-thalidomide or bortezomib-prednisone, respectively. Among 92 nonhyperdiploid patients (which are enriched for patients with high-risk genetics), the 3-year OS was 72% vs 52% (P = .1) for the patients randomized to the melphalan versus the thalidomide arm. In 44 patients with compared with 188 without high-risk FISH, the median OS was 38 months versus not reached (P = .001).58 The Italian group also investigated the outcome of high-risk patients in the context of a protocol comparing VMP versus VMP plus thalidomide (VMPT).59 In this study, high-risk patients had a similar PFS to that of standard-risk patients with VMP (P = .83) as well as VMPT (P = .43), but further follow up and effects on OS need to be clarified.
Conclusion
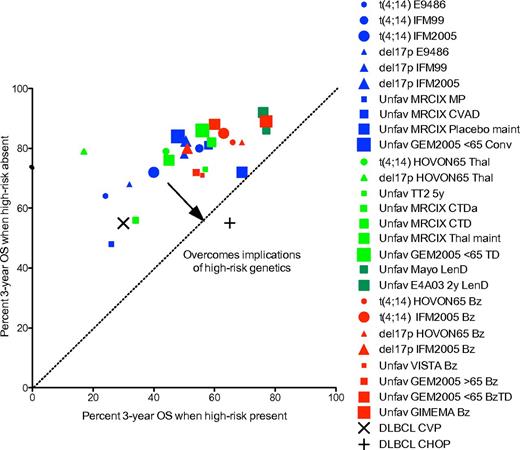
In conclusion, we have highlighted several important concepts in this paper. First, we show that adverse prognosis probably occurs via a number of different mechanisms (Table 1; Figure 1) and that strategies to overcome the poor prognostic impact of a given factor must critically examine the mechanism by which each variable exerts its poor prognostic effect. Second, we discuss the importance of cytogenetic markers that result in poor prognosis by virtue of being associated with aggressive disease biology, and highlight risk stratification models that can evolve over time as new data emerge. Third, we make a distinction between strategies that overcome the poor prognostic effects of high-risk biomarkers (overcome adverse prognosis) and strategies that result in improved outcome for high-risk patients, even if the overall result is still suboptimal compared with standard risk patients (improve outcome). The type of data, evidence, and trial designs for these 2 approaches are different. The difference between these 2 types of analyses is graphically demonstrated in Figures 2 and 3, respectively; and although the impression from examining Figure 2 is that this analysis (overcoming high risk) does not provide a clear picture of the role of different drugs in the treatment of high-risk MM, a much clearer picture arises from the analysis depicted in Figure 3 (improving high risk). The rational conclusion from this analysis is that bortezomib is indicated in the treatment of high-risk MM, whereas thalidomide used as maintenance therapy has been associated with inferior outcome in these patients. Further studies on the optimal use of lenalidomide in high-risk MM are needed.
Identification of regimens that overcome the poor prognostic impact of various genetic lesions in MM. xy plot of percent 3-year OS for patients with and without different genetic lesions treated with conventional, thalidomide or bortezomib-based therapies. Data from Table 3 for MM patients with (x-value) or without (y-value): t(4;14) (circles), del17p (triangles), unfavorable FISH (squares) treated with conventional (blue), thalidomide (light green), lenalidomide (dark green), or bortezomib (red) based regimens. The larger studies are represented with larger symbols. The regimens with data points closest to the dotted line have the most similar survival rates for patients with or without a given abnormality and are best at overcoming the implications of high-risk genetics. A conceptual example is given of the introduction of doxorubicin in the treatment of non-Hodgkin lymphomas. Diffuse large B-cell lymphoma, which had a shorter survival then other lymphomas, was suddenly found to have a better survival after the introduction of doxorubicin. None of the drugs in MM has such a dramatic effect on any genetic subtype of the disease. M indicates melphalan; P, prednisone; C, cyclophosphamide; V or O, vincristine; A or H, doxorubicin; D, dexamethasone; Da, attenuated-dose dexamethasone; Conv, conventional chemotherapy arm of GEM2005 for patients younger than 65 years (VBMCP/VBAD + Bz × 2 + ASCT × 1); T or Thal, thalidomide; Len, lenalidomide; Bz, bortezomib; and maint, maintenance.
Identification of regimens that overcome the poor prognostic impact of various genetic lesions in MM. xy plot of percent 3-year OS for patients with and without different genetic lesions treated with conventional, thalidomide or bortezomib-based therapies. Data from Table 3 for MM patients with (x-value) or without (y-value): t(4;14) (circles), del17p (triangles), unfavorable FISH (squares) treated with conventional (blue), thalidomide (light green), lenalidomide (dark green), or bortezomib (red) based regimens. The larger studies are represented with larger symbols. The regimens with data points closest to the dotted line have the most similar survival rates for patients with or without a given abnormality and are best at overcoming the implications of high-risk genetics. A conceptual example is given of the introduction of doxorubicin in the treatment of non-Hodgkin lymphomas. Diffuse large B-cell lymphoma, which had a shorter survival then other lymphomas, was suddenly found to have a better survival after the introduction of doxorubicin. None of the drugs in MM has such a dramatic effect on any genetic subtype of the disease. M indicates melphalan; P, prednisone; C, cyclophosphamide; V or O, vincristine; A or H, doxorubicin; D, dexamethasone; Da, attenuated-dose dexamethasone; Conv, conventional chemotherapy arm of GEM2005 for patients younger than 65 years (VBMCP/VBAD + Bz × 2 + ASCT × 1); T or Thal, thalidomide; Len, lenalidomide; Bz, bortezomib; and maint, maintenance.
Identification of novel agents that improve the survival of patients with high-risk MM. xy plot of percent OS for the 2 arms of randomized controlled clinical trials for patients with different genetic lesions. Data from Table 4 for MM patients with t(4;14) (circles), del17p (triangles), unfavorable FISH (squares) treated with thalidomide (red) or bortezomib (green) based regimens. The x-value is the survival for the arm with the novel agent, and the y-value for the survival of the control arm. The regimens with data points above the dotted line are better than control, whereas those below are worse. In this analysis, regimens that improve survival have points that fall in the upper left, whereas those that worsen survival fall in the lower left. One sees clearly that all of the bortezomib arms (red) fall in the upper left, whether bortezomib is being used in induction or maintenance and whether it is being compared with placebo, vincristine-adriamycin, or thalidomide. In contrast, the thalidomide arms (green) almost all fall in the lower right, whether thalidomide is being used for induction or maintenance and whether it is being compared with placebo, melphalan, or bortezomib. Abbreviations are as in Figure 2.
Identification of novel agents that improve the survival of patients with high-risk MM. xy plot of percent OS for the 2 arms of randomized controlled clinical trials for patients with different genetic lesions. Data from Table 4 for MM patients with t(4;14) (circles), del17p (triangles), unfavorable FISH (squares) treated with thalidomide (red) or bortezomib (green) based regimens. The x-value is the survival for the arm with the novel agent, and the y-value for the survival of the control arm. The regimens with data points above the dotted line are better than control, whereas those below are worse. In this analysis, regimens that improve survival have points that fall in the upper left, whereas those that worsen survival fall in the lower left. One sees clearly that all of the bortezomib arms (red) fall in the upper left, whether bortezomib is being used in induction or maintenance and whether it is being compared with placebo, vincristine-adriamycin, or thalidomide. In contrast, the thalidomide arms (green) almost all fall in the lower right, whether thalidomide is being used for induction or maintenance and whether it is being compared with placebo, melphalan, or bortezomib. Abbreviations are as in Figure 2.
We can only speculate as to the mechanism underlying these important clinical observations. The action of bortezomib is probably multifactorial, including targeting proliferation, ER stress, the unfolded protein response, and activating mutations of the NF-κB pathway.60,61 Targeting some or all of these may play an important role in the ability of bortezomib to abrogate the early mortality seen in high-risk MM. Furthermore, given that high-risk MM has recently been associated with increased genomic instability and clonal heterogeneity,62 it is probable that the deeper and faster responses seen with the addition of bortezomib may be particularly important. In contrast, the use of chronic, suboptimal doses of thalidomide as maintenance in such patients may more quickly lead to the selection of resistant clones, compared with thalidomide use at full doses in the relapsed setting.
To make progress in the future, our strategies will need to be quite focused and may need to vary depending on the particular adverse prognostic marker. At present, we may be able to overcome the poor prognostic effect of t(4;14), whereas overcoming other high-risk cytogenetic markers is proving to be quite challenging given the current repertoire of active MM drugs. Finally, we highlight that certain strategies (eg, thalidomide maintenance) that may be harmless and even of benefit in standard-risk patients may be ineffective or even harmful in patients with high-risk disease. Although our paper is focused on MM, we think that several of the principles apply to other similar malignancies as well. High-risk MM is a complex entity, and the eventual solutions are probably not simple or easy to come by. We urge that investigators work together, pool data and studies, and work with a singular focus on improving OS in this patient population.
Acknowledgments
This work was supported by the National Institutes of Health (grant CA100707, P.L.B., S.V.R.; grants CA136671 and CA133966, P.L.B.; grants CA107476 and CA 83724, S.V.R.) and El Fondo de Investigación Sanitaria (grant PS09/1897; J.F.S.M.).
National Institutes of Health
Authorship
Contribution: P.L.B., M.-V.M., N.C.G., S.V.R., and J.F.S.M. did the required background research and wrote the paper.
Conflict-of-interest disclosure: P.L.B. has served on advisory boards for Celgene and Onyx Pharmaceuticals. J.F.S.M. has served on advisory boards for Celgene, Millenium, Jansen, Novartis, and BMS. The remaining authors declare no competing financial interests.
Correspondence: P. Leif Bergsagel, Division of Hematology-Oncology, Mayo Clinic in Arizona, 13400 E Shea Blvd, Scottsdale, AZ 85259; e-mail: bergsagel.leif@mayo.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal