Key Points
Autoimmune diseases do occur after CBT in approximately 5% of patients.
Of these, AIHA or ITP were observed the most often and were treated with prednisone, CSA, and RTX.
Abstract
To describe the incidence, risk factors, and treatment of autoimmune diseases (ADs) occurring after cord blood transplantation (CBT), we analyzed both CBT recipients reported to EUROCORD who had developed at least 1 new AD and those who had not. Fifty-two of 726 reported patients developed at least 1 AD within 212 days (range, 27-4267) after CBT. Cumulative incidence of ADs after CBT was 5.0% ± 1% at 1 year and 6.6% ± 1% at 5 years. Patients developing ADs were younger and had more nonmalignant diseases (P < .001). ADs target hematopoietic (autoimmune hemolytic anemia, n = 20; Evans syndrome, n = 9; autoimmune thrombocytopenia, n = 11; and immune neutropenia, n = 1) and other tissues (thyroiditis, n = 3; psoriasis, n = 2; Graves disease, n = 1; membranous glomerulonephritis, n = 2; rheumatoid arthritis, n = 1; ulcerative colitis, n = 1; and systemic lupus erythematosus, n = 1). Four patients developed 2 ADs (3 cases of immune thrombocytopenia followed by autoimmune hemolytic anemia and 1 Evans syndrome with rheumatoid arthritis). By multivariate analysis, the main risk factor for developing an AD was nonmalignant disease as an indication for CBT (P = .0001). Hematologic ADs were most often treated with steroids, rituximab, and cyclosporine. With a median follow-up of 26 months (range, 2-91), 6 of 52 patients died as a consequence of ADs. We conclude that CBT may be followed by potentially life-threatening, mainly hematologic ADs.
Introduction
Existing data on the nature, treatment, and outcome of autoimmune diseases (ADs) occurring after autologous and/or allogeneic hematopoietic stem cell transplantation (HSCT) for different indications have been summarized in recent reviews.1,2 Mostly, these “new” ADs are autoantibody-mediated and organ specific (eg, autoimmune thyroiditis, autoimmune hemolytic anemia [AIHA], or immune thrombocytopenia [ITP]); more rarely, they are multisystemic (eg, systemic lupus erythematosus [SLE] or rheumatoid arthritis [RA]). Data on incidence and risk factors are only emerging, and this has been first investigated in a larger cohort for ADs occurring after HSCT used as treatment option for primary AD.3,4
In addition to BM and peripheral blood, HSCs originating from umbilical cord blood are being used for HSCT. Cord blood–derived lymphocytes are naive unlike adult lymphocytes, resulting in decreased stringency of HLA match and the relatively lower risk of GVHD. The application of “double” cord blood transplantation (CBT) to overcome the dose limit of single units in adults might modify the early lymphoid reconstitution.5 These immunologic and genetic properties may affect the development of ADs after CBT. Reports of ADs occurring after CBT are scarce and based on single case reports and small series, the largest including 10 patients.6 The most prevalent ADs reported are AIHA,6,7 Evans syndrome,8 ITP,6,9 thyroiditis,10 and bullous dermatoses (pemphigus and pemphigoid).11,12
This study was undertaken to characterize the nature, incidence, and risk factors for ADs developing after CBT in a large patient population.
Methods
This retrospective study, which was conducted in accordance with the Declaration of Helsinki, was approved and conducted by EUROCORD and the European Group for Blood and Marrow Transplantation (EBMT) Autoimmune Disease Working Party (ADWP) following the EBMT study guidelines. All centers reporting to EUROCORD were invited to participate. Centers agreeing to participate were asked to follow-up on all patients who received CBT before January 1, 2009 and to identify those who had developed an AD after CBT. To compare data from patients developing ADs after CBT with those who did not, we only included in the control group the CBT recipients confirmed not to have developed ADs after CBT. A detailed questionnaire including the diagnosis of AD, disease extent if applicable, therapy, and outcome of AD was sent to the referring physicians. Informed consent to share clinical and outcome data were obtained from all patients before CBT.
The primary end point of the study was the incidence of ADs occurring after CBT. Patient-, disease-, and transplantation-related variables of the 2 groups were compared using the χ2 statistic or the Fisher exact test for categorical variables and the Mann-Whitney test for continuous variables.
Engraftment-related data were as follows: neutrophil recovery was defined as achieving an absolute neutrophil count ≥ 0.5 × 109/L for 3 consecutive days. Full donor chimerism was defined as ≥ 95% of leukocytes being of donor origin in peripheral blood or BM samples measured by different techniques according to transplantation centers. Autologous reconstitution was defined as ≥ 95% of leukocytes being of recipient origin. Mixed chimerism was defined as the presence of > 5% but < 95% of leukocytes of donor origin. The diagnosis and grading of acute and chronic GVHD were assigned by the transplantation center using standard criteria. Overall survival (OS) was calculated from the date of CBT until death or last observation alive. Cumulative incidence curves were used for estimating incidence of ADs considering death as a competing event. The Gray test was used for univariate comparisons.13 Associations of patient, disease, and graft characteristics with outcomes were evaluated in univariate and multivariate analyses using proportional subdistribution hazard regression model of Fine and Gray. Acute and chronic GVHD were analyzed as risk factors for AD as time-dependent covariates. A stepwise backward procedure was used to construct a set of independent predictors of new AD. All predictors achieving P < .10 were considered for multivariate analysis and these results are provided in “Risk factors for developing an AD after CBT.”
The impact of new AD on OS was studied using a Cox proportional hazard model including time-dependent variable. All tests were 2-sided. The type I error rate was fixed at 0.05 for determination of factors associated with time to event outcomes. Statistical analyses were performed with SPSS Version 19 and R Version 2.13.2 software.
Results
The 37 participating centers from 18 countries provided complete updates on all CBT patients at their institution before January 1, 2009. Among 778 patients included, 52 had developed at least 1 new AD after CBT in a median of 212 days (range, 27-4267). Data from these 52 patients were compared with those from the remaining 726 reported patients who had not developed an AD (the control group). All patients were transplanted between 1992 and 2008. Follow-up for all patients was 43 months (range, 3.5-217); 37 months (range, 7-74) for patients having developed an AD, and 43 months (range, 3.5-217) for the control group.
Patient characteristics
Detailed description of patient and graft characteristics, original disease, and conditioning for all patients are provided in Table 1. Patients having developed a new AD after CBT were younger at the time of CBT (P < .0001) and were more often transplanted for nonmalignant primary diseases (P < .0001).
Patient characteristics
| Patient characteristics . | With new ADs after CBT (n = 52) . | Without new ADs after CBT (n = 726) . | P . | |
|---|---|---|---|---|
| Age at CBT, y (range) | 5 (0.2-45) | 16 (0.11-67) | < .0001 | |
| Male sex, n (%) | 33 (63%) | 408 (56%) | .33 | |
| CMV+ prior to CBT, n (%) | 23 (46%) | 417 (60%) | .05 | |
| Reason for CBT malignant disease, n (%) | Acute leukemia | 16 (31%) | 418 (58%) | Malignant versus nonmalignant disease < .0001 |
| MDS | 4 (8%) | 80 (11%) | ||
| CML | 5 (10%) | 36 (5%) | ||
| Lymphoma | 1 (2%) | 33 (5%) | ||
| Myeloma | 0 | 3 (0.5%) | ||
| Nonmalignant disease, n (%) | Aplastic anemia | 6 (12%) | 55 (8%) | |
| Hemoglobinopathy | 0 | 15 (2%) | ||
| SCID | 6 (12%) | 51 (7%) | ||
| Histiocytosis | 2 (4%) | 13 (2%) | ||
| Metabolic disease | 12 (23%) | 18 (3%) | ||
| Other* | 0 | 3 | ||
| Time from diagnosis to CBT, mo (range) | 8 (2-87) | 12 (0.5-247) | .04 | |
| Graft characteristics, n (%) | Unrelated donor | 50 (96%) | 665 (92%) | .25 |
| HLA match 5/6 and 6/6 | 33 (66%) | 323 (48%) | .02 | |
| More than 1 CB unit transplanted | 8 (15%) | 141 (19%) | .48 | |
| TNC infused × 107/kg (median) | 5.9 (1.4-25) | 3.7 (0.2-31) | .005 | |
| ABO matched | 19 (40%) | 250 (39%) | .91 | |
| ABO minor mismatch | 11 (22%) | 166 (26%) | ||
| ABO major mismatch | 18 (38%) | 233 (36%) | ||
| Conditioning regimen, n (%) | RIC | 9 (18%) | 168 (24%) | .32 |
| TBI | 9 (18%) | 254 (35%) | .01 | |
| ATG/alemtuzumab | 38 (76%) | 527 (78%) | .73 | |
| GVHD prophylaxis, n (%) | CsA + steroids | 26 (52%) | 443 (63%) | .23 |
| CsA + MMF + steroids | 7 (14%) | 162 (23%) | ||
| Patient characteristics . | With new ADs after CBT (n = 52) . | Without new ADs after CBT (n = 726) . | P . | |
|---|---|---|---|---|
| Age at CBT, y (range) | 5 (0.2-45) | 16 (0.11-67) | < .0001 | |
| Male sex, n (%) | 33 (63%) | 408 (56%) | .33 | |
| CMV+ prior to CBT, n (%) | 23 (46%) | 417 (60%) | .05 | |
| Reason for CBT malignant disease, n (%) | Acute leukemia | 16 (31%) | 418 (58%) | Malignant versus nonmalignant disease < .0001 |
| MDS | 4 (8%) | 80 (11%) | ||
| CML | 5 (10%) | 36 (5%) | ||
| Lymphoma | 1 (2%) | 33 (5%) | ||
| Myeloma | 0 | 3 (0.5%) | ||
| Nonmalignant disease, n (%) | Aplastic anemia | 6 (12%) | 55 (8%) | |
| Hemoglobinopathy | 0 | 15 (2%) | ||
| SCID | 6 (12%) | 51 (7%) | ||
| Histiocytosis | 2 (4%) | 13 (2%) | ||
| Metabolic disease | 12 (23%) | 18 (3%) | ||
| Other* | 0 | 3 | ||
| Time from diagnosis to CBT, mo (range) | 8 (2-87) | 12 (0.5-247) | .04 | |
| Graft characteristics, n (%) | Unrelated donor | 50 (96%) | 665 (92%) | .25 |
| HLA match 5/6 and 6/6 | 33 (66%) | 323 (48%) | .02 | |
| More than 1 CB unit transplanted | 8 (15%) | 141 (19%) | .48 | |
| TNC infused × 107/kg (median) | 5.9 (1.4-25) | 3.7 (0.2-31) | .005 | |
| ABO matched | 19 (40%) | 250 (39%) | .91 | |
| ABO minor mismatch | 11 (22%) | 166 (26%) | ||
| ABO major mismatch | 18 (38%) | 233 (36%) | ||
| Conditioning regimen, n (%) | RIC | 9 (18%) | 168 (24%) | .32 |
| TBI | 9 (18%) | 254 (35%) | .01 | |
| ATG/alemtuzumab | 38 (76%) | 527 (78%) | .73 | |
| GVHD prophylaxis, n (%) | CsA + steroids | 26 (52%) | 443 (63%) | .23 |
| CsA + MMF + steroids | 7 (14%) | 162 (23%) | ||
Other includes congenital erythropoietic porphyria, autoimmune hemolytic anemia, and dyserythropoietic anemia.
CMV+ indicates CMV-specific IgG present at CBT; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; TNC, total nucleated cell; RIC, reduced-intensity conditioning; TBI, total body irradiation; ATG, antithymocyte globulin; and MMF, mycophenolate mofetil.
Incidence of newly diagnosed ADs after CBT
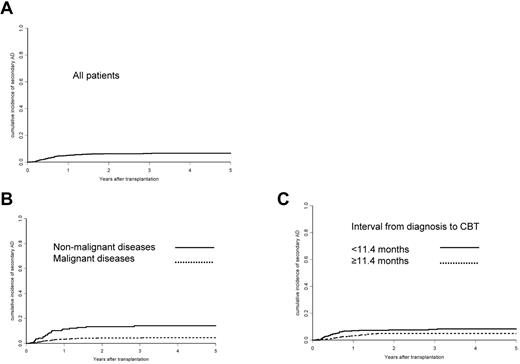
The cumulative incidence of ADs after CBT was 5% ± 1% after 1 year and 6.6% ± 1% after 5 years (Figure 1). Most ADs (in 41 patients) were hematologic (ie, cytopenias such as AIHA, n = 20; Evans syndrome, n = 9; ITP, n = 11; and immune neutropenia, n = 1). Thyroiditis (n = 3), psoriasis (n = 2), Graves disease (n = 1), membranous glomerulonephritis (n = 2), RA (n = 1), ulcerative colitis (n = 1), and SLE (n = 1) were also diagnosed. Four patients developed sequentially 2 ADs: 3 had ITP followed by AIHA 3, 7, and 12 months later and 1 patient with Evans syndrome developed RA 26 months later.
Cumulative incidences for the development of an AD after CBT. Shown are incidences for all patients (A), for patients with a malignant disease versus those with a nonmalignant disease (B), and for patients with an interval from diagnosis of the disease leading to transplantation until CBT < median (11.4 months) versus patients with a longer or equal interval as the median (C).
Cumulative incidences for the development of an AD after CBT. Shown are incidences for all patients (A), for patients with a malignant disease versus those with a nonmalignant disease (B), and for patients with an interval from diagnosis of the disease leading to transplantation until CBT < median (11.4 months) versus patients with a longer or equal interval as the median (C).
The median time to the occurrence of AD was 191 days (range, 27-4267) after CBT for hematologic diseases (n = 41) and 367 days (range, 70-116) for other ADs (n = 11; P = .09).
Disease-specific diagnostic criteria were fulfilled for all AD diagnoses (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Risk factors for developing an AD after CBT
The following parameters were evaluated as potential risk factors for developing an AD after CBT: age at transplantation, interval from disease (indication for CBT) to diagnosis, sex, reduced-intensity conditioning, serotherapy (use of ATG or alemtuzumab during conditioning), total body irradiation, year of CBT, number of CB units transplanted (1 vs > 1), donor choice (related vs unrelated), HLA matching (5/6 and 6/6 HLA match vs < 5/6), ABO blood-group match, number of nucleated cells infused at CBT, type of GVHD prophylaxis, and diagnosis of the disease leading to CBT. Acute and chronic GVHD were analyzed as time-dependent covariates.
Using univariate analysis, median age at CBT < 15 years (P = .002), from the primary disease diagnosis to CBT < 11.4 months (median interval; P = .03), diagnosis of a nonmalignant disease (P < .0001), HLA match ≥ 5/6 (P = .017), and total body irradiation not included in the conditioning regimen (P = .011) appeared as risk factors for the development of AD after CBT. The median number of infused nucleated cells was higher in patients who developed an AD after CBT than in those who did not (P < .005; Table 1).
After multivariate analysis, diagnosis of a nonmalignant disease versus a malignant disease as the reason for CBT (hazard ratio [HR] = 3.36; 95% confidence interval [CI], 1.95-5.78; P < .001) and interval from diagnosis to CBT < 11.4 months (median interval; HR = 1.85; 95% CI, 1.05-3.28; P = .034) were independently associated with the occurrence of ADs after CBT. The 5-year cumulative risk for the development of ADs in patients with a nonmalignant disease was 14%, and 8.3% for patients having received CBT earlier after diagnosis (Figure 1). The same risk factors were found when a subgroup analysis was performed for the 41 patients developing a hematologic AD (data not shown).
Engraftment, GVHD, and infection at diagnosis of AD
Of 778 patients included in this analysis, 123 did not engraft and chimerism data during the 100 days after CBT were available for 605 patients (120 with primary nonmalignant disorders and 485 with malignant disorders). Autologous recovery was observed in 11% of patients with nonmalignant disorders and in 8% of patients with malignant disorders; however, mixed chimerism was observed in 28% and 7%, respectively. Among the 52 patients having developed a new AD after CBT, none had autologous recovery of the hematopoiesis, 92% had full donor chimerism, and 8% had mixed chimerism compared with 9% of patients with autologous recovery, 80% with full donor chimerism, and 11% with mixed chimerism in the control group.
Platelet recovery for patients with and without AD was low (59% ± 2% at 180 days). At the time of onset of a new AD, acute GVHD was present in 8 patients. Of the 19 patients who developed chronic GVHD, 5 were transplanted for a nonhematologic malignancy and 14 for a hematologic malignancy, 14 had chronic GVHD before the diagnosis of AD, and 5 developed chronic GVHD after the diagnosis of AD was made.
There was a statistical trend for the association of chronic GVHD (analyzed as a time-dependent variable) with an increased risk of developing AD (HR = 1.83; 95% CI, 0.85-3.5; P = .07).
In 15 patients, an infection or a reactivation of a chronic virus infection was diagnosed in the 3 months preceding the diagnosis of AD: 2 EBV (1 EBV reactivation and 1 posttransplant lymphoproliferative disorder), 6 CMV (1 reactivation, 4 pneumonia, and 1 encephalitis), 3 HHV6 reactivation, 1 adenovirus infection, 1 hepatitis C infection, 2 infectious diarrhea, and 2 sepsis. One patient had 4 infections (CMV, hepatitis C, pneumonia, and diarrhea), during the 3 months preceding AD; 1 patient had 3 infections (EBV, adenovirus, and HHV6), and 2 patients had 2 infections (CMV and sepsis and CMV and HHV6).
Outcome of patients having developed an AD
The estimated OS for all patients (N = 778) was 48% ± 2% at 5 years with a median follow-up of 46 months (range, 3-216). Forty of 52 patients who developed an AD were alive with a median follow-up of 26 months (range, 2-91) after the diagnosis of AD.
When selecting only patients transplanted for nonmalignant diseases, the occurrence of ADs was not associated with a lower survival (HR = 1.09; 95% CI, 0.33-3.6; P = .89). The causes of death in the 12 patients who died after diagnosis of an AD were as follows: 6 patients died from AD (3 AIHA, 2 Evans syndrome, and 1 ITP); death in 4 patients was attributed to complications related to CBT and in 2 to relapses of their original disease. None of the 6 patients who died of AD were in remission of their AD at the time of death (4 in partial remission and 2 with uncontrolled AD). However, the occurrence of AD (as a time-dependent variable) was not associated with a higher overall mortality (HR = 1.05; 95% CI, 0.58-1.88; P = .86).
Treatment of patients having developed hematologic AD
From the 40 patients with AIHA, ITP, or Evans syndrome, 7 received steroids only and 33 additional immunosuppressive treatment, mainly rituximab (RTX) and cyclosporine A (CSA). Response to treatment was as follows: ITP: n = 4 complete responses (CRs), n = 3 partial responses (PRs), and n = 3, no responses (NRs); AIHA: n = 9 CRs, n = 8 PRs, and n = 3 NRs; and Evans syndrome: n = 3 CRs, n = 3 PRs, and n =3 NRs (Table 2). Nineteen of these patients needed a second line treatment and 13 of 19 patients responded (PR and CR). RTX was used most often (Table 3). The patient who developed autoimmune neutropenia did not receive specific treatment. The second hematologic AD in patients having developed 2 ADs responded completely (2 to steroids and 1 to steroids + RTX). The estimated 5-year OS was 91% ± 9% for patients who developed ITP, 59% ± 11% for those with AIHA, and 67% ± 16% for those with Evans syndrome.
Response of patients with hematological AD to first-line treatment
| . | Steroids only . | RTX . | CSA . | RTX + CSA . | IVIg . | Others . |
|---|---|---|---|---|---|---|
| ITP | NR (n = 1) CR (n = 2) | NR (n = 1) CR (n = 2) | NR (n = 1) | NR (n = 1) PR (n = 3) | ||
| AIHA | NR (n = 1) PR (n = 2) CR (n = 1) | NR (n = 1) PR (n = 1) PR (n = 2)* CR (n = 5) | CR (n = 2) | PR (n = 1) | NR (n = 1)* PR (n = 1) PR (n = 1)† | CSA + tacrolimus CR (n = 1) |
| Evans | NR‡ (n = 1) CR (n = 1) | NR (n = 2) PR (n = 1) CR (n = 1) | PR (n = 1) CR (n = 1) | Tacrolimus PR (n = 1) |
| . | Steroids only . | RTX . | CSA . | RTX + CSA . | IVIg . | Others . |
|---|---|---|---|---|---|---|
| ITP | NR (n = 1) CR (n = 2) | NR (n = 1) CR (n = 2) | NR (n = 1) | NR (n = 1) PR (n = 3) | ||
| AIHA | NR (n = 1) PR (n = 2) CR (n = 1) | NR (n = 1) PR (n = 1) PR (n = 2)* CR (n = 5) | CR (n = 2) | PR (n = 1) | NR (n = 1)* PR (n = 1) PR (n = 1)† | CSA + tacrolimus CR (n = 1) |
| Evans | NR‡ (n = 1) CR (n = 1) | NR (n = 2) PR (n = 1) CR (n = 1) | PR (n = 1) CR (n = 1) | Tacrolimus PR (n = 1) |
ITP indicates immune thrombocytopenia; AIHA, autoimmune hemolytic anemia; RTX, rituximab; CSA, cyclosporine A; NR, no response; PR, partial response; and CR, complete response.
Received additional plasmapheresis.
Received additional CSA.
Received additional cyclophosphamide.
Response of patients with hematological AD to second-line therapy
| . | Steroids only . | RTX . | Other . |
|---|---|---|---|
| ITP | CR (n = 1) | PR (n = 1) NN (n = 1) NN (n = 1) + azathioprine CR (n = 1) | PR (n = 1) plasma exchange |
| AIHA | NR (n = 1) PR (n = 2) CR (n = 2) | Azathioprine (n = 1) NN CSA (n = 1) CR | |
| Evans | NR (n = 1) + cyclophosphamide PR (n = 1) + CSA + tacrolimus CR (n = 1) + CSA + MMF PR (n = 1) CR (n = 2) + tacrolimus |
| . | Steroids only . | RTX . | Other . |
|---|---|---|---|
| ITP | CR (n = 1) | PR (n = 1) NN (n = 1) NN (n = 1) + azathioprine CR (n = 1) | PR (n = 1) plasma exchange |
| AIHA | NR (n = 1) PR (n = 2) CR (n = 2) | Azathioprine (n = 1) NN CSA (n = 1) CR | |
| Evans | NR (n = 1) + cyclophosphamide PR (n = 1) + CSA + tacrolimus CR (n = 1) + CSA + MMF PR (n = 1) CR (n = 2) + tacrolimus |
MMF indicates mycophenolate mofetil; and NN, response not known.
Treatment of patients having developed a nonhematologic AD
Two of the 3 patients with autoimmune thyroiditis (hypothyroidism) received replacement therapy with thyroxin. One patient who developed psoriasis received CSA and prednisone and the other was treated with topical tacrolimus. One patient with membranous glomerulonephritis received prednisone and the other received CSA with CR. The patient with SLE responded to steroid and CSA treatment (CR); ulcerative colitis was successfully treated with steroids and tacrolimus and RA with prednisone and methotrexate.
Discussion
AD occurred in more than 6% of patients from 5 weeks up to more than 10 years after CBT. Most were organ-specific ADs such as cytopenias followed by ADs of the thyroid and a few cases of miscellaneous multisystemic AD. The diagnoses of ADs in our cohort are comparable to those after CBT reported in the literature6-12 and to ADs after allogeneic HSCT.1,14 AIHA, ITP, and, more rarely, autoimmune neutropenia have been reported after allogeneic, noncord-blood–derived SCT with an incidence of 3%-4.5% in 3 single-center studies14-16 The published cases have been reviewed recently.2 Unrelated donor status, chronic GVHD, and indication of HSCT for nonmalignant disorders have been proposed in small single-center studies to be positively associated with the development of AIHA after allogeneic HSCT.14,17
A large cohort of patients having received autologous HSCT for an AD is so far the only other multicenter cohort in which incidence, risk factors, and outcome of new ADs after HSCT has been retrospectively investigated. Within this cohort, the cumulative incidence of new ADs was > 7% after 3 years.4 All ADs within our study occurred in cord blood recipients having achieved at least mixed chimerism. However, complete or mixed chimerism after SCT does not seem be essential for the development of ADs, because ADs have been reported to occur after autologous HSCT.4 It might be that the incidence of ITP was underestimated because of the low incidence of platelet recovery in our population.
The diagnosis of a nonmalignant disease as an indication for CBT emerged as the main risk factor for developing a new AD in multivariate analysis. Because these often hereditary diseases manifest early in life, patients are young at diagnosis. This is reflected by the fact that the median age of the patients at CBT was younger in the AD than in the control group.
A previous single-center study reported a very high incidence of ADs in newborns after CBT for metabolic diseases.6 We previously identified younger age at transplantation as a risk factor for future development of an AD in patients having received autologous HSCT for an AD.4 Therefore, younger age and diagnosis of a nonmalignant disease might both contribute to the development of ADs after HSCT. This is contradictory to the fact that thymic function is better in younger patients and declines with age and after thymic damage, for example, by radiation or chemotherapy in malignant diseases.
A shorter interval from diagnosis of the initial disease to CBT appeared as the second risk factor for developing an AD after CBT. How this is related to the diagnosis remains unclear. However, young patients with metabolic diseases are generally transplanted early after diagnosis because a therapeutic alternative is missing. This might relate diagnosis of a nonmalignant disease and time to CBT as risk factors, although this could not be proven in multivariate analysis.
A single-center study suggested that GVHD is a risk factor for the development of AIHA after allogeneic HSCT.14 Although acute or chronic GVHD was absent in 34 of 52 patients at diagnosis of AD in our study, there was a trend toward an association between chronic GVHD and the development of new ADs (P = .07). GVHD is thought to be the consequence of donor T cells reacting with host-specific tissue markers (eg, HLA).18 In contrast, AIHA and ITP, the most prevalent ADs occurring after CBT, are caused by autoantibodies reacting with specific tissue antigens on platelets and RBCs that are not necessarily host specific. Moreover, our patients (with or without ADs) did not differ with respect to ABO compatibility to their respective donors. In 15 patients, one or more infections preceded the diagnosis of post-CBT AD and viruses, especially replication of endogenous herpes viruses, were most prevalent. These “danger signals” may shift the balance between autoreactive and regulatory elements toward autoimmunity.19 Therefore, we could speculate that viral infections may be involved in the development of ADs; however, we were not able to study infectious disease in the control group because of the retrospective design of the study.
Most ADs within our cohort were organ specific (eg, AIHA or ITP) and known to be associated with the presence of autoantibodies. An imbalance between the development of autoreactive cells (eg, autoantibody-producing plasma cell precursors) and that of regulatory active cells (eg, Tregs) after CBT might favor the development of autoantibody-producing plasma cells.2
At least 6 of 41 patients who developed a new hematologic AD after CBT died as a consequence of the AD. It is not unlikely that ADs contributed to death in the remaining 6 patients, because no CR of the new AD was achieved by the time of death in any of these patients. The 5-year OS (59%) after development of AIHA in our cohort was a major concern and the outcome of AIHA after allogeneic HSCT according to existing data are poor, with a mortality rate of up to 50%.2
Treatment of AIHA, ITP, and Evans syndrome was based on prednisone and RTX and CSA were most often used as immunosuppressive agents. Half of these patients needed second-line treatment and most responded to RTX, which was used in all but one patient as it has been shown to induce remissions of ITP20 and AIHA21 outside of the setting of HSCT. However, controlled data on the treatment for ADs after CBT are lacking, so more specific recommendations cannot be given at this stage.
Conclusion
In this large cohort of more than 700 patients having received CBT, new, posttransplantation ADs occurred in more than 6%. Patients transplanted for nonmalignant diseases are at an increased risk for developing posttransplantation ADs. AIHA and ITP were observed most commonly and therefore have to be considered in any patient at any time after CBT as a possible reason for an unexplained cytopenia. In patients not responding to initial treatment with steroids, RTX may be an effective second-line treatment option. Both the clinical and the mechanistic aspects of new ADs occurring after HSCT merit further attention and research.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following institutions for providing follow-up data on their patients: Department of Hematology, University Hospital Basel, Basel, Switzerland (CIC 202); Paediatric Clinic II, Rigshospitalet Copenhagen, Copenhagen, Denmark (CIC 206); Children‘s Center for Allogeneic Stem Cell Transplantation, Hospital Karolinska University Hospital Huddinge Stockholm, Stockholm, Sweden (CIC 212); Institut d'Hématologie et d'Oncologie Pédiatrique Unité Lyon, Lyon, France (CIC 241); Department Hématologie, Hopital A. Michallon, Grenoble, France (CIC 270); Ospedale Infantile Regina Margherita Onco-Ematologia Pediatrica, Torino, Italy (CIC 305); St László Hospital, Budapest, Hungary (CIC 556); Department of Hematology, George Papanicolaou General Hospital, Thessaloniki, Greece (CIC561); Department of Hematology, Hospital Vall d'Hebron, Barcelona, Spain (CIC 584); Department of Hematology, Evangelismos Hospital, Athens, Greece (CIC 622); Department of Hematology, Universitair Ziekenhuis Brussel, Brussels, Belgium (CIC 630); Pediatric Hematopoietic Cell Transplantation Unit, Valencia Hospital Infantil, La Fe, Spain (CIC 663); Department of Hematology, University of Liege, Liege, Belgium (CIC 726); Chaim Sheba Medical Center, Tel-Hashomer, Israel (CIC 754); and Canterbury Health Laboratories, Christchurch, New Zealand (CIC 798).
T.D. was supported by a European League Against Rheumatism grant and by the Freiwillige Akademische Gesellschaft, Basel, Switzerland.
Authorship
Contribution: T.D., A.-L.H., E.G., D.G., and V.R. designed the study; T.D., M.L., A.R., and V.R. analyzed the data and performed the statistical analysis; and A.C., M.A., A.A.H., K.C., J.C., J.L.D.-M., V.G., M.F., C.L., A.O., V.M., M.R., P.S., A.S., G.S., F.S., S.V., J.S., J.V., A.V., M.A.Y., A.-L.H., E.G., and D.F. contributed data and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas Daikeler, Department of Rheumatology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; e-mail: tdaikeler@uhbs.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal