Key Points
Targeted deletion of the gene for macrophage migration inhibitory factor (MIF) delays development of chronic lymphocytic leukemia and prolongs survival in mice.
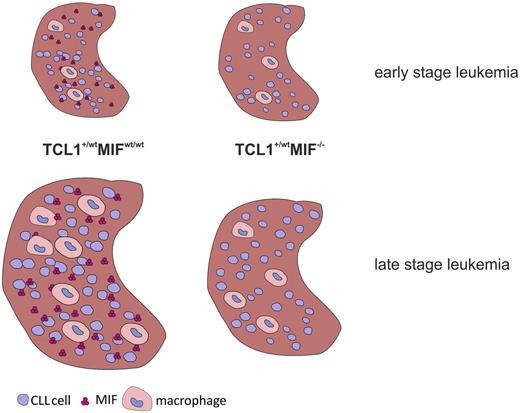
MIF recruits leukemia-associated macrophages to spleen or liver.
Abstract
Survival of chronic lymphocytic leukemia (CLL) cells depends on stimuli provided by a suitable microenvironment. The factors and mechanisms providing this growth support for CLL cells are not fully understood. We found that plasma levels of macrophage migration inhibitory factor (MIF), a proinflammatory and immunoregulatory chemokine, were elevated in CLL patients. Therefore, we characterized the functional role of MIF in a CLL mouse model. For this purpose, we crossed Eμ-TCL1 mice with MIF knockout (MIF−/−) mice. The resulting TCL1+/wtMIF−/− mice showed a delayed onset of leukemia, reduced splenomegaly and hepatomegaly, and a longer survival than TCL1+/wtMIFwt/wt controls. Immunohistochemical examination of the lymphoid organs showed that the numbers of macrophages were significantly reduced in the spleen and bone marrow of TCL1+/wtMIF−/− mice compared with TCL1+/wtMIFwt/wt controls. Mechanistic studies in vitro revealed that the absence of MIF rendered CLL cells more susceptible to apoptosis. Accordingly, incubation with an anti-MIF antibody reduced the survival of CLL cells on a macrophage feeder layer. In addition, the migratory activity of TCL1+/wtMIF−/− macrophages was decreased compared with TCL1+/wtMIFwt/wt macrophages. Taken together, our results provide evidence that MIF supports the development of CLL by enhancing the interaction of CLL cells with macrophages.
Introduction
Chronic lymphocytic leukemia (CLL) is a clonal B-cell disorder that is not curable by conventional chemoimmunotherapies. The leukemic transformation may be initiated by specific genomic alterations (eg, del13q) that may cause the deletion of specific micro-RNA genes (eg, miR15 and miR16) and increase the resistance of B cells toward apoptosis.1,2 Survival of CLL cells depends on a permissive microenvironment composed of cellular components, such as macrophages, T cells, or stromal follicular dendritic cells.3-5 This microenvironment provides various chemokines and angiogenic factors, which interact with leukemic cells via appropriate surface receptors and adhesion molecules.2,5
Macrophage migration inhibitory factor (MIF) is a proinflammatory and immunoregulatory cytokine that seems to be involved in the pathogenesis of various malignant diseases.6-9 MIF was identified as a product of T cells10 but also other cells of the immune system (B cells, monocytes/macrophages).11 Later, MIF was found to be an almost ubiquitous mediator secreted by a wide variety of cells in the mammalian organism, such as endothelial cells, epithelial cells, or fibroblasts.12 Macrophages are considered to be a prime source for MIF, as they are able to secrete large amounts of MIF in response to various stimuli.13 MIF binds to the surface receptors CD74 and CXCR2/CXCR4, thereby stimulating signaling pathways, such as MAPK, NF-κB, and AKT.14-16 In B cells, activation of the surface receptor complex CD74/CD44 by MIF induces the proteolytic release of the intracellular domain of CD74, which in turn initiates a signaling cascade composing Syk, AKT, and NF-κB; this leads to the production of IL-8 and to an increased resistance to apoptosis via the up-regulation of BCL-2.17,18 Thus, the MIF-MIF receptor system may be seen as a part of the B-cell costimulatory signals that are required for full B-cell activation and maturation. MIF-deficient mice do not show developmental abnormalities and appear to have normal numbers of B cells.6 However, they exhibit a number of immune dysfunctions when challenged by antigens or infectious agents.19-22 Even more importantly, MIF seems to be required for bone marrow-derived dendritic cells to maintain mature B cells in the bone marrow compartment.23
MIF is overexpressed in a variety of malignancies compared with the respective primary tissues (eg, prostate,24 colon,25 melanoma,26 glioblastoma,27 breast cancer28,29 ). This overexpression might be caused by the tumor-activated HSP90 chaperone complex that protects MIF from degradation, as shown in an ErbB2 transgenic mouse model of human HER2-positive breast cancer.28 In a mouse model of myc-driven B-cell lymphoma, genetic ablation of MIF was shown to delay the onset of B-cell lymphoma development.6 MIF-deficient premalignant Eμ-myc B cells showed a delayed lymphoma progression and increased apoptosis, which seemed to be explained, at least in part, by a dysregulation of E2F factors and an enhanced activity of the p53 tumor suppressor pathway.6 A number of other mechanisms of how MIF may influence tumor growth have been proposed, including altered signal transduction through ERK or AKT, neoangiogenesis and altered response to hypoxia, or different immune effector functions.26,30
MIF has been shown to promote the survival of human CLL cells in vitro via CD74.18 Given the complexity of MIF biology, we made use of a CLL animal model, the Eμ-TCL1 mouse,31 to examine the functional relevance of MIF in CLL. Eμ-TCL1 mice were crossed with MIF-deficient mice (MIF−/−),32 allowing us to study the role of MIF for CLL development over the entire life span of a mammalian organism.
Methods
Human malignant and healthy B cells for RNA/protein expression studies
Malignant B cells were obtained from 30 patients treated at the Department of Internal Medicine I of Cologne University Hospital with a diagnosis of CLL and a peripheral blood lymphocyte count of ≥ 25 × 109/L or from healthy donors. B cells were purified (> 95%) using Ficoll sucrose gradient and depletion of non-B cells by the RosetteSep Human B Cell enrichment cocktail (StemCell Technologies) for the isolation of CLL B cells and the Miltenyi Human B Cell Isolation Kit II (Miltenyi Biotec) for the healthy B cells.
MIF plasma levels in healthy controls and CLL
MIF plasma levels from patients with CLL (n = 49) treated at the Department of Internal Medicine I of Cologne University Hospital (all with informed consent per the Declaration of Helsinki as approved by the Institutional Ethics Committee no. 08-275) or age-matched healthy donors (n = 50) were analyzed by R&D MIF ELISA according to the manufacturer's instructions.
MIF levels in supernatants of cultured B cells
Malignant B cells were obtained from 5 CLL patients treated at the Department of Internal Medicine I of Cologne University Hospital with a peripheral blood lymphocyte count > 30 × 109/L. Healthy B cells were obtained from 5 buffy coats obtained from blood donors at the Department of Transfusion Medicine of Cologne University Hospital (Institutional Ethics Committee approval no 03-143). B cells were purified as described in “Human malignant and healthy B cells for RNA/protein expression studies.” Isolated B cells were cultured in RPMI media without FCS at a density of 2.5 × 106 cells/500 μL per well in 48-well plates. Cell culture supernatants were gently aspirated at the indicated times at 0, 12, 24, and 48 hours of incubation time. Clear supernatants after centrifugation were frozen at −80°C. The levels of MIF expression in supernatants were detected by R&D human MIF ELISA according to the manufacturer. Each patient sample was analyzed in triplicates.
Mice
Eμ-TCL1 transgenic mice31 were crossed with C57Bl/6 MIF knockout mice32 for 2 generations. Mice were kept in a conventional animal facility under virus-free conditions. For determination of overall survival, TCL1+/wtMIFwt/wt and their TCL1+/wtMIF−/− littermates (n = 60 per group) were bred and observed from birth until death or euthanization (when required by internationally endorsed principles of experimental procedures for laboratory animals). Only mice dying of leukemia or lymphoma were included into the analysis of overall survival. Hematologic screening was performed at months 3, 6, 9, and 12. All animal experiments were approved by the state authorities of North Rhine–Westphalia (no. K17, 12/04).
RNA-isolation and quantitative RT-PCR
RNA was isolated using Trizol reagent (Invitrogen). Reverse transcription and whole transcriptome amplification were done using Quantitect Whole Transcriptome Kit (QIAGEN). Quantitative real-time PCR for MIF was run on a Light Cycler 480 system (Roche Diagnostics) using Express SYBR GreenERqPCRSupermix (Invitrogen). mRNA levels were normalized to peptidylprolylisomerase A (cyclophilin A = peptidylprolylisomerase A).
Western blotting
Purified CD19+ B cells, CD11b+ macrophages, or total splenocytes (crude spleen suspension after erythrocyte lysis with ACK buffer) were lysed and cytoplasmic and nuclear fractions separated using the NE-PER kit (Thermo Fisher Scientific). A total of 30 μg of protein was separated by SDS-PAGE and probed with a polyclonal rabbit–anti-MIF antibody (Ka565) and goat–anti-β-actin (Santa Cruz Biotechnology). Secondary antibodies used for detection were donkey–anti-rabbit HRP and donkey–anti-goat HRP (Santa Cruz Biotechnology). Splenic cells were purified using Miltenyi Biotec CD19 microbeads or CD11b microbeads according to the manufacturer's instructions. Quantification of the MIF protein was done by densitometric comparison of MIF protein with β-actin protein levels using the program Image J.
Flow cytometric analysis of MIF receptor expression
Tail vein blood was collected from 3- and 12-month-old TCL1+/wt mice. The surface expression of CD74 (clone In-1), CD44 (clone IM7), and CXCR2 (clone 242216) on IgM+CD5− nonleukemic or IgM+CD5+ (IgM clone II/41; CD5 clone 53-7.3) leukemic B cells was measured using fluorescently labeled monoclonal antibodies compared with the respective isotype controls (BD Biosciences PharMingen).
Analysis of leukocyte and IgM+CD5+ counts
Tail vein blood was collected from 3-, 6-, 9-, and 12-month-old TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− mice. White blood cell count (WBC) was measured with a SYSMEX XE-5000 hemacytometer. The numbers of normal (IgM+CD5−) and malignant B cells (IgM+CD5+) were analyzed by flow cytometry using anti-IgM–FITC and anti-CD5–peridinin chlorophyll protein (BD Biosciences PharMingen) on a BD Canto flow cytometer.
Immunohistochemistry
Organs were fixed in 4% buffered paraformaldehyde and paraffin-embedded. Sections were stained using primary antibodies against CD3, CD5, CD68, CD79a, CD163, and Ki67 (all Thermo Fisher Scientific), a universal secondary antibody conjugated to a polymer recognizing rabbit and mouse immunoglobulins with detection by FastRed (UltraVision LP Detection Kit, Thermo Fisher Scientific). Numbers of CD68+ macrophages were counted at 40× magnification by a blinded pathologist under a Leica microscope.
Detection of apoptotic cells in vivo by TUNEL
Apoptotic cells in paraffin-embedded spleen sections were detected by dT-mediated dUTP-biotin nick end labeling (TUNEL) according to the manufacturer's instructions (Promega).
Spontaneous and drug-induced splenocyte apoptosis ex vivo
Splenocytes from leukemic and nonleukemic animals were treated with the indicated drug concentrations (50μM fludarabine, 61nM vincristine, 1μM prednisolone). The rate of apoptosis was determined using flow cytometry with annexin-V/7-amino-actinomycin D (BD Biosciences PharMingen) after 24 hours.
Ex vivo cocultures
Thioglycollate-elicited peritoneal macrophages from MIF+/+ and MIF−/− mice were cocultured with malignant B cells from TCL1+/wtMIFwt/wt or TCL1+/wt MIF−/− mice. Viability was measured by flow cytometric analysis of annexin-V/propidium iodide (BD Biosciences PharMingen). Cells were treated with mouse antimurine MIF monoclonal antibody (IgG1) IIID9 (100 μg/mL) and isotype control IgG1 (100 μg/mL).
Macrophage migration assay
Thioglycollate-elicited peritoneal macrophages were plated into transwell chambers (BioCat) and migration toward various chemokines [MIF (100 ng/mL], CCL2 (100 ng/mL), CCL3 (10 ng/mL), CCL4 (100 ng/mL), and SDF1 (100 ng/mL)] was measured after 15 hours according to the manufacturer in a fluorescence plate reader (PerkinElmer Life and Analytical Sciences). Cells were treated with mouse anti–murine MIF monoclonal antibody (IgG1) IIID9 (100 μg/mL) and isotype control IgG1 (100 μg/mL).
Determination of IGHV mutational status
RNA was extracted from peripheral blood cells with Trizol (Invitrogen). Mouse IgH rearrangements were analyzed on an ABI 3130 sequencer (Applied Biosystems). Sequences were analyzed using Lasergene Version 9 software (DNAStar) and manual review. Sequences were compared with mouse germline Ig gene sequences with the IMGT database.33
SKY
For spectral karyotyping (SKY) analysis (Table 1), metaphase chromosomes were prepared from splenocytes by treatment with colcemid (0.035 μg/mL overnight), incubation in KCl (0.075M, 20 minutes, 37°C) and fixation in a freshly prepared mixture of methanol/acetic acid (3:1; room temperature). Cell suspension was dropped onto glass slides in a climate chamber (Polymer, Kassel, Germany; 22°C, 48% humidity). SKY analysis was performed as described previously,34 followed by hybridization with the SKY probe mixture for mouse chromosomes (Applied Spectral Imaging). Spectral images were acquired using an epifluorescence microscope equipped with an interferometer (SpectraCube Applied Spectral Imaging), a custom-designed optical filter, and SkyView Version 1.6 software (Applied Spectral Imaging).
Chromosomal abnormalities revealed by SKY in TCL1+/wtMIFwt/wt (n = 3) and TCL1+/wtMIF−/− (n = 4) mice
| Genotype . | Assessment by SKY . |
|---|---|
| TCL1+/wtMIFwt/wt | |
| 1 | 41,XY,+15 [6]/ 42–43,XY,+10,+Del(15) [cp5]/ 40,XY [4] |
| 2 | 80 < 4n > ,XXYY [8]/ 40,XY [7] |
| 3 | 41,XY,+15 [14] |
| TCL1+/wtMIF−/− | |
| 1 | 40,X,-X,+15 [2]/ 41,XX,+15 [1]/ 40,XX [8] |
| 2 | 71∼75,XXY,-Y,Der(1)T(1;?10),Der(4)T(4;?),Der(15)T(7;15),mar [cp9] /40,XY [4]/ 80 < 4n > ,XXYY [2] |
| 3 | 40,XY [10] |
| 4 | 40,X,-Y,+15 [9]/ 41,XY,+15 [5] |
| Genotype . | Assessment by SKY . |
|---|---|
| TCL1+/wtMIFwt/wt | |
| 1 | 41,XY,+15 [6]/ 42–43,XY,+10,+Del(15) [cp5]/ 40,XY [4] |
| 2 | 80 < 4n > ,XXYY [8]/ 40,XY [7] |
| 3 | 41,XY,+15 [14] |
| TCL1+/wtMIF−/− | |
| 1 | 40,X,-X,+15 [2]/ 41,XX,+15 [1]/ 40,XX [8] |
| 2 | 71∼75,XXY,-Y,Der(1)T(1;?10),Der(4)T(4;?),Der(15)T(7;15),mar [cp9] /40,XY [4]/ 80 < 4n > ,XXYY [2] |
| 3 | 40,XY [10] |
| 4 | 40,X,-Y,+15 [9]/ 41,XY,+15 [5] |
Statistics
Data are expressed as mean ± SEM. Unless indicated, statistical analysis was performed using a 2-tailed unpaired Student t test. Overall survival and tumor incidence were tested using Kaplan-Meier analysis with a log-rank test. P values < .05 were considered significant.
Results
CLL patients show increased cellular and plasma levels of MIF
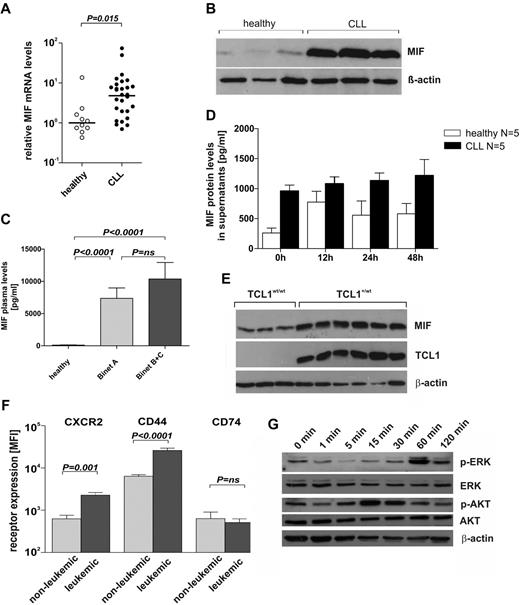
To investigate MIF mRNA and protein expression levels in CLL patients, we performed quantitative RT-PCR analyses and immunoblots using RNA preparations and cell lysates obtained from purified B cells from CLL patients and healthy donors. Results were compared with both CD19+CD5− and CD19+CD5+ B cells from healthy donors. The levels of MIF mRNA and MIF protein were 4 times (P = .015) and 1.4 times (P = .0007) higher in CLL cells than in B cells of healthy donors (Figure 1A-B), respectively. The analysis of MIF mRNA levels in CD5+ B cells of 5 healthy donors showed slightly increased values (mean ± SEM 4.8 ± 2.4) compared with CD5− B cells (2.3 ± 1.3, n = 10) but lower levels than leukemic CLL cells (8.9 ± 2.8, n = 30). Moreover, MIF plasma levels were strongly increased in 49 CLL patients (8763 ± 1457 pg/mL) compared with 50 age-matched healthy controls (105 ± 17 pg/mL; Figure 1C). MIF levels also showed some trend to higher levels in patients with advanced stage CLL (Binet B + C) compared with patients with early-stage CLL (Binet A: n = 26; 7353 ± 1618 pg/mL and Binet B + C: n = 23; 10 360 ± 2508 pg/mL (Figure 1C). Purified CD19+ B cells obtained from CLL patients (n = 5) released significantly more MIF into the supernatant than CD19+ B cells from healthy donors (n = 5; Figure 1D), suggesting that CLL cells contributed to the increased MIF levels found in CLL patients.
MIF and MIF receptor expression in human and murine CLL. (A) Quantitative RT-PCR from purified B cells of healthy donors (n = 10) and of CLL patients (n = 30). Mean ± SEM of MIF mRNA expression in CLL patients was 8.9 ± 2.8 compared with 2.3 ± 1.3 in healthy donors relative to peptidylprolylisomerase A mRNA levels (P = .015, Mann-Whitney U test). (B) Representative immunoblot for MIF and β-actin protein in lysates of B cells from healthy donors (n = 5; > 95% CD19+ cells) and leukemic B cells from CLL patients (n = 10; > 95% CD19+ cells). Overexpression of MIF protein in human CLL cells (P = .0007). (C) MIF plasma levels in 49 patients with CLL and 50 age-matched healthy donors. Significantly elevated MIF plasma levels were found in CLL patients (7353 ± 1618 pg/mL for Binet A, n = 26; and 10 360 ± 2508 pg/mL for Binet B + C, n = 23) compared with healthy controls (105 ± 17 pg/mL; P < .0001). The difference of MIF levels in early stage (Binet A) versus advanced stage (Binet B + C) CLL patients was not significant. (D) MIF protein levels by ELISA in cell culture supernatants of purified CLL cells and CD19+ peripheral blood B cells (n = 5 each) cultured for 0-48 hours. CLL cells release more MIF than healthy B cells (P < .01 by a 2-way ANOVA). (E) Representative immunoblot for MIF, TCL1, and β-actin in lysates of splenocytes from leukemic Eμ-TCL1+ mice (n = 6) and nonleukemic wild-type control animals (n = 3). Overexpression of MIF protein in murine CLL cells (P = .0017). (F) Surface expression of MIF receptors CXCR2, CD44, and CD74 on TCL1+/wt nonleukemic (IgM+CD5−; n = 5) and leukemic (IgM+CD5+; n = 5) murine B cells. Increased receptor expression on leukemic versus nonleukemic B cells seen for CXCR2 and CD44. No difference in CD74 receptor expression in leukemic and nonleukemic B cells. MFI indicates mean fluorescence intensity. (G) Representative immunoblot for p-ERK, t-ERK, p-AKT, t-AKT, and β-actin in lysates of isolated splenic B cells from Eμ-TCL1 mice, stimulated with 100 ng/mL recombinant MIF for 0, 1, 5, 15, 30, 60, and 120 minutes.
MIF and MIF receptor expression in human and murine CLL. (A) Quantitative RT-PCR from purified B cells of healthy donors (n = 10) and of CLL patients (n = 30). Mean ± SEM of MIF mRNA expression in CLL patients was 8.9 ± 2.8 compared with 2.3 ± 1.3 in healthy donors relative to peptidylprolylisomerase A mRNA levels (P = .015, Mann-Whitney U test). (B) Representative immunoblot for MIF and β-actin protein in lysates of B cells from healthy donors (n = 5; > 95% CD19+ cells) and leukemic B cells from CLL patients (n = 10; > 95% CD19+ cells). Overexpression of MIF protein in human CLL cells (P = .0007). (C) MIF plasma levels in 49 patients with CLL and 50 age-matched healthy donors. Significantly elevated MIF plasma levels were found in CLL patients (7353 ± 1618 pg/mL for Binet A, n = 26; and 10 360 ± 2508 pg/mL for Binet B + C, n = 23) compared with healthy controls (105 ± 17 pg/mL; P < .0001). The difference of MIF levels in early stage (Binet A) versus advanced stage (Binet B + C) CLL patients was not significant. (D) MIF protein levels by ELISA in cell culture supernatants of purified CLL cells and CD19+ peripheral blood B cells (n = 5 each) cultured for 0-48 hours. CLL cells release more MIF than healthy B cells (P < .01 by a 2-way ANOVA). (E) Representative immunoblot for MIF, TCL1, and β-actin in lysates of splenocytes from leukemic Eμ-TCL1+ mice (n = 6) and nonleukemic wild-type control animals (n = 3). Overexpression of MIF protein in murine CLL cells (P = .0017). (F) Surface expression of MIF receptors CXCR2, CD44, and CD74 on TCL1+/wt nonleukemic (IgM+CD5−; n = 5) and leukemic (IgM+CD5+; n = 5) murine B cells. Increased receptor expression on leukemic versus nonleukemic B cells seen for CXCR2 and CD44. No difference in CD74 receptor expression in leukemic and nonleukemic B cells. MFI indicates mean fluorescence intensity. (G) Representative immunoblot for p-ERK, t-ERK, p-AKT, t-AKT, and β-actin in lysates of isolated splenic B cells from Eμ-TCL1 mice, stimulated with 100 ng/mL recombinant MIF for 0, 1, 5, 15, 30, 60, and 120 minutes.
MIF and MIF receptors are expressed in murine CLL
The results showing increased MIF levels in CLL patients led us to investigate the role of MIF in an established animal model for CLL, the Eμ-TCL1 mouse, where the T-cell leukemia-1 (TCL1) oncogene is overexpressed in a B cell–restricted manner.31 As in human CLL, B cells obtained from spleens of leukemic Eμ-TCL1 mice showed a significantly higher expression of MIF protein compared with B cells from TCL1 wild-type mice (P = .0017, Figure 1E). The known receptors of MIF, CXCR2,14 and CD7417 as well as the coreceptor CD4415 were expressed on murine CLL cells. Moreover, the expression of CXCR2 was increased on leukemic B cells (MFI CLL cells: 2276 ± 345; B cells: 624 ± 137; P = .001; Figure 1F). Similarly, the CD74 coreceptor CD44 was expressed at higher levels on CLL cells (MFI 25 980 ± 3501 versus 6379 ± 568; P < .0001; Figure 1F). In some contrast, CD74, the surface form of MHC-associated invariant chain, was expressed at relatively low levels both on leukemic and nonleukemic B cells (Figure 1F). Taken together, murine CLL cells expressed essential receptors of MIF (CXCR2, CD74/CD44). We also analyzed selected signaling pathways in leukemic splenocytes from Eμ-TCL1 mice by immunoblotting with phosphospecific antibodies. On stimulation of cells with 100 ng/mL MIF for 0-120 minutes, cytosolic Akt and MAPK were phosphorylated, peaking at 15 minutes after stimulation for Akt and at 60 minutes after stimulation for ERK (Figure 1G), in agreement with previously published results in various cells in vitro.35
CLL development in MIF-deficient TCL1+/wt mice
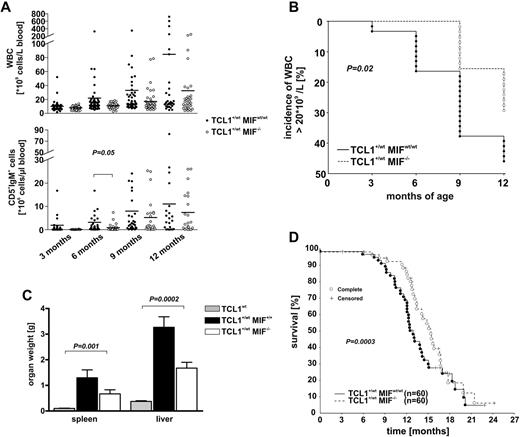
To investigate a functional role of MIF for CLL development, we crossed MIF−/− mice32 with Eμ-TCL1 mice.31 In the F2 generation, TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− mice were followed for clinical signs of leukemia (leukocytosis, CD5+ lymphocytosis, hepatomegaly, or splenomegaly) and overall survival. Overt leukemia in Eμ-TCL1 mice was defined as > 20 × 109 WBC/L (2 times upper limit of normal). Sequential analyses of WBC and IgM+CD5+ lymphocyte counts in peripheral blood of both genotypes demonstrated that the absence of MIF led to a delayed development of overt leukemia (Figure 2A-B). TCL1wt littermates did not show an elevated WBC or an increase of the IgM+CD5+ clone. At 6 months after birth, TCL1+/wtMIFwt/wt animals showed higher IgM+CD5+ cell counts (3148 ± 1084 cells/μL) than TCL1+/wtMIF−/− mice (891 ± 271 cells/μL; P = .05). Similarly, WBC was higher in TCL1+/wtMIFwt/wt mice at 6, 9, and 12 months after birth (Figure 2A). Finally, CLL development was enhanced in TCL1+/wtMIFwt/wt animals, with 2 and 8 animals showing overt leukemia at 3 and 6 months of age, compared with none in TCL1+/wtMIF−/− mice (Figure 2B; P = .02).
Absence of MIF delays CLL development and prolongs overall survival. (A) WBC (top panel) and malignant (IgM+CD5+) B cells (bottom panel) over time in TCL1+/wtMIFwt/wt (●) and TCL1+/wtMIF−/− (○) mice. Y-axis modified to accommodate high cell counts in leukemic mice. TCL1+/wtMIFwt/wt mice have higher WBC and malignant B cells than TCL1+/wtMIF−/− mice. (B) Incidence of CLL over time. TCL1+/wtMIFwt/wt mice develop leukemia with a WBC > 20 × 109/L more frequently than TCL1+/wtMIF−/− mice (P = .02, log rank test). Y-axis truncated at 50% because not all animals develop WBC > 20 × 109/L. (C) Increased spleen and liver weight in TCL1+/wtMIFwt/wt versus TCL1+/wtMIF−/− mice at month 12. (D) Prolonged overall survival of TCL1+/wtMIF−/− (n = 60) versus TCL1+/wtMIFwt/wt mice (n = 60). The difference of 80 days was significant with P = .0003 (log-rank test). + indicates censored events (not caused by CLL).
Absence of MIF delays CLL development and prolongs overall survival. (A) WBC (top panel) and malignant (IgM+CD5+) B cells (bottom panel) over time in TCL1+/wtMIFwt/wt (●) and TCL1+/wtMIF−/− (○) mice. Y-axis modified to accommodate high cell counts in leukemic mice. TCL1+/wtMIFwt/wt mice have higher WBC and malignant B cells than TCL1+/wtMIF−/− mice. (B) Incidence of CLL over time. TCL1+/wtMIFwt/wt mice develop leukemia with a WBC > 20 × 109/L more frequently than TCL1+/wtMIF−/− mice (P = .02, log rank test). Y-axis truncated at 50% because not all animals develop WBC > 20 × 109/L. (C) Increased spleen and liver weight in TCL1+/wtMIFwt/wt versus TCL1+/wtMIF−/− mice at month 12. (D) Prolonged overall survival of TCL1+/wtMIF−/− (n = 60) versus TCL1+/wtMIFwt/wt mice (n = 60). The difference of 80 days was significant with P = .0003 (log-rank test). + indicates censored events (not caused by CLL).
To determine the tumor burden in leukemic animals, 10 mice of each genotype were killed at the age of 12 months. Organ weights of wild-type littermates were determined as a reference (spleen: 0.16 ± 0.04 g; liver: 0.7 ± 0.16 g). Eμ-TCL1 mice showed the expected hepatomegaly and splenomegaly. Comparative analyses of TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− mice showed that the absence of MIF was associated with a decreased spleen weight of 1.3 ± 0.2 g versus 0.8 ± 0.1 g (P = .07) and liver weight of 3.3 ± 0.3 g to 1.7 ± 0.2 g (P = .028; Figure 2C). Livers and spleens were diffusely infiltrated by small CD79a+ lymphocytes coexpressing CD5 with a predominantly mature morphology, consistent with the diagnosis of CLL (supplemental Figure 2F, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Giemsa staining of spleens showed a disrupted organ architecture with a dense infiltration by small lymphocytes (supplemental Figure 2E). A marked lymphocytic infiltration of the marrow was also observed in both genotypes. In leukemic mice, erythrocyte and platelet counts decreased over time, without detectable differences between both genotypes (supplemental Figure 2G). Finally, in a long-term follow-up experiment, TCL1+/wtMIFwt/wt mice showed a shorter overall survival (median, 380 days) than TCL1+/wtMIF−/− mice (460 days, P = .0003; Figure 2D). All deaths were caused by CLL as shown by postmortem pathology examination.
We then asked whether the presence of MIF had an impact on major genetic events in CLL, in particular those with prognostic relevance. For this purpose, murine leukemic splenocytes of both genotypes were analyzed for IGHV mutational status, for chromosomal aberrations by SKY, and for p53, MDM2, c-myc, and BCL-2 expression by immunoblotting. However, these studies did not reveal any consistent difference between the 2 genotypes (supplemental Table 1; supplemental Figure 1H-I). Leukemic cells were unanimously of the unmutated CLL type as previously reported for the Eμ-TCL1 model.36
The absence of MIF reduces the numbers and changes the distribution of macrophages in leukemic mice
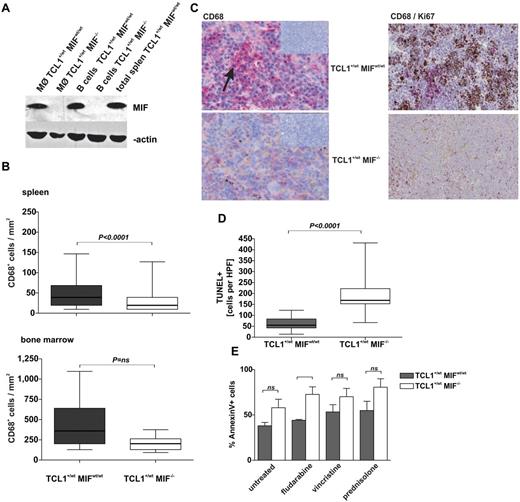
Macrophages were described as a prominent source of MIF.13 Accordingly, purified splenic macrophages of TCL1+/wtMIFwt/wt mice contained substantial amounts of MIF, at levels comparable with isolated splenic B cells or total splenocytes (Figure 3A). Immunohistochemical staining of 12-month-old mice showed reduced numbers of CD68+ macrophages in spleens and marrows of TCL1+/wtMIF−/− compared with TCL1+/wtMIFwt/wt mice (spleen: 50 ± 2 vs 30 ± 2 CD68+ cells per mm2; P < .0001; marrow: 452 ± 119 vs 206 ± 33 CD68+ cells per mm2; P = not significant; Figure 3B). In TCL1+/wtMIFwt/wt mice, splenic macrophages formed zonal aggregates, whereas splenic TCL1+/wtMIF−/− macrophages showed a scattered, nonaggregating distribution (Figure 3C). Interestingly, costaining of Ki-67 and CD68 showed that Ki67+ cells clustered near macrophages and at considerably higher density in TCL1+/wtMIFwt/wt than in TCL1+/wtMIF−/− mice (Figure 3C).
MIF deficiency leads to reduced numbers of TAMs and increased apoptosis of CLL cells. (A) MIF protein content determined by Western blotting in purified splenic macrophages and B cells and total splenocytes as control from TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− mice. β-actin as loading control. (B) Absolute numbers of CD68+ TAMs per mm2 in sections of enlarged spleens (top panel) and bone marrow (bottom panel) are higher in TCL1+/wtMIFwt/wt (n = 18) versus TCL1+/wtMIF−/− (n = 14) mice (spleen: P < .0001; bone marrow: P = .066). (C) Representative immunohistochemistry for CD68 in splenic sections of TCL1+/wtMIFwt/wt (top panel) and TCL1+/wtMIF−/− mice (bottom panel). Macrophage agglomerates (red, arrow) are present in spleens of TCL1+/wtMIFwt/wt mice, whereas macrophages are only sparsely distributed in TCL1+/wtMIF−/− mice. Insets represent negative control without primary antibody (10×magnification). Representative CD68/Ki67 double staining of TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− spleen showing accumulation of proliferating cells (brown) in areas of macrophage agglomeration in TCL1+/wtMIFwt/wt but not in TCL1+/wtMIF−/− spleen. (D) TUNEL-positive cells in splenic sections of TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− mice (n = 5 per genotype; 62 ± 7 in TCL1+/wtMIFwt/wt mice vs 192 ± 20 in TCL1+/wt MIF−/− mice; P < .0001). (E) Spontaneous and drug-induced rate of apoptosis as detected by flow cytometric positivity for annexin V/7-amino-actinomycin D in cultured nonleukemic splenocytes of TCL1+/wtMIF−/− versus TCL1+/wtMIFwt/wt mice. Cells were treated ex vivo with cytostatic drugs (50μM fludarabine, 61nM vincristine, 1μM prednisolone) for 24 hours. For each group and genotype, n = 5 independent splenocyte preparations were analyzed.
MIF deficiency leads to reduced numbers of TAMs and increased apoptosis of CLL cells. (A) MIF protein content determined by Western blotting in purified splenic macrophages and B cells and total splenocytes as control from TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− mice. β-actin as loading control. (B) Absolute numbers of CD68+ TAMs per mm2 in sections of enlarged spleens (top panel) and bone marrow (bottom panel) are higher in TCL1+/wtMIFwt/wt (n = 18) versus TCL1+/wtMIF−/− (n = 14) mice (spleen: P < .0001; bone marrow: P = .066). (C) Representative immunohistochemistry for CD68 in splenic sections of TCL1+/wtMIFwt/wt (top panel) and TCL1+/wtMIF−/− mice (bottom panel). Macrophage agglomerates (red, arrow) are present in spleens of TCL1+/wtMIFwt/wt mice, whereas macrophages are only sparsely distributed in TCL1+/wtMIF−/− mice. Insets represent negative control without primary antibody (10×magnification). Representative CD68/Ki67 double staining of TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− spleen showing accumulation of proliferating cells (brown) in areas of macrophage agglomeration in TCL1+/wtMIFwt/wt but not in TCL1+/wtMIF−/− spleen. (D) TUNEL-positive cells in splenic sections of TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− mice (n = 5 per genotype; 62 ± 7 in TCL1+/wtMIFwt/wt mice vs 192 ± 20 in TCL1+/wt MIF−/− mice; P < .0001). (E) Spontaneous and drug-induced rate of apoptosis as detected by flow cytometric positivity for annexin V/7-amino-actinomycin D in cultured nonleukemic splenocytes of TCL1+/wtMIF−/− versus TCL1+/wtMIFwt/wt mice. Cells were treated ex vivo with cytostatic drugs (50μM fludarabine, 61nM vincristine, 1μM prednisolone) for 24 hours. For each group and genotype, n = 5 independent splenocyte preparations were analyzed.
CLL cells from TCL1+/wtMIF−/− mice show an increased rate of apoptosis
Leukemic growth depends on the rate of cellular proliferation and apoptosis. TUNEL staining of spleen sections from leukemic TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− animals demonstrated that the absence of MIF was associated with increased rates of spontaneous CLL cell apoptosis. The number of TUNEL-positive splenocytes was ∼ 3-fold lower in TCL1+/wtMIFwt/wt than in TCL1+/wtMIF−/− mice (62 ± 7 vs 192 ± 20 cells per HPF; P < .0001; Figure 3D; supplemental Figure 3F). Moreover, TCL1+/wtMIF−/− splenocytes were more sensitive to spontaneous and drug-induced (fludarabine, vincristine, prednisolone) apoptosis than TCL1+/wtMIFwt/wt splenocytes in vitro (Figure 3E). Inhibition of DNA damage pathways with the p53-antagonist pifithrin-α,37 the CDC25-inhibitor NSC-663284,38 as well as proteasome inhibition by bortezomib did not abolish the differences of fludarabine-induced apoptosis between TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− splenocytes, suggesting that these pathways were not responsible for the observed differences (supplemental Figure 3G).
Reduced survival of CLL cells in the absence of MIF
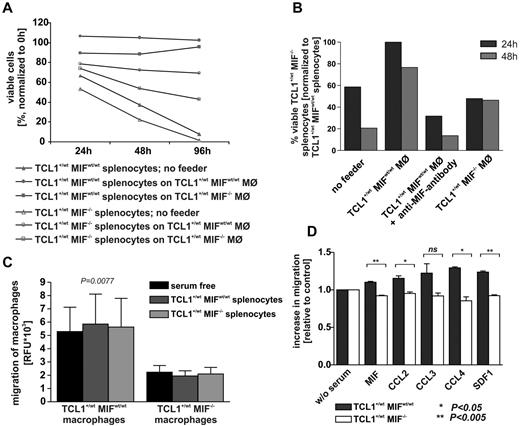
We next asked whether soluble MIF could promote the survival of murine leukemia cells in vitro. Recombinant MIF alone did not induce a significantly longer survival of CLL cells in vitro. However, when leukemic splenocytes were cultured on a layer of MIFwt/wt murine embryonic fibroblasts, cells survived at least 3 days longer than on MIF−/− murine embryonic fibroblasts. Similarly, coculture of leukemic splenocytes with thioglycollate-elicited peritoneal macrophages showed that the presence of MIF originating from macrophages or splenocytes extended the survival of CLL cells in vitro (Figure 4A). Finally, we performed inhibition experiments using a neutralizing anti-MIF antibody IIID9 (100 μg/mL), which reduced the survival of TCL1+/wtMIFwt/wt leukemic cells to levels comparable with TCL1+/wtMIF−/− cells (Figure 4B).
Macrophages support CLL cells in culture. (A) Viable (annexin V/propidium iodide-negative) cells in coculture of murine TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− CLL cells with thioglycollate-elicited peritoneal macrophages from TCL1+/wtMIFwt/wt or TCL1+/wtMIF−/− mice. The figure shows 1 representative example of n = 5 independent experiments. (B) Viable (annexin V/propidium iodide-negative) cells in coculture of murine TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− CLL cells with thioglycollate-elicited peritoneal macrophages from TCL1+/wtMIFwt/wt or TCL1+/wtMIF−/− mice. Addition of neutralizing anti-MIF antibody (IIID9, 100 μg/mL) leads to reduced viability. Representative experiment from a total of 2 independent experiments. (C) Migratory activity of peritoneal macrophages in trans-well experiments toward TCL1+/wtMIFwt/wt or TCL1+/wtMIF−/− B cells depends on MIF status of the macrophages. RFU indicates relative fluorescence units. P = .0077. Figure shows representative results of n = 5 experiments. (D) Gradient-triggered migratory activity of murine TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− peritoneal macrophages in the presence of MIF (100 ng/mL), CCL2 (100 ng/mL), CCL3 (10 ng/mL), CCL4 (100 ng/mL), and SDF-1 (100 ng/mL). Only TCL1+/wtMIFwt/wt macrophages are reactive to the action of chemokines (n = 3).
Macrophages support CLL cells in culture. (A) Viable (annexin V/propidium iodide-negative) cells in coculture of murine TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− CLL cells with thioglycollate-elicited peritoneal macrophages from TCL1+/wtMIFwt/wt or TCL1+/wtMIF−/− mice. The figure shows 1 representative example of n = 5 independent experiments. (B) Viable (annexin V/propidium iodide-negative) cells in coculture of murine TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− CLL cells with thioglycollate-elicited peritoneal macrophages from TCL1+/wtMIFwt/wt or TCL1+/wtMIF−/− mice. Addition of neutralizing anti-MIF antibody (IIID9, 100 μg/mL) leads to reduced viability. Representative experiment from a total of 2 independent experiments. (C) Migratory activity of peritoneal macrophages in trans-well experiments toward TCL1+/wtMIFwt/wt or TCL1+/wtMIF−/− B cells depends on MIF status of the macrophages. RFU indicates relative fluorescence units. P = .0077. Figure shows representative results of n = 5 experiments. (D) Gradient-triggered migratory activity of murine TCL1+/wtMIFwt/wt and TCL1+/wtMIF−/− peritoneal macrophages in the presence of MIF (100 ng/mL), CCL2 (100 ng/mL), CCL3 (10 ng/mL), CCL4 (100 ng/mL), and SDF-1 (100 ng/mL). Only TCL1+/wtMIFwt/wt macrophages are reactive to the action of chemokines (n = 3).
Absence or inhibition of MIF reduces the migratory activity of macrophages in CLL-diseased mice
Tumor-associated macrophages (TAMs) possess tumor-promoting capacity.39 Given the known properties of MIF and the elevated number of macrophages in MIF-proficient Eμ-TCL1 spleens, we hypothesized that MIF might induce the homing of splenic macrophages. To corroborate this concept, we performed Boyden chamber experiments. Thioglycollate-elicited TCL1+/wtMIFwt/wt macrophages showed increased spontaneous motility in serum-free media compared with MIF-deficient controls (Figure 4C). Similarly, the incubation with the anti-MIF antibody IIID9 (100 μg/mL) slightly reduced the migratory capacity of TCL1+/wtMIFwt/wt macrophages by 20%. In contrast, coculture with TCL1+/wtMIFwt/wt or TCL1+/wtMIF−/− splenocytes did not affect the motility of TCL1+/wt MIFwt/wt or TCL1+/wtMIF−/− macrophages. TCL1+/wtMIFwt/wt macrophages also showed an increased migratory activity toward different chemokines compared with TCL1+/wtMIF−/− macrophages. Recombinant MIF and various other chemokines (CCL2, CCL3, CCL4, and SDF-1) were able to induce a higher migratory activity of TCL1+/wt macrophages only in the MIFwt/wt, but not in the MIF−/− genotype (Figure 4D).
Discussion
The survival of CLL cells depends on the support of stromal cells, soluble factors, and surface receptors.5 None of these factors has been validated in an autochthonous mouse model for CLL so far. Using a gene knockout strategy in a well-established model for CLL, the Eμ-TCL1 mouse, we could provide genetic evidence that the absence of MIF delays the development of CLL by reducing the survival of CLL cells and the number of macrophages in homing organs of CLL cells, such as spleen, liver, and marrow. MIF secretion of macrophages was required for full migratory activity of macrophages. Macrophages seemed to accumulate at lower density in leukemic homing organs, such as spleen, liver, and bone marrow, thereby providing an explanation for the delayed CLL development in the absence of MIF. The theory that macrophage MIF supports the survival of CLL cells is corroborated most impressively by the fact that CLL-diseased mice live longer in the absence of MIF (Figure 2D).
Our results are in agreement with previously published effects of MIF in other cancers: MIF is overexpressed in various human and murine cancers.40 Various murine tumor models, such as myc-induced lymphoma, antigen-presenting cell-induced colon carcinoma, and nitrosamine-induced bladder carcinoma,6,8,41 have provided genetic evidence that MIF promotes tumor growth. Several mechanisms have been proposed to explain the tumor-promoting effects of MIF. MIF may functionally interfere with tumor suppressor pathways, such as p53 and Rb/E2F.42,43 So far, we have not been able to identify such mechanisms in our CLL model. The rate of p53 mutations in murine CLL did not appear to be changed in MIF knockout animals. MIF is known to affect signaling of growth and apoptosis-regulating pathways.40 In our studies, we could confirm the activation of some known pathways (Akt, MAPK) by MIF in CLL cells. The Akt and MAPK pathways are known to be important for the survival of human CLL cells.44,45 In addition, the function of MIF as a proinflammatory cytokine may support the development of cancer by creating an inflammatory, favorable microenvironment for cancer development.46 This may be of particular importance in CLL where tumor cells do not survive longer than 1-3 days ex vivo without support by nurse-like cells or survival-promoting cytokines and chemokines.5 The results described in this manuscript suggest that MIF acts as a macrophage recruiting and activating chemokine in CLL.
The functional association of MIF with macrophage migration is well established: The regulation of macrophage migration by lymphocyte-derived MIF led to the initial functional characterization of this protein.10 MIF is an essential factor for delayed-type hypersensitivity reactions and for regulating neutrophil and eosinophil migration.47,48 In arteriosclerosis, MIF is required for monocyte/macrophage migration into atherosclerotic plaques and to accelerate the progression of vessel wall inflammation.14 Our observation that the presence of MIF augments the number of macrophages in CLL homing organs is novel and supports the concept that TAMs are stimulated by MIF and have a functional role for CLL growth.49 In CLL, cells that support the survival of CLL cells in vitro are also referred to as nurse-like cells.50,51 Nurse-like cells were generated by in vitro culture from peripheral blood or spleen mononuclear cells and were identified as macrophages based on plastic adherence and CD68 expression.51 The CD68+ cells analyzed in this work were tissue resident macrophages that were not generated by in vitro culture. We cannot exclude that these cells correspond to nurse-like cells. However, to avoid inappropriate claims and to keep consistent with the current nomenclature in the research field on the tumor microenvironment,52 we preferred the term TAMs for the resident CD68+ cells found in our investigations.
Two subtypes of TAM exist: M1 macrophages with cytotoxic effects on tumor cells and M2 macrophages with poor antigen-presenting capacity, which may suppress the antitumor immune response and promote tumor progression and invasion.39,49 The delay of CLL development and the longer survival of mice in the absence of MIF suggest that MIF-recruited macrophages rather belong to the M2 subtype. This is in keeping with gene expression studies in follicular lymphoma and classic Hodgkin lymphoma, indicating that a macrophage/dendritic cell-associated signature correlates with an inferior outcome.53,54 The role of MIF in the regulation of B cells is also highlighted by the finding that MIF produced by bone marrow resident dendritic cells promotes the survival of murine recirculating B cells in the bone marrow,23 as well as the survival of human B cells via the CD74-NF-κB-BCL2 pathway.17,53
We have not yet studied the phagocytic properties of TAMs in our model. Therefore, the increased numbers of apoptotic cells observed in TCL1+/wtMIF−/− animals may have resulted from a decreased phagocytic activity in MIF-negative macrophages because MIF is known to stimulate the phagocytic properties of macrophages in an autocrine and paracrine fashion.55 However, we think that this mechanism is of minor importance because the spontaneous rate of apoptosis of MIF-deficient splenocytes was also increased in the absence of macrophages (Figures 3E and 4A-B). Another potential mode of action might be the interaction of TAMs with CLL cells generating survival-promoting effects for CLL cells. It is well described in the literature that TAMs promote tumor growth by producing tumor cell supporting factors.52
The earlier onset of CLL in MIF-competent animals may provoke the question whether MIF is a causative factor for CLL. However, there is no evidence to suggest that MIF alone would be sufficient to cause CLL. Transgenic mice, which ubiquitously overexpress MIF, do not show an increased incidence of tumors, including lymphoma or CLL. These mice have high turnover osteoporosis, a condition in which activated macrophages play an important role.56 Moreover, CLL occurred in TCL1+/wtMIF−/− mice with a similar incidence compared with TCL1+/wtMIFwt/wt mice, albeit with a delayed onset and a lower tumor burden. Therefore, MIF seems to act rather as a cofactor for CLL development by supporting the expansion of the malignant clone via the accumulation of TAMs in leukemia homing organs (Figure 5).
Effects of MIF in CLL. The chemokine MIF induces an increased recruitment of TAMs into the homing organs of CLL cells, such as the spleen. Macrophages support the survival of murine CLL cells (via MIF and other mediators), thereby generating a vicious circle that enhances the development of CLL.
Effects of MIF in CLL. The chemokine MIF induces an increased recruitment of TAMs into the homing organs of CLL cells, such as the spleen. Macrophages support the survival of murine CLL cells (via MIF and other mediators), thereby generating a vicious circle that enhances the development of CLL.
Taken together, our results show that the absence of MIF delays the development of CLL by reducing the survival of CLL cells and the number and migratory capacity of macrophages in leukemic homing organs. These results seem to be of clinical relevance because the absence or inhibition of MIF may result in a slower progression of CLL. Therefore, these findings may contribute to design new strategies targeting the microenvironment of this leukemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the blood donors and Prof Dr B. Gathof (University of Cologne Institute for Transfusion Medicine, Cologne, Germany) for donation and collection of plasma samples; Prof C. Croce (The Ohio State University, Columbus, OH) for the Eμ-TCL1 transgenic breeder mice; and Prof J. Bernhagen (RWTH Aachen University, Department of Biochemistry and Molecular Cell Biology, Aachen, Germany) for recombinant MIF and anti-MIF III.D.9 and isotype controls.
This work was supported by the Deutsche Forschungsgemeinschaft (German Research Council; grant A16, SFB 832, M. Hallek and N. Reinart; grant FI 712/2-2, G.F.-R.), the German Jose Carreras Leukemia Foundation (grant R06/20, G.F.-R.), and the Köln Fortune program (grant 88/2009; M.M.-R.).
Authorship
Contribution: N. Reinart designed and executed experiments, analyzed data, and wrote the manuscript; P.-H.N., J.B., N. Rosen, C.R., V.R., T.V., C.M., K.S.R., and M.M.-R. designed and executed the experiments; H.-M.K. and L.H. analyzed the pathologic sections; E.P.v.S., G.K., B.S., and M. Herling analyzed data; and M. Hallek and G.F.-R. designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Hallek, University Hospital Cologne, Department I of Internal Medicine, Center for Integrated Oncology, Kerpener Strasse 62, 50924 Cologne, Germany; e-mail: michael.hallek@uni-koeln.de.