In this issue of Blood, Cieri and colleagues elucidate the instructive signals that guide naive T lymphocytes to differentiate into memory stem cells (TSCM) in vitro, paving the way for a rapid translation of TSCM into new clinical trials.1
Memory T lymphocytes play a critical role in the immune response against infectious disease and cancer.2,3 The memory T-cell compartment is heterogeneous but it can be divided into 3 main subsets, with the recently identified T memory stem cells (TSCM) joining the well-established central memory (TCM) and effector memory (TEM) T-cell subsets (see figure).3-7 TSCM are capable of reconstituting the full diversity of memory and effector T lymphocytes on serial transplantation in mice, indicating that these cells are endowed with the stem cell–like attributes of self-renewal capacity and multipotency.5 These qualities make TSCM a particularly attractive subset to employ in adoptive immunotherapies for cancer as they might overcome current limitations, including inefficient T-cell engraftment, poor persistence, and inability to mediate a prolonged immune attack. Indeed, TSCM displayed robust proliferative and survival capacities and were superior to other memory T-cell subsets in causing regression of large, established tumors in preclinical models.5,6 While the instructive signals guiding the formation of TSCM have just begun to be investigated,5 clinical-grade protocols to facilitate translation of TSCM into the clinic remain undefined.
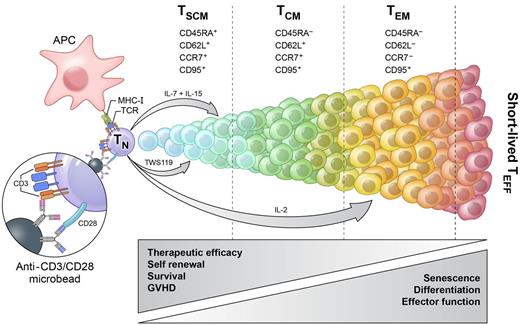
Programming T-cell fates for therapeutic use. After antigen encounter or stimulation with anti-CD3 and anti-CD28 antibody-conjugated microbeads, naive T cells (TN) enter a program of proliferation and differentiation that culminates in the generation of terminally differentiated short-lived effector T cells (TEFF). During this process of maturation, T cells progressively acquire effector functions but simultaneously lose their capacities for self-renewal and survival, diminishing their therapeutic effectiveness. Cytokines and small molecules can be used to modulate this process and preferentially generate a desired T-cell subset. IL-2 induces effector memory T cells (TEM), while IL-7 and IL-15 can be used in combination to generate T memory stem cells (TSCM). TSCM can also be induced by targeting the Wnt/β-catenin signaling pathway with TWS119 or other inhibitors of GSK-3β. APC indicates antigen presenting cell; and TCM, central memory T cell.
Programming T-cell fates for therapeutic use. After antigen encounter or stimulation with anti-CD3 and anti-CD28 antibody-conjugated microbeads, naive T cells (TN) enter a program of proliferation and differentiation that culminates in the generation of terminally differentiated short-lived effector T cells (TEFF). During this process of maturation, T cells progressively acquire effector functions but simultaneously lose their capacities for self-renewal and survival, diminishing their therapeutic effectiveness. Cytokines and small molecules can be used to modulate this process and preferentially generate a desired T-cell subset. IL-2 induces effector memory T cells (TEM), while IL-7 and IL-15 can be used in combination to generate T memory stem cells (TSCM). TSCM can also be induced by targeting the Wnt/β-catenin signaling pathway with TWS119 or other inhibitors of GSK-3β. APC indicates antigen presenting cell; and TCM, central memory T cell.
Cieri and colleagues now demonstrate that it is possible to generate, expand, and gene engineer TSCM in vitro starting from naive precursors using reagents that are currently manufactured under GMP-quality conditions. The authors found that activation of naive T cells with anti-CD3 and anti-CD28 antibody-conjugated beads in the presence of low doses of IL-7 and IL-15 promoted the generation of CD45RA+CD62L+CCR7+ CD95+ T cells phenotypically resembling the TSCM cell population recently described by our group (see figure).6 Cells generated under these conditions, however, also expressed CD45RO, a prototypical marker of memory and effector T lymphocytes, which is absent from the surface of TSCM as originally reported.6 This phenotypic discrepancy likely resulted from differences in the expression of HNRPLL (encoding heterogeneous nuclear ribonucleoprotein L-like),6 a key regulator of the alternative splicing of the CD45 pre-mRNA required for efficient CD45RO expression. This difference places IL-7/IL-15–generated cells in a more differentiated position in the spectrum of differentiation (see figure). However, in vitro–generated TSCM closely clustered with naturally occurring TSCM when analyzed using a set of 65 genes differentially regulated between naive and memory T lymphocytes. Furthermore, in vitro–generated TSCM displayed an enhanced proliferative capacity on adoptive transfer into immunodeficient mice, a finding consistent with those using naturally occurring TSCM.6 Most importantly, Cieri et al in a brilliant set of experiments showed for the first time that TSCM were the only T-cell subset capable of expanding and mediating GVHD on serial transplantation. These findings represent the most compelling evidence that TSCM preferentially retain stem cell–like attributes among all human T lymphocytes and position researchers in the field to formally test the “stemness” of TSCM in human clinical trials.
By dissecting the relative contribution of the biologic signals required for the in vitro generation of TSCM, the authors found that IL-7 was indispensable for the formation of these cells, while IL-15 was necessary to sustain their expansion. In vivo, elevated levels of IL-7 and IL-15 are found in hosts receiving lymphodepleting conditioning regimens for hematopoietic stem cell (HSC) transplantation or adoptive T-cell immunotherapy as a result of transient eradication of cellular sinks for homeostatic cytokines.8 Consistent with this notion, Cieri and colleagues found that after allogeneic HSC transplantation, virtually all seemingly naive T cells were actually TSCM, because CD45RA+CD62L+ T cells also expressed high levels of CD95. Although the instructive signals guiding TSCM cell formation during physiologic immune responses are yet to be elucidated, these findings provide the first glimpse on how TSCM can be formed in vivo in a clinically relevant setting.
The identification of clinically compliant conditions for the efficient generation and genetic manipulation of TSCM also has important implications for the development of new T cell–based immunotherapies. Despite the potent anti-tumor activity of TSCM in preclinical animal tumor models,5,6 it is currently not feasible to treat patients with naturally occurring TSCM because these cells represent only a small portion of circulating lymphocytes. Adoptive immunotherapy may require larger number of transferred cells than can be obtained from the naturally occurring TSCM compartment. Therefore, the identification of strategies that generate, expand, and enable redirecting of TSCM against cancer cells is crucial. We have previously shown that programming naive T cells in the presence of small molecules targeting the Wnt/β-catenin pathway, such as glycogen synthase-3β (GSK-3 β) inhibitors, promotes the generation of TSCM (see figure).5,6 Although inhibitors of GSK-3β are effective at arresting T-cell differentiation, they also inhibit T-cell proliferation.5 For this reason, finding alternative approaches that uncouple cell expansion and differentiation is desirable. Cieri and colleagues now describe how priming T cells in the presence of low doses of IL-7 and IL-15 can generate larger numbers of TSCM than previously reported with GSK-3β inhibitors.
Finally, the findings reported here by Cieri et al provide new experimental evidence that helps to resolve the ongoing debates regarding the ontogeny of memory cells9 and which T-cell subset needs to be isolated to generate more potent anti-tumor T cells for human clinical trials.10 Whole genome profiling as well as phenotypic and functional data described in this issue of Blood1 are consistent with a linear model of differentiation in which naive T cells differentiate first into TSCM cells and then into TCM and TEM.2,3 Consistent with this model and a progressive loss of therapeutic potential with differentiation (see figure),3 T cells expanded from sorted TCM were less potent at mediating GVHD compared with naive-derived T cells and were unable to reconstitute immunodeficient animals on serial transplantation. These data indicate that TCM-derived T cells are relatively ineffective and suggest that new adoptive immunotherapies will greatly benefit from the generation of tumor-reactive TSCM from sorted naive precursors.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal