Abstract
Graft-versus-host disease (GVHD) induced by transplant-derived T cells represents a major complication after allogeneic bone marrow transplantation (BMT). However, these T cells support engraftment, early T-cell immunity, and mediate the graft-versus-tumor (GVT) effect. Cytotoxic effector functions by transplanted T cells are predominantly mediated by the perforin/granzyme and the CD95/CD95L system. APG101, a novel recombinant human fusion protein consisting of the extracellular domain of CD95 and the Fc domain of an IgG1 antibody inhibited CD95L-induced apoptosis without interfering with T-cell function in vitro and was therefore tested for its ability to prevent GVHD in murine BMT models across minor or major histocompatibility barriers. Starting APG101 treatment either 1 day before or 6 days after transplantation effectively reduced clinical GVHD and rescued survival between 60% and 100% if GVHD was CD95L mediated. APG101 did not interfere with the GVT effect, because P815 mastocytoma and most importantly primary Bcr-Abl–transformed B-cell leukemias were completely eradicated by the alloantigen-specific T cells. Phenotype and homing of alloantigen-specific T cells or their perforin/granzyme-mediated cytotoxicity and proliferative capacity were not affected by APG101 treatment suggesting that APG101 therapy might be useful in GVHD prophylaxis without impairing T-cell function and most importantly preserving GVT activity.
Key Points
The novel recombinant protein APG101 preventing CD95/CD95L interaction inhibits GVHD without abrogating the GVT effect or T-cell functions.
APG101 might be incorporated into protocols of GVHD prevention and treatment during bone marrow transplantation.
Introduction
Allogeneic bone marrow transplantation (BMT) is a potentially curative treatment modality for hematologic malignancies, which involves the graft-versus-tumor or graft-versus-leukemia effect, that is, elimination of residual tumor cells by mature allogeneic T cells in the transplant. However, these T cells are also responsible for the induction of graft-versus-host disease (GVHD), which is still a major complication after allogeneic BMT leading to significant morbidity and mortality despite routine immune suppression used in all protocols.1 T-cell depletion of the graft significantly decreases the risk of GVHD, however it also delays engraftment, sensitizes the host for opportunistic infections, and abrogates the GVT effect.2 Therefore, preventing GVHD while maintaining GVT activity and early T-cell immunity is a major goal in allogeneic BMT.
GVHD represents a complex disease depending on alloantigen-specific T-cell activation, proliferation, and subsequent induction of T-cell effector functions, which are attempt to be inhibited by routinely given immunosuppressive therapy. However, this causes general immunosuppression leading to an increased risk for opportunistic infections, cancer development and also severely suppresses the GVT effect.3 Acquiring effector functions is associated with versatile molecular and cellular changes in the T cell, for example, the expression of activation markers, homing molecules, cytokines, and cytotoxic molecules that represent possible targets for new therapeutic approaches in GVHD-prevention and treatment.4 Analysis of T-cell subsets showed that memory T cells are unable to induce GVHD but efficiently eradicate tumor cells.5,6 We recently found that even in vitro-generated alloantigen-specific cytotoxic T cells (CTLs) mediate antitumor cytotoxicity in the absence of GVHD.7 Interfering with T-cell trafficking or cytokine production offers other treatment strategies.8,9 The primary goal of most trials is the inhibition of host tissue destruction by attacking alloantigen-specific T cells. Destructive function of T cells is mediated either by death ligands CD95L, TRAIL, and TNF-α, or by the perforin/granzyme system. BMT-models using T cells either deficient for CD95L or perforin or treatment with anti–TNF-α antibodies demonstrated that all 3 molecules are involved in GVHD induction although their contribution to GVHD pathology is different. Although TNF-α and CD95L are indispensable for hepatic, intestinal, and cutaneous GVHD, perforin plays an important role in the kinetics of GVHD pathophysiology.10-12 Efficient antitumor cytotoxicity, however, is predominantly dependent on TNF-α and perforin.11,13 The role of TRAIL in GVHD induction and GVT effect is controversially discussed.5,14,15 These studies indicate that interference with the CD95/CD95L pathway might be a successful treatment option to inhibit GVHD without abrogating the desired GVT effect after allogeneic BMT. APG101 is a human soluble fusion protein consisting of the extracellular domain of CD95 and the Fc portion of IgG1 thereby blocking the interaction of CD95L with its cognate receptor. APG101 was shown to be well tolerated up to high doses in a phase 1 first-in-man study,16 and may therefore be applicable in GVHD prophylaxis. Because the CD95 system can mediate other cellular functions in addition to apoptosis, such as migration, invasiveness, and tumor promotion, dependent on the specific cellular systems,17,18 APG101 is also currently exploited in the treatment of gliomas.19 To test whether the CD95L-mediated inhibition of tissue destruction by APG101 offers a new therapeutic approach to treat and/or prevent GVHD, we treated allogeneic BM-transplanted mice on a regular basis with APG101. Because CD95L is not only known for its function as an apoptosis inducer, but may also transmit signals into the CD95L-expressing cells, a process known as reverse signaling and predominantly affecting T-cell proliferation,20-22 we further analyzed the effect of APG101 on T-cell function, antitumor cytotoxicity, and homing.
In a parent into F1 MHC class I and II–mismatched BMT model and a BMT model differing in minor histocompatibility antigens (miHAg), we found that APG101 efficiently prevents clinical, CD95L-mediated GVHD. APG101 inhibited CD95-mediated apoptosis without disabling T-cell functions because donor-derived T cells maintained their migratory ability, proliferated after activation, and most importantly exhibited efficient antitumor cytotoxicity with complete elimination of residual tumor cells. Thus, inhibiting CD95/CD95L interaction by APG101 might represent a clinically applicable strategy in allogeneic BMT to inhibit GVHD without disabling T-cell functions indispensable for antitumor cytotoxicity and early T-cell immunity.
Methods
Cell culture
All cell lines were grown in complete RPMI 1640 medium (GIBCO-BRL) supplemented with 10% FCS (Lonza), 2mM l-glutamine, 1mM sodium pyruvate at 37°C in a humidified atmosphere containing 7.5% CO2. CTLs were cultured in α-MEM (Lonza), 10%FCS (PAA), 2mM l-glutamine, 1mM sodium-pyruvate, 0.05mM 2-ME, 5% vol/vol of 0.5M methyl-α-D-mannopyranoside, and 5% vol/vol ConA supernatant.
Mice and BMT
Female C57BL/6 (B6; H-2b, CD45.2), B6D2F1 (H-2bxd, CD45.2), DBA/2 (H-2d; Harlan-Winkelmann), BALB/c (H-2d; Janvier), B10.D2/nSnJ (H-2d; The Jackson Laboratory), and B6.SJL-PtprcaPepcb/BoyJ (B6.SJL; H-2b, CD45.1; breeding pairs from The Jackson Laboratory and bred at the University of Ulm) were used at age 6 to 10 weeks. On day 1, recipient mice were lethally irradiated with 13 Gy split into 2 doses 3 hours apart from a 137Cs source and were reconstituted on day 0 with 5 × 106 BM cells via tail-vein injection. If indicated, mature T cells were depleted from BM by incubation with hybridoma supernatant 30-H12 (anti–Thy-1.2) and subsequently lysed by low-TOX-M rabbit complement (Cedarlane; TCD BM = T cell–depleted BM). BM was coinjected with 2 × 107 spleen cells in B6D2F1 mice or with 3 × 107 spleen cells in BALB/c mice. APG101 treatment started either 1 day before BMT (day −1), or 6 or 13 days after BMT (day 6, day 13). APG101 was injected at a fixed dose of 1 mg/mouse twice a week intraperitoneally in PBS. Weight loss, decreased activity, skin lesions, fur ruffling, and hunched posture were scored on a scale from 0 to 2 in the recipient mice every 2 days and the GVHD-score was determined by summation of the 5 criteria.23 Animals dying during the experiment remained included in the calculation until the end of the experiment with their final body weights and GVHD-scores at day of death. Mice were killed when more than 20% of their original weight was lost or if they became moribund. Survival was analyzed using Kaplan-Meier method. Tumor cells were injected intravenously either at the day of BMT (P815 [H-2d], 2 × 103/mouse) or 7 days after BMT (GFP-Bcr-Abl-transformed B-ALL [H-2d], 3 × 102/mouse).24 All animal studies were approved by the Regierungspräsidium, Tübingen, Germany.
Histopathology and immunohistochemistry
Liver, intestine, and skin from the interscapular region were fixed in 4% formalin, paraffin-embedded, sectioned, and stained with H&E. Slides were coded and examined by a pathologist who was blinded for the experimental history of the animals. Histopathology of GVHD was graded according to Kaplan et al.25 To determine target cell apoptosis paraffin sections were stained at room temperature with a polyclonal cleaved caspase-3 (Asp175) antibody (Cell Signaling Technology), followed by anti–rabbit biotin (Jackson ImmunoResearch Labortories) and subsequently REAL detection system (Dako) according to the manufacturer's protocol, nuclear counter-labeled with hemalaun, and embedded in Aquatex medium (Merck).
Induction of apoptosis
CD95-sensitivity was determined by incubating cells with 100 ng/mL CD95L and 1 μg/mL enhancer of ligands (Alexis Biochemicals). After 24 hours, specific apoptosis was determined by 7-AAD staining on LSRII flow cytometer.
Generation of alloantigen-specific T cells
Spleen cells from B6 mice were weekly stimulated at 1:1 ratio with irradiated spleen cells from B6D2F1 mice as previously described.7 If indicated, APG101 at a concentration of 100 ng/mL was present during the whole stimulation time.
Chromium release assay
Chromium release assay was described previously.26 Assays were set up in triplicates and performed at day 6 after T-cell stimulation. Cytotoxic effector mechanisms of CTLs were identified by 2-hour preincubation of CTLs with CMA (10nM, Sigma-Aldrich) or APG101 (100 ng/mL). CMA and APG101 were constantly present during the whole assay. To determine cytotoxic activity of transplanted CD45.1+ T cells, spleen cells of transplanted mice were stained for CD45.1 expression before the assay and the total number of CD45.1+ T cells was calculated. Effector-target ratios were then adjusted for a ratio of CD45.1+ T cells:target cells.
CFSE-labeling
Two × 106 cells/mL were labeled with 100 μL 50μM CFSE (Invitrogen) for 10 minutes at 37°C. Cells were immediately washed and subsequently used for IV injection or in vitro assays.
Proliferation
Two × 105 CFSE-labeled cells were stimulated either with anti-CD3 and CD28 antibodies (0.0125 μg/mL, eBioscience) or 2 × 105 irradiated allogeneic spleen cells and proliferation was measured on day 4 (after CD3/28 activation) or day 6 (after alloantigen or third-party stimulation) on LSRII flow cytometer.
Flow cytometry
Two × 105 cells were stained with the following antibodies: CD3, CD4, CD25, NK1.1, TCRβ, CD122, CCR7, CD45.1, FoxP3, H-2Kd (eBioscience), H-2Kb, CD3, CD8, CD44, CD62L (BD Bioscience), and analyzed by flow cytometry on LSRII flow cytometer.
Isolation of CD45.1+ T cells
Spleen cells from transplanted mice were stained with CD45.2, CD11b, CD16/32 antibody (eBioscience) and supernatant from the hybridoma PK136 (anti-NK1.1) and were subsequently eliminated with goat-anti–mouse IgG and goat-anti–rat IgG magnetic beads (Invitrogen). Purity of the CD45.1+ T-cell population was always > 87%.
Statistical analysis
Differences between groups in survival studies were determined using log-rank test. For all other data, a student t test was used. Results were considered significant if P ≤ .05.
Results
APG101 inhibits apoptosis but does not interfere with T-cell function in vitro
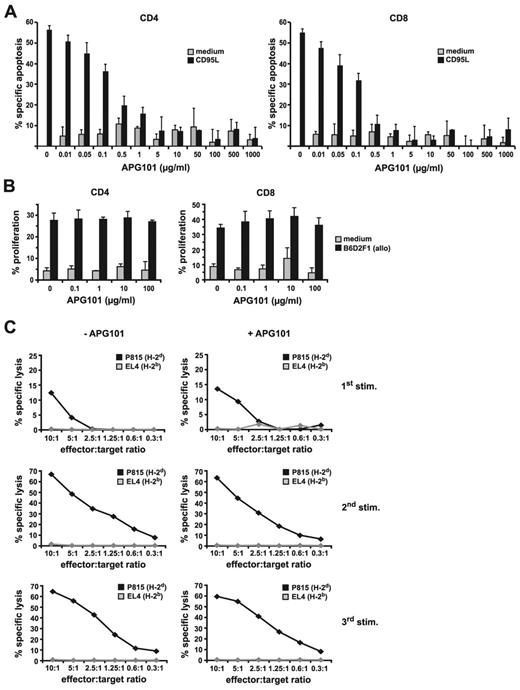
APG101 is a recombinant human fusion protein binding to CD95L and thereby inhibiting apoptosis induction in CD95-expressing target cells. Although CD95L is best known for its role in apoptosis induction, it may also serve a function in reverse signaling, thereby modulating the immune function of CD95L-expressing cells.21,22 Therefore, we first tested the effect of APG101 on apoptosis inhibition and T-cell function in vitro. B6-derived spleen cells were treated with recombinant CD95L and enhancer in the presence of increasing concentrations of APG101. After 24 hours CD95L-mediated apoptosis of CD4+ and CD8+ T cells was efficiently blocked with APG101 concentrations ranging from 5 to 1000 μg/mL. APG101 by itself did not induce apoptosis (Figure 1A). Because CD95L can modulate T-cell function as a reverse signaling costimulator,21 we tested the effect of APG101 on T-cell proliferation and T-cell cytotoxicity. CFSE-labeled B6-derived (H-2b) splenic CD4+ and CD8+ T cells activated for 6 days with irradiated allogeneic spleen cells from B6D2F1 mice (H-2bxd) efficiently proliferated independent of the presence or absence of APG101 (Figure 1B). To exclude that APG101 interferes with the development of alloantigen-specific CTLs, B6-derived spleen cells were activated with irradiated B6D2F1 spleen cells in the presence or absence of APG101. After 1 week (1st stim) the lytic capacity of induced CTLs was tested on H-2d–expressing target cells (P815). P815 cells were effectively lysed independent whether or not APG101 was added to the cultures, whereas syngeneic H-2b–expressing cells (EL4) were not recognized. Cytotoxicity of H-2d–specific CTLs increased when CTLs (1st stim) were subsequently restimulated by alloantigen-expressing cells (2nd and 3rd stim) independent of the presence of APG101 (Figure 1C). Taken together, these data clearly indicate that APG101 effectively prevents cell death induction but does not interfere with the proliferative or cytotoxic capacity of T cells in vitro.
APG101 inhibits CD95-mediated apoptosis but does not prevent T-cell proliferation or cytotoxicity in vitro. (A) Spleen cells from B6 mice were incubated with medium or recombinant CD95L plus enhancer in the presence or absence of increasing concentrations of APG101. After 24 hours cells were stained for CD4+ and CD8+ expression and apoptosis was determined in both T-cell populations by measuring 7-AAD–positive cells. Data present the mean of 3 independent experiments with triplicates ± SD. (B) CFSE-labeled B6-derived spleen cells were stimulated with medium or irradiated B6D2F1-derived allogeneic spleen cells in the presence or absence of increasing concentrations of APG101. After 6 days, proliferation of CD4+ and CD8+ T cells was measured. Data represent the mean of triplicates ± SD of 1 representative experiment of 3 experiments performed. (C) H-2d–specific CTLs were established by coincubation of B6-derived spleen cells with irradiated B6D2F1 spleen cells in the presence or absence of APG101 (100 ng/mL; 1st stim). After each week, living cells were isolated and restimulated with irradiated B6D2F1 spleen cells (2nd stim, 3rd stim) in the presence or absence of APG101. Six days after each stimulation, H-2d–specific cytotoxicity of CTLs was tested on P815 (H-2d) or EL4 (H-2b) target cells in a chromium release assay. Data are representative for 1 experiment of 3 done.
APG101 inhibits CD95-mediated apoptosis but does not prevent T-cell proliferation or cytotoxicity in vitro. (A) Spleen cells from B6 mice were incubated with medium or recombinant CD95L plus enhancer in the presence or absence of increasing concentrations of APG101. After 24 hours cells were stained for CD4+ and CD8+ expression and apoptosis was determined in both T-cell populations by measuring 7-AAD–positive cells. Data present the mean of 3 independent experiments with triplicates ± SD. (B) CFSE-labeled B6-derived spleen cells were stimulated with medium or irradiated B6D2F1-derived allogeneic spleen cells in the presence or absence of increasing concentrations of APG101. After 6 days, proliferation of CD4+ and CD8+ T cells was measured. Data represent the mean of triplicates ± SD of 1 representative experiment of 3 experiments performed. (C) H-2d–specific CTLs were established by coincubation of B6-derived spleen cells with irradiated B6D2F1 spleen cells in the presence or absence of APG101 (100 ng/mL; 1st stim). After each week, living cells were isolated and restimulated with irradiated B6D2F1 spleen cells (2nd stim, 3rd stim) in the presence or absence of APG101. Six days after each stimulation, H-2d–specific cytotoxicity of CTLs was tested on P815 (H-2d) or EL4 (H-2b) target cells in a chromium release assay. Data are representative for 1 experiment of 3 done.
APG101 treatment prevents clinical GVHD in a parent → F1 BMT model
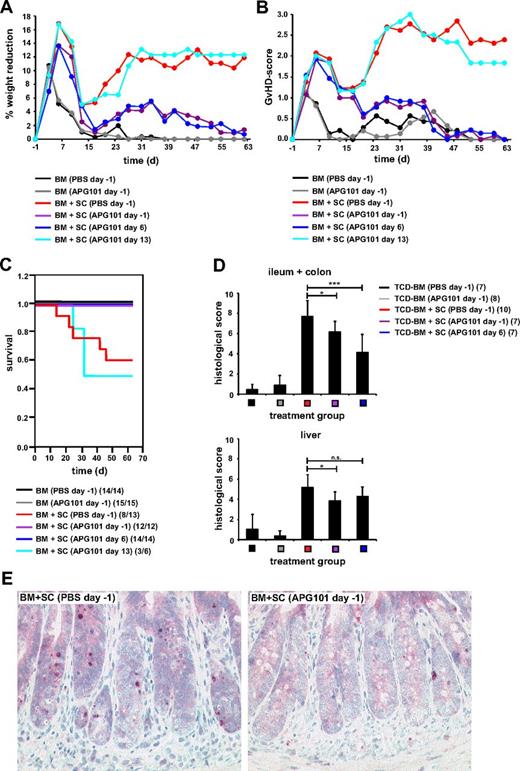
In allogeneic BMT models where GVHD development depends on the presence of CD4+ T cells in the transplant recipient tissue destruction is predominantly mediated by CD95L-expressing allogeneic T cells attacking CD95-positive target cells.27 Because APG101 efficiently inhibited apoptosis induction without abrogating T-cell proliferation or cytotoxicity in vitro, we studied whether APG101 might be a useful therapeutic agent in preventing GVHD after allogeneic BMT in a CD4+-dependent parent → F1 BMT model with a full mismatch for MHC class I and II molecules (B6 → B6D2F1). Lethally irradiated B6D2F1 mice were reconstituted with BM alone or together with spleen cells from B6 mice. APG101 treatment started 1 day before BMT (day −1) or 6 or 13 days after transplantation (day 6, day 13). Mice were treated twice a week with a fixed dose of 1 mg APG101/mouse intraperitoneally. Induction of GVHD was determined by weight loss, a clinical scoring system,23 and survival. Because of the toxic effect of irradiation, GVHD scores increased and body weight decreased initially between days 4 and 10 after transplantation. After a short recovery phase, mice reconstituted with BM and spleen cells exhibited severe weight loss, increased GVHD-scores, and a mortality of 38%. Importantly, mice treated with APG101 prophylactically at day −1 and even therapeutically at day 6 showed only a modest weight loss and increase in GVHD-scores and a 100% survival comparable with mice transplanted with BM alone. However, starting treatment 13 days after transplantation APG101 did not prevent GVHD induction (Figure 2A-C). Mice injected with BM alone and treated with APG101 were indistinguishable from mice receiving BM and PBS. No differences in time to cellular reconstitution or the composition of repopulating cell subsets were observed (data not shown) indicating that APG101 does not influence donor chimerism. Although clinical GVHD was efficiently prevented by APG101 treatment starting at day −1 or 6, histologic GVHD was not totally inhibited. Treatment start at day −1 or day 6 significantly inhibited GVHD of the intestine, whereas liver damage was only reduced in transplanted mice when APG101 treatment started 1 day before transplantation (Figure 2D). Histology of the ileum and colon of APG101-treated mice showed reduced mononuclear inflammatory infiltrates in the lamina propria, decreased numbers of intraepithelial lymphocytes, and reduced apoptosis of crypt epithelium depicted by active caspase-3 staining (Figure 2E). In the liver reduced inflammation of the portal field and bile duct injury was observed in mice treated with APG101 starting day −1. To prove the specificity of APG101 and to exclude an off-target effect we tested the effect of APG101 in an allogeneic BMT model where GVHD induction is preferentially dependent on the perforin/granzyme system and CD8+ T cells in the transplant (B6 [H-2Kb] into B6.bm1 [H-2Kbm1]).27 B6.bm1 mice transplanted with BM and spleen cells and treated with APG101 on day −1 or day 6 developed clinical GVHD and showed no improved survival compared with the PBS-treated groups (supplemental Figure 1A-B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These data clearly show that CD95L-mediated GVHD can be specifically inhibited by APG101 treatment starting either 1 day before or 6 days after transplantation.
APG101 prevents GVHD in a MHC class I and II–mismatched parent → F1 BMT model. (A-E) Lethally irradiated B6D2F1 recipient mice were reconstituted with BM from B6 mice without or with B6-derived spleen cells (SCs) and were treated with PBS or APG101 starting 1 day before transplantation (day −1) or with APG101 starting 6 (day 6) or 13 days (day 13) after transplantation. Weight reduction (A), GVHD-scores (B), and survival (C) were determined. (C): BM+SC (PBS, day −1) versus BM+SC (APG101, day −1), *P < .05; versus BM+SC (APG101, day 6), **P < .01; versus BM+SC (APG101, day 13), P = .12ns. Surviving animals/total animals treated are indicated in (C). On day 16 after transplantation paraffin sections of ileum, colon, and liver of 7 to 10 animals/treatment group were analyzed for histologic signs of GVHD, that is, inflammation, active caspase-3 (*P < .05, ***P < .001). (D) On day 16 after transplantation active caspase-3 was stained at room temperature on colon sections of BM + spleen cells transplanted mice treated with PBS or APG101 on day −1 (Zeiss Axiphot microscope, Plan-Neofluar objective lens, 20× magnification, 0.50 numeric aperture, JVC-KY-F75U digital camera and DISKUS Version 4.5 software). (E) Data represent the combination of 2 individual experiments.
APG101 prevents GVHD in a MHC class I and II–mismatched parent → F1 BMT model. (A-E) Lethally irradiated B6D2F1 recipient mice were reconstituted with BM from B6 mice without or with B6-derived spleen cells (SCs) and were treated with PBS or APG101 starting 1 day before transplantation (day −1) or with APG101 starting 6 (day 6) or 13 days (day 13) after transplantation. Weight reduction (A), GVHD-scores (B), and survival (C) were determined. (C): BM+SC (PBS, day −1) versus BM+SC (APG101, day −1), *P < .05; versus BM+SC (APG101, day 6), **P < .01; versus BM+SC (APG101, day 13), P = .12ns. Surviving animals/total animals treated are indicated in (C). On day 16 after transplantation paraffin sections of ileum, colon, and liver of 7 to 10 animals/treatment group were analyzed for histologic signs of GVHD, that is, inflammation, active caspase-3 (*P < .05, ***P < .001). (D) On day 16 after transplantation active caspase-3 was stained at room temperature on colon sections of BM + spleen cells transplanted mice treated with PBS or APG101 on day −1 (Zeiss Axiphot microscope, Plan-Neofluar objective lens, 20× magnification, 0.50 numeric aperture, JVC-KY-F75U digital camera and DISKUS Version 4.5 software). (E) Data represent the combination of 2 individual experiments.
APG101 treatment strongly attenuates clinical GVHD in a minor histocompatibility antigen mismatched BMT model
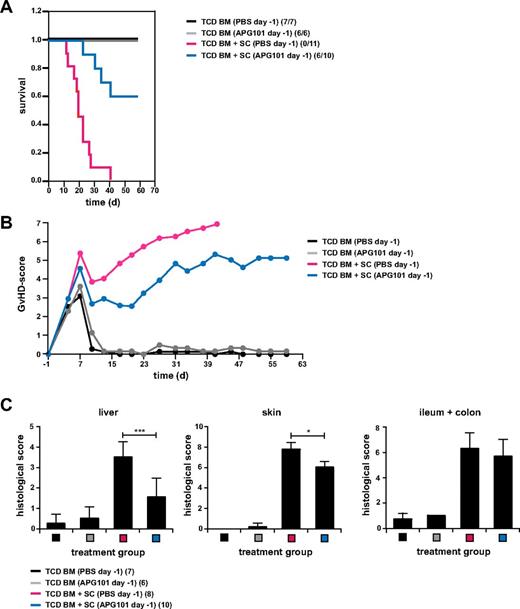
The B6→B6D2F1 BMT model resembles the haploidentical BMT, but in humans transplantations with differences in only miHAgs are more frequently performed. Therefore, we tested the effect of APG101 in a miHAg-mismatched model (B10.D2→BALB/c). Lethally irradiated BALB/c mice reconstituted with B10.D2-derived TCD BM and spleen cells and treated with PBS developed severe GVHD with increased GVHD scores and a 100% mortality after 41 days. Treatment with APG101 starting 1 day before transplantation diminished GVHD severity and most importantly rescued mice from lethal GVHD with 60% (Figure 3A-B). Mice transplanted with TCD BM alone and treated with PBS or APG101 survived and exhibited no signs of GVHD. Histologic liver GVHD was significantly decreased by APG101 treatment, whereas skin GVHD was only slightly diminished and no effect was observed in the intestine (Figure 3C). These data show that in 2 clinically relevant BMT models APG101 attenuated clinical and histologic GVHD and most importantly significantly improved the survival of transplanted animals.
APG101 significantly decreased GVHD-induced death in a minor histocompatibility-mismatched BMT model. (A-C) Lethally irradiated BALB/c recipient mice were reconstituted with TCD BM from B10.D2 mice with or without B10.D2-derived spleen cells (SCs) and were treated with PBS or APG101 starting 1 day before transplantation (day −1). Survival (A) and GVHD-scores (B) were determined and surviving animals/total animals treated are indicated in panel A. TCD BM+SC (PBS) versus TCD BM+SC (APG101; ***P < .001). Histologic signs of GVHD in liver, skin, and intestine of 6 to 10 animals/ treatment group were either determined the day mice were killed because of their moribund state and for all surviving mice at the end of the experiment. Data are from 1 experiment performed.
APG101 significantly decreased GVHD-induced death in a minor histocompatibility-mismatched BMT model. (A-C) Lethally irradiated BALB/c recipient mice were reconstituted with TCD BM from B10.D2 mice with or without B10.D2-derived spleen cells (SCs) and were treated with PBS or APG101 starting 1 day before transplantation (day −1). Survival (A) and GVHD-scores (B) were determined and surviving animals/total animals treated are indicated in panel A. TCD BM+SC (PBS) versus TCD BM+SC (APG101; ***P < .001). Histologic signs of GVHD in liver, skin, and intestine of 6 to 10 animals/ treatment group were either determined the day mice were killed because of their moribund state and for all surviving mice at the end of the experiment. Data are from 1 experiment performed.
APG101 treatment does not influence engraftment, homing, phenotype, or function of allogeneic T cells
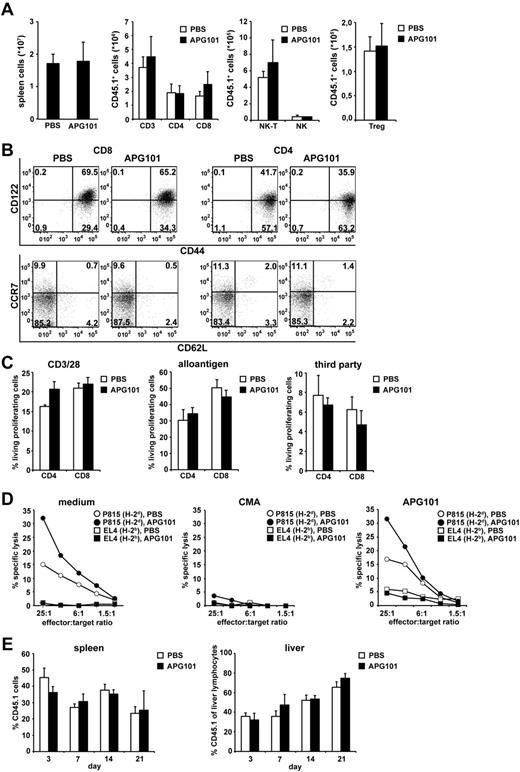
Because transplanted mature T cells support engraftment, promote early T-cell immunity and mediate the GVT effect, we tested whether APG101 treatment in vivo interferes with reconstitution of the hematopoietic compartment, homing, phenotype, and functionality of transplanted allogeneic T cells. Therefore, lethally irradiated B6D2F1 mice (H-2bxd, CD45.2+) were transplanted with B6-derived BM (H-2b, CD45.2+) and spleen cells from B6.SJL mice (H-2b, CD45.1+), and treated with APG101 or PBS starting on day −1. Using B6.SJL-derived spleen cells allows discrimination between transplanted mature allogeneic T cells and T cells developing of the transplanted hematopoietic stem cells by the congenic marker CD45.1. Fourteen days after transplantation mice were killed and T-cell numbers, phenotype, and function were analyzed. Independent of whether mice were treated with APG101 or PBS no differences in total spleen cell count or changes in the number of CD45.1+ CD4+, CD8+, regulatory T cells, NK, or NK-T cells were detected (Figure 4A). Transplanted CD4+ and CD8+ CD45.1+ T cells developed into effector/memory T cells (CD44+, CD122+, CCR7−, CD62L−; Figure 4B). No differences in the expression of adhesion molecules CCR9, LPAM-1, LFA-1, and CXCR3 were observed in transplanted CD45.1+CD4+ and CD8+ T cells independent whether mice were treated with APG101 or PBS (supplemental Figure 2A). Transplanted CD45.1+ T cells maintained their proliferative capacity toward CD3/CD28 activation, alloantigen, or third-party stimulation (Figure 4C) and produced Th1 and Th2 cytokines after allogeneic or PMA/Iono stimulation irrespective whether APG101 was present or not (supplemental Figure 2B). Most importantly, CD45.1+ splenic T cells exhibited efficient cytotoxic capacity toward the alloantigen-expressing tumor line P815, which was even slightly increased compared with CD45.1+ T cells from PBS-treated mice. Target-cell lysis was mediated by the perforin/granzyme system because addition of the vacuolar type H+-ATPase Concanamycin A (CMA), which inhibits the perforin-dependent killing pathway,28 abrogated specific cytotoxicity whereas addition of APG101 showed no effect (Figure 4D). APG101 treatment did not interfere with the homing capacity of CD45.1+ T cells because 3, 7, 14, or 21 days after transplantation the percentage of CD45.1+ T cells in spleen and liver (Figure 4E) as well as lymph nodes, thymus, bone marrow, and lung (data not shown) was comparable between PBS and APG101-treated mice. All together, these data clearly show that despite the inhibitory effects of APG101 on GVHD, alloantigen-specific T-cell function required for tumor cell rejection or early T-cell immunity is not impaired by APG101.
APG101 treatment did not affect donor engraftment, T-cell phenotype, function, or homing in allogeneic BM-transplanted animals. (A-E) Lethally irradiated B6D2F1 recipient mice (H-2bxd, CD45.2+) were reconstituted with BM from B6 mice (H-2b, CD45.2+) plus B6.SJL-derived spleen cells (H-2b, CD45.1+) and were treated with PBS or APG101 starting 1 day before transplantation. Fourteen days after transplantation mice were killed and spleen cells were analyzed for total cell count and the numbers of CD45.1+ T, NK, NK-T cells, and Treg (A) and T-cell phenotype (B) by flow cytometry. To measure the proliferative capacity and cytotoxicity of allogeneic T cells, CD45.1+ T cells were purified from spleens of PBS and APG101-treated mice 14 days after transplantation. CFSE-labeled T cells were stimulated in vitro with anti-CD3 and CD28 antibodies, irradiated DBA/2 (H-2d) spleen cells (alloantigen) or C3H (H-2k) spleen cells (third party). Proliferation of CD3/CD28-activated CD4+ and CD8+ CD45.1+ T cells was measured after 4 days, whereas allogeneic and third-party proliferation was determined after 6 days (C). Cytotoxic specificity of splenic CD45.1+ T cells was analyzed on P815 and EL4 cells in chromium release assays. Addition of CMA (10nM) or APG101 (100 ng/mL) during the assay indicates the cytotoxic effector mechanisms used (D). Different days after transplantation, spleen, and liver of transplanted mice were analyzed for the percentage of infiltrating CD45.1+ T cells by flow cytometry (E). Data represent the mean value ± SD of 6 mice analyzed in panel A, of triplicates from CD45.1+ T cells, which were re-isolated and pooled from spleens of 30 mice treated in panel C, of triplicates from spleen cells, which were pooled from 30 mice treated and adjusted for an effector-target ration of CD45.1+ T cells:target cells as described in M+M in panel D, and the mean values ± SD of 3 mice analyzed in panel E. (B) is representative for 1 mouse of 6 analyzed. All data are representative for 1 experiment of at least 2 experiments performed. No statistical significant differences between APG101 or PBS-treated animals were detected in all experiments performed.
APG101 treatment did not affect donor engraftment, T-cell phenotype, function, or homing in allogeneic BM-transplanted animals. (A-E) Lethally irradiated B6D2F1 recipient mice (H-2bxd, CD45.2+) were reconstituted with BM from B6 mice (H-2b, CD45.2+) plus B6.SJL-derived spleen cells (H-2b, CD45.1+) and were treated with PBS or APG101 starting 1 day before transplantation. Fourteen days after transplantation mice were killed and spleen cells were analyzed for total cell count and the numbers of CD45.1+ T, NK, NK-T cells, and Treg (A) and T-cell phenotype (B) by flow cytometry. To measure the proliferative capacity and cytotoxicity of allogeneic T cells, CD45.1+ T cells were purified from spleens of PBS and APG101-treated mice 14 days after transplantation. CFSE-labeled T cells were stimulated in vitro with anti-CD3 and CD28 antibodies, irradiated DBA/2 (H-2d) spleen cells (alloantigen) or C3H (H-2k) spleen cells (third party). Proliferation of CD3/CD28-activated CD4+ and CD8+ CD45.1+ T cells was measured after 4 days, whereas allogeneic and third-party proliferation was determined after 6 days (C). Cytotoxic specificity of splenic CD45.1+ T cells was analyzed on P815 and EL4 cells in chromium release assays. Addition of CMA (10nM) or APG101 (100 ng/mL) during the assay indicates the cytotoxic effector mechanisms used (D). Different days after transplantation, spleen, and liver of transplanted mice were analyzed for the percentage of infiltrating CD45.1+ T cells by flow cytometry (E). Data represent the mean value ± SD of 6 mice analyzed in panel A, of triplicates from CD45.1+ T cells, which were re-isolated and pooled from spleens of 30 mice treated in panel C, of triplicates from spleen cells, which were pooled from 30 mice treated and adjusted for an effector-target ration of CD45.1+ T cells:target cells as described in M+M in panel D, and the mean values ± SD of 3 mice analyzed in panel E. (B) is representative for 1 mouse of 6 analyzed. All data are representative for 1 experiment of at least 2 experiments performed. No statistical significant differences between APG101 or PBS-treated animals were detected in all experiments performed.
GVT effect is maintained in APG101-treated mice after allogeneic BMT
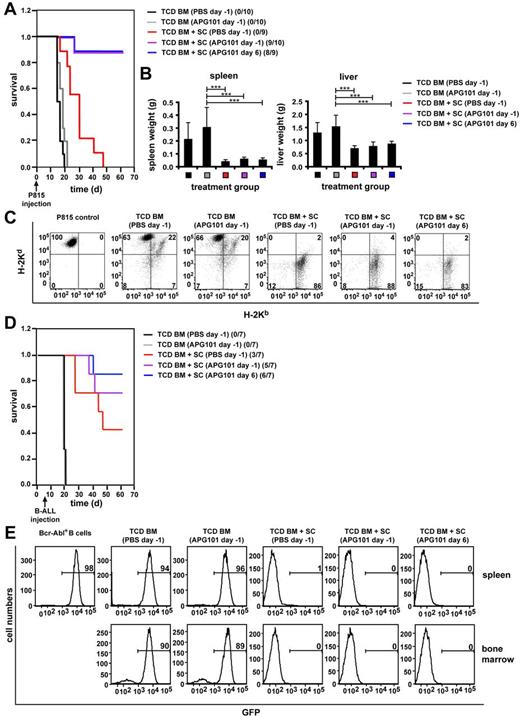
Because APG101 did not abrogate effector functions of transplanted allogeneic T cells and the CD95/CD95L system may be dispensable for tumor rejection11,13 we tested the antitumor effect of alloantigen-specific T cells in APG101-treated mice. Lethally irradiated B6D2F1 mice were reconstituted with T cell–depleted BM (TCD BM) and spleen cells from B6 mice and coinjected with P815 (H-2d) mastocytoma cells, which preferentially home to BM, spleen, and liver. APG101 treatment was either started 1 day before BMT (day −1) or 6 days after transplantation (day 6). H-2d–expressing P815 cells were detected by flow cytometry and an increase in liver and spleen weights. Between day 17 and 20 after transplantation all mice injected with P815 cells and TCD BM in the absence of immunocompetent spleen cells died (Figure 5A) because of tumor burden reflected by significantly increased liver and spleen weights (Figure 5B) and detection of H-2d single-positive cells in liver (Figure 5C) and spleen (data not shown). APG101 had no direct effect on tumor growth because survival times in APG101-treated and PBS-treated TCD BM-transplanted mice were similar. Most importantly, mice receiving APG101 together with TCD BM and allogeneic spleen cells exhibited a survival of 95% independent whether treatment was started on day −1 or 6 (Figure 5A). Death of 1 mouse in each APG101-treated group was not caused by tumor growth because no tumor cells could be detected in target organs by flow cytometry but increased GVHD-scores indicating GVHD development (data not shown). Analysis of all surviving animals at the end of the experiment showed no increase in organ weights and no detection of tumor cells by flow cytometry (Figure 5B-C). Mice reconstituted with TCD BM plus spleen cells and treated with PBS were tumor-free but died until day 50 because of GVHD induction indicated by elevated GVHD scores (supplemental Figure 3A). As BMT is a curative therapy for leukemias, we tested whether APG101 treatment did also not affect the antitumor response toward Bcr-Abl–transformed primary B-ALL cells coexpressing GFP.24 B-ALL cells are highly aggressive because injection of 40 to 600 cells/mouse kills all animals between days 10 and 23 dependent on the individual experiment (data not shown). To prevent that tumor-mediated death is induced before the animals start to reconstitute their hematopoietic compartment we injected the tumor cells 7 days after transplantation. Treatment of APG101 was started either 1 day before (day −1) or 6 days (day 6) after transplantation. Independent whether APG101 or PBS was injected, mice receiving B-ALL cells in the absence of transplanted immunocompetent spleen cells died around 13 days after tumor injection at day 20 or 21 (Figure 5D) confirmed by high tumor loads of GFP+ cells in spleen and bone marrow (Figure 5E). Most importantly, all animals receiving APG101 treatment and transplanted with BM and spleen cells remained 100% tumor-free indicated by the absence of tumor cells in spleen and bone marrow (Figure 5E). Survival was improved (71% day −1, 86% day 6) compared with PBS-treated mice (43%). Death of APG101-treated mice was not because of tumor growth but induced by GVHD. Mice receiving BM and spleen cells and treated with PBS were also all tumor-free, but developed GVHD indicated by elevated GVHD scores (supplemental Figure 3B). No interference of APG101 with the GVT effect was also detected in the miHAg-mismatch BMT model. Lethally irradiated BALB/c mice reconstituted with TCD BM in the absence of immunocompetent cells, coinjected with Bcr-Abl+ B-ALL, and treated with PBS or APG101 died of tumor development indicated by more than 75% of tumor cells in spleen, BM, and liver. Cotransplantation of spleen cells totally prevented tumor development independent whether mice were treated with PBS or APG101 (supplemental Figure 4). All tumor experiments convincingly show that APG101 treatment does not abrogate the antitumor effect of transplanted allogeneic T cells. Taken together, our experiments show that the CD95/CD95L-pathway inhibitor APG101 efficiently prevents CD95L-mediated GVHD development in allogeneic BM-transplanted animals, whereas GVT activity and T-cell functions are preserved.
APG101 treatment does not prevent GVT activity. (A-E) Lethally irradiated B6D2F1 mice were transplanted with B6-derived TCD BM in the presence or absence of B6-derived spleen cells (SCs) and treated with PBS starting 1 day before transplantation (day −1) or APG101 starting 1 day before (day −1) or 6 days after (day 6) transplantation. P815 mastocytoma cells were coinjected on day 0 (A-C), whereas injection of GFP-expressing B-ALL cells was performed 7 days after transplantation (D-E). P815-injected mice were analyzed for survival (A) and surviving animals/total animals treated are noted. TCD BM+SC (PBS day −1) versus TCD BM+SC (APG101 day −1) ***P < .001; versus TCD BM+SC (APG101 day 6), ***P < .001. Spleen and liver weights (B) were determined either the day mice were killed because of their moribund state or at the end of the experiment (***P < .001). Tumor manifestation was also determined by flow cytometry of liver lymphocytes. (C) FACS analysis is shown for 1 representative mouse of at least 3 mice analyzed at the end of the experiment (for mice transplanted with TCD BM plus SCs and treated with APG101) or the day mice were killed because of their moribund state (for mice transplanted with TCD BM alone or TCD BM plus SCs and treated with PBS). (D) B-ALL injected mice were analyzed for survival and surviving animals/total animals treated are indicated. TCD BM+SC (PBS day−1) versus TCD BM+SC (APG101 day −1), P = .3ns; versus TCD BM+SC (APG101 day 6), P = .1ns. (E) Tumor manifestation by GFP-expression in bone marrow and spleen is shown by flow cytometry for 1 representative mouse of at least 3 mice analyzed either at the end of the experiment (for mice transplanted with TCD BM plus SCs and treated with APG101) or the day mice were killed because of their moribund state (for mice transplanted with TCD BM alone or TCD BM plus SCs and treated with PBS).
APG101 treatment does not prevent GVT activity. (A-E) Lethally irradiated B6D2F1 mice were transplanted with B6-derived TCD BM in the presence or absence of B6-derived spleen cells (SCs) and treated with PBS starting 1 day before transplantation (day −1) or APG101 starting 1 day before (day −1) or 6 days after (day 6) transplantation. P815 mastocytoma cells were coinjected on day 0 (A-C), whereas injection of GFP-expressing B-ALL cells was performed 7 days after transplantation (D-E). P815-injected mice were analyzed for survival (A) and surviving animals/total animals treated are noted. TCD BM+SC (PBS day −1) versus TCD BM+SC (APG101 day −1) ***P < .001; versus TCD BM+SC (APG101 day 6), ***P < .001. Spleen and liver weights (B) were determined either the day mice were killed because of their moribund state or at the end of the experiment (***P < .001). Tumor manifestation was also determined by flow cytometry of liver lymphocytes. (C) FACS analysis is shown for 1 representative mouse of at least 3 mice analyzed at the end of the experiment (for mice transplanted with TCD BM plus SCs and treated with APG101) or the day mice were killed because of their moribund state (for mice transplanted with TCD BM alone or TCD BM plus SCs and treated with PBS). (D) B-ALL injected mice were analyzed for survival and surviving animals/total animals treated are indicated. TCD BM+SC (PBS day−1) versus TCD BM+SC (APG101 day −1), P = .3ns; versus TCD BM+SC (APG101 day 6), P = .1ns. (E) Tumor manifestation by GFP-expression in bone marrow and spleen is shown by flow cytometry for 1 representative mouse of at least 3 mice analyzed either at the end of the experiment (for mice transplanted with TCD BM plus SCs and treated with APG101) or the day mice were killed because of their moribund state (for mice transplanted with TCD BM alone or TCD BM plus SCs and treated with PBS).
Discussion
The presence of allogeneic T cells in BM transplants ensures early T-cell immunity and antitumor cytotoxicity but severely increases the risk of GVHD. Interfering with the CD95/CD95L pathway represents one possibility to dissect these 2 T-cell effector functions. Treatment of allogeneic BM-transplanted animals with APG101, a human fusion protein, which prevents CD95L binding to its cognate receptor and therefore disrupts cell death induction in CD95-expressing target cells successfully prevented GVHD development in BMT models across major and minor histocompatibility barriers. Most importantly, however, transplanted allogeneic T cells remained functional because graft-versus-tumor activity and effector functions were preserved. To our knowledge, we show here for the first time, that a therapeutically applicable CD95/CD95L pathway inhibitor, which is already used for treating glioma patients in a clinical phase 2 study, can be applied successfully in GVHD prophylaxis without abrogating GVT activity and T-cell immunity.
Treatment of allogeneic BM-transplanted animals with 2 weekly injections of APG101 efficiently prevented clinical GVHD and disease-induced morbidity in a major and a minor HAg-mismatched BMT model. APG101 treatment was successful when started 1 day before or 6 days after transplantation. Interestingly, T-cell infiltration and GVHD-associated histologic damages were not completely prevented. In line with this finding, clinical symptoms do not reliably correlate with the presence of gastrointestinal histopathology in human GVHD. This may indicate that the injected dose was not sufficient to completely eliminate all features of GVHD, or alternatively, that mechanisms other than CD95-mediated apoptosis contribute to histologic and/or clinical GVHD. APG101 treatment starting at day 13 after BMT was completely inefficient to prevent GVHD. This suggests either that target tissue destruction at day 13 is already advanced to such an extent that APG101 treatment cannot rescue organ function anymore or that GVHD at this time point is already driven by T cell– and CD95-independent mechanisms. In earlier studies prevention of CD95/CD95L-induced GVHD was achieved to some extent with anti-CD95L antibodies or using CD95L-deficient T cells as allogeneic effector cells.10,11,13,29 Although anti-CD95L antibody treatment reduced morbidity to 50% and was accompanied by a strongly extenuated weight loss, marked hepatic lymphocyte infiltration was still present in antibody-treated mice.29 In this study CD95L antibody injections were superior to human Fas-Fc treatment.29 In our experiments, however, APG101 treatment rescued 60% to 100% of the mice from GVHD-induced cell death. Inhibition of GVHD was also reported after injections of a nontoxic anti-CD95 antibody mediating apoptosis of transplanted recipient-activated T cells30 suggesting that interference with the CD95 system in fact is effective in several models.
Defining effector mechanisms for GVHD induction, however, appears to be model system dependent. Contributions of the CD95/CD95L or the perforin/granzyme system to GVHD induction are partially defined by the type of MHC disparities between host and recipient. Whereas the CD95/CD95L pathway plays a predominant role in class II mismatches, the perforin/granzyme system contributes efficiently to GVHD induction in class I differences.27,31 APG101 efficiently prevents GVHD in major and minor histocompatibility mismatched models where GVHD development is dependent on the presence of CD4+ T cells in the transplant31-33 but is inefficient in the B6→B6.bm1 model, in which GVHD induction is preferentially mediated by perforin/granzyme and CD8+ T cells.27,34,35 However, T-cell deficiency in perforin and CD95L does not necessarily abrogate GVHD development36,37 indicating that other cytotoxic molecules, such as TRAIL or TNF-α, might also contribute to disease development.10,11,15,37
To our knowledge none of the studies analyzed the effect of disrupting CD95/CD95L interaction on antitumor cytotoxicity, donor engraftment or T-cell effector function. Here, we show that preventing CD95-mediated apoptosis by APG101 had no effect on the GVT activity of transplanted allogeneic T cells. Independent whether or not mice were treated with APG101, tumor cells were totally eradicated as soon as the transplant contained mature T cells. Not only the mouse mastocytoma cell line P815 was efficiently rejected but also primary Bcr-Abl transduced B cells, which are highly aggressive because injection of 40 tumor cells induced 100% mortality after 14 days.7 Differences in the effect of APG101 on allogeneic T-cell effector functions, which induce host tissue destruction on the one hand and tumor cell eradication on the other hand, can probably be explained by the requirement of specific effector molecules for cell death in certain cell types. Tumor-cell destruction can be predominantly dependent on the presence of one cytotoxic mechanism but often CD95L and perforin-dependent killing can compensate for the absence of the other.14,38,39 Apparently, P815 and Bcr-Abl+ B-ALL tumor-cell eradication does not predominantly require CD95L, because APG101 did not interfere with antitumor cytotoxicity, which was further supported by our in vitro data showing that allogeneic CTL-mediated tumor cell lysis cannot be blocked by APG101 but is totally abrogated by CMA. Although the contribution of TRAIL in GVHD and GVT effect is still under discussion,5,14,15 TRAIL seems to play only an inferior role in our BMT system.
Because the CD95/CD95L complex is now increasingly recognized as a system that does not only signal apoptosis but also mediates nonapoptotic cell-type specific suppression or activation depending on the environment and costimulation,18,20,40,41 we investigated the effect of APG101 on donor engraftment and T-cell function and homing. Allogeneic T cells from APG101-treated mice were phenotypically indistinguishable from T cells developing in PBS-injected animals and donor engraftment of CD4+ and CD8+ T, NK, and NK-T cells was similar. Even Treg numbers were identical although these cells are susceptible to homeostatic control by CD95.42 Although CD95L activation is known to be a possible modulator of T-cell expansion,21,22 re-isolated allogeneic T cells from APG101-treated mice showed no changes in proliferation toward alloantigen or third-party antigens compared with T cells isolated from GVHD suffering mice. T-cell expansion in APG101-treated mice was further confirmed by efficient T-cell invasion of host tissues in vivo, which was reported to be impaired if CD95L-deficient T cells were used as allogeneic effector cells.43,44 Taken together, these data indicate that APG101 predominantly prevents apoptosis of CD95-expressing target organs, which was confirmed by a decrease of active capsase-3–expressing cells in liver and intestine and the inefficiency of APG101 to prevent GVHD in a perforin/granzyme-dependent BMT model.
Because APG101 prevents CD95L-mediated GVHD in murine allogeneic BMT models and does not inhibit the antitumor cytotoxicity of allogeneic T cells it might be a potential therapeutic reagent for prevention or even treatment of human GVHD after allogeneic BMT. However, although murine GVHD models are well defined by their dependence on either the CD95/CD95L or perforin/granzyme system,27 the situation in humans appears to be more complex. Elevated CD95L expression was detected in patients with acute and chronic GVHD, however perforin and granzyme B serum levels were also increased indicating that both effector pathways contribute to human GVHD.45-48 Applying APG101 as a mono therapy in humans might therefore not be sufficient for GVHD inhibition and probably requires the administration of other immunosuppressive drugs.
In summary, we showed that APG101 is an effective therapeutic reagent to prevent CD95L-mediated GVHD but preserves antitumor cytotoxicity and early T-cell immunity. These data suggest that APG101 could be incorporated into protocols for GVHD prevention and treatment during BMT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sarah Gehring for excellent technical assistance.
This work was supported in part by research funding from Apogenix GmbH, Heidelberg, Germany.
Authorship
Contribution: N.H. performed experiments, analyzed data, and reviewed the paper; J.J.M. performed experiments, analyzed data, prepared the figures, and reviewed the paper; F.L. performed histologic analysis, analyzed data, and reviewed the paper; M.W. performed experiments; M.K. provided APG101, designed research, and reviewed the paper; H.F. provided APG101 and reviewed the paper; K.-M.D. obtained funding, designed research, and reviewed the paper; and G.S. designed research, wrote the paper, and obtained funding for the project.
Conflict-of-interest disclosure: N.H. received research funding from Apogenix GmbH; M.K. is employed by Apogenix GmbH; H.F. is employed by Apogenix GmbH and has ownership interests; K.-M.D. obtained research funding and has consulted within the past 2 years for Apogenix GmbH; K.-M.D. received research funding and has consulted within the past 2 years for Apogenix GmbH; and G.S. obtained research funding from Apogenix GmbH. The remaining authors declare no competing financial interests.
Correspondence: Gudrun Strauss, University Medical Center Ulm, Dept of Pediatrics and Adolescent Medicine, Eythstr 24, 89075 Ulm, Germany; e-mail: gudrun.strauss@uniklinik-ulm.de.
References
Author notes
N.H. and J.J.M contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal