Key Points
Polymorphisms in the MTX pathway genes substantially influence the kinetics and response to high-dose MTX therapy in childhood ALL.
Germline variants in SLCO1B1, thymidylate synthase, and methylenetetrahydrofolate reductase are significantly associated with kinetics, toxicity, and outcome.
Abstract
The pharmacogenetics of methotrexate (MTX) was investigated in a large cohort of pediatric patients with acute lymphoblastic leukemia (ALL). Four hundred ninety-nine children with ALL from the ALL-BFM (Berlin–Frankfurt–Münster) 2000 trial who received 1996 courses of MTX at 5 g/m2 were genotyped for 8 single nucleotide polymorphisms in 5 candidate genes of the MTX/folate pathway. Patients’ MTX pharmacokinetics, MTX toxicities, and outcomes were correlated with the genotypes. The interindividual variability in MTX kinetics had a substantial genetic component between 68% and 75%. The SLCO1B1 rs4149056 variant was significantly associated with MTX kinetics. In a multiple regression model, MTX area under the concentration time curve (AUC)0-48h increased by 26% (P < .001) per SLCO1B1 rs4149056 C allele. MTX AUC0-48h was a significant predictor of overall toxic adverse events during MTX courses (R2 = 0.043; P < .001), whereas the thymidylate synthase rs34743033 tandem repeat polymorphism was predictive of stomatitis (R2 = 0.018; P = .009), a frequent side effect of high-dose MTX. Multiple Cox regression analyses revealed an association of minimal residual disease (hazard ratio 7.3; P < .001) and methylenetetrahydrofolate reductase rs1801131 (hazard ratio 3.1; P = .015) with event-free survival in the ALL-BFM 2000 study population. Genetic variations substantially influence the kinetics and response to high-dose MTX therapy in childhood ALL.
Introduction
The outcome for pediatric cancer patients has improved dramatically over the past 6 decades, with 5-year survival rates currently at 75% vs 20% in the 1950s.1,2 Accordingly, the cure rates of acute lymphoblastic leukemia (ALL), which is the most common childhood cancer, are higher than 80% due to optimized chemotherapy protocols.3,4 Most protocols consist of methotrexate (MTX) as a key chemotherapeutic agent with high therapeutic efficiency. Nevertheless, MTX causes severe dose-limiting adverse events and organ toxicities.5 The present study performed by the Late Effects Surveillance System study group aimed to identify genetic polymorphisms in candidate genes of the MTX pathway associated with MTX pharmacokinetics, toxicity, and outcome.
MTX enters the cell via the reduced folate carrier (solute carrier family 19 member 1, SLC19A1) or the solute carrier organic anion transporter 1B1 (SLCO1B1).6,7 In the cytoplasm, MTX is polyglutamated by folylpolyglutamate synthase, which enhances its retention inside the cell.8 Both MTX and MTX–polyglutamate inhibit dihydrofolate reductase, an enzyme that catalyzes the conversion of dihydrofolate into tetrahydrofolate, which is the active form of folic acid. Tetrahydrofolate is involved in many single-carbon transfer reactions, including the synthesis of DNA and RNA nucleotides. Inhibition of dihydrofolate reductase causes depletion of intracellular tetrahydrofolate, which has a cytotoxic effect, particularly on rapidly dividing cells.9 MTX-polyglutamate further inhibits thymidylate synthase (TYMS) and can also interfere with methylenetetrahydrofolate reductase (MTHFR), both of which contribute to MTX’s cytotoxic effects.10,11 MTX is eliminated from the cell via cellular efflux transporters, such as the ATP-binding cassette transporter ABCC2, also known as multidrug-resistance–associated protein 2, which export drugs against concentration gradients at the expense of ATP hydrolysis.12
Several single nucleotide polymorphisms (SNPs) in these candidate genes that are involved in the folate pathway have been implicated in the kinetics and effects of MTX in previous studies. Although previous work has constituted an important step in unraveling the pharmacogenetics of MTX, reduced statistical power due to small study cohorts, heterogeneous study populations, and/or treatment protocols or replication failure limit its significance.13-16 The aim of the present study was to confirm positive associations in a large homogenous subpopulation from the multicenter ALL-BFM (Berlin–Frankfurt–Münster) 2000 trial. The primary endpoint was the effect of genetic variants in candidate genes on MTX pharmacokinetics. Because altered MTX pharmacokinetics may affect adverse drug reactions and treatment efficacy, overall toxicity, stomatitis (which is a frequent adverse event specifically of high-dose MTX), and event-free survival (EFS, time from diagnosis to relapse, secondary neoplasm, or death from any cause) were included as secondary endpoints in an explorative analysis. Based on the strength of evidence of previously published association studies, the following candidate genetic variants were selected for our analysis: rs4149056, rs2306283, and rs11045879 in SLCO1B1; rs717620 in ABCC2; rs1051266 in SLC19A1; rs1801131 and rs1801133 in MTHFR; and rs34743033 in TYMS.
All patients of our study cohort were similarly treated with MTX regarding the ALL-BFM 2000 protocol for patients within the standard risk (SR), medium risk, and slow early responder (SER) minimal residual disease (MRD) class, thereby excluding interacting effects of protocol inconsistency on MTX pharmacokinetics and response.
Methods
Study population
Between August 1, 1999, and November 30, 2005, 2300 patients with ALL (aged ≥1 to <18 years) were enrolled in the ALL-BFM 2000 trial (ClinicalTrials.gov: NCT00430118) in Germany. The institutional review board of the Hannover Medical School approved the study and informed consent was obtained from patients and/or their legal guardians in accordance with the Declaration of Helsinki. Diagnostics and treatment arm assignments were performed according to the ALL-BFM 2000 protocol.17 Treatment arm assignment was based on MRD analysis after induction (day 33) and consolidation therapy (day 78) and the presence of clinical high-risk (HR) criteria (Table 1). MRD stratification required at least 2 polymerase chain reaction (PCR)-MRD targets with a sensitivity of at least 10−4. MRD was analyzed by real-time quantitative–PCR reaction analysis of “leukemia-specific” junctional regions of rearranged immunoglobulin genes and T-cell receptor genes, which can be considered as “DNA fingerprints” of the leukemic cells. Molecular marker identification was patient specific and failed in some patients. If MRD evaluation was not available, patients were assigned to the intermediate treatment arm or, based on clinical parameters, to the HR group. A detailed description is provided in the supplemental data.
MRD class stratification and postinduction therapy assignment
| MRD class . | MRD day 33 (TP1) . | MRD day 79 (TP2) . | HR criteria* . | Postinduction treatment arm . |
|---|---|---|---|---|
| MRD-SR | Negative | Negative | No | SR protocol |
| MRD-MR | Positive | Negative | No | IR protocol |
| Positive, low MRD level (<10−3) | Positive, low MRD level (<10−3) | No | ||
| MRD-SER | Positive, high MRD level (≥10−3) | Positive, low MRD level (<10−3) | No | |
| −/− | MRD not available | MRD not available | No | |
| MRD-HR | Positive | Positive, high MRD level (≥10−3) | No | HR protocol |
| Variable | Negative or positive or not available | Negative or positive or not available | Yes |
| MRD class . | MRD day 33 (TP1) . | MRD day 79 (TP2) . | HR criteria* . | Postinduction treatment arm . |
|---|---|---|---|---|
| MRD-SR | Negative | Negative | No | SR protocol |
| MRD-MR | Positive | Negative | No | IR protocol |
| Positive, low MRD level (<10−3) | Positive, low MRD level (<10−3) | No | ||
| MRD-SER | Positive, high MRD level (≥10−3) | Positive, low MRD level (<10−3) | No | |
| −/− | MRD not available | MRD not available | No | |
| MRD-HR | Positive | Positive, high MRD level (≥10−3) | No | HR protocol |
| Variable | Negative or positive or not available | Negative or positive or not available | Yes |
HR, high risk; IR, intermediate risk.
HR criteria: prednisone poor response (≥1000 leukemic blasts per microliter in the peripheral blood on day 8), or failure to achieve remission (ie, with >5% leukemic blasts in the BM on day 33, or persistent extramedullary disease) after induction phase IA (induction failure), or positivity for MLL/AF4 fusion transcript.
Standard- and intermediate-risk patients from the ALL-BFM 2000 cohort who received 4 courses of high-dose MTX (protocol M) were included if diagnostic bone marrow (BM) samples were obtained during phase I/2 of remission-induction therapy (study day 52) and DNA extracted from these BM samples was available from the German ALL-BFM biological specimen bank. A total of 499 patients were eligible according to these criteria (Figure 1).
Consolidated Standards of Reporting Trials (CONSORT) diagram of entry criteria and study cohort characteristics for the study population.
Consolidated Standards of Reporting Trials (CONSORT) diagram of entry criteria and study cohort characteristics for the study population.
The racial/ancestral status of the patients was not recorded and thus is unknown. Extrapolating from the proportions of ethnic groups in the general German population, we would expect that the vast majority (>95%) of the study participants are white. Consequently, stratification according to the racial/ancestral status was not expected to have a major impact on our results.
Treatment and clinical data
Patients were treated according to the ALL-BFM 2000 protocol. During the extracompartment phase (protocol M), patients received 4 courses of MTX every 2 weeks at a dose of 5000 mg/m2 body surface area in each course. MTX was given as a 500-mg/m2 loading infusion over 0.5 hour followed by a 4500-mg/m2 intravenous infusion for another 23.5 hours. MTX administration, leucovorin rescue, intravenous hydration, urinary alkalinization therapy, and monitoring were standardized according to the ALL-BFM 2000 study protocol (supplemental Table 1). The MTX dose was reduced in children with Down syndrome (trisomy 21) because of their suspected poor tolerance to antineoplastic drugs (n = 5). In cases (n = 19) when children experienced severe MTX toxicity with the standard dose, dose adjustments were made in subsequent courses.
MTX toxicities during each MTX course were reported by applying a 5-step scoring scheme (0 = none to 4 = highest grade of toxicity) based on the National Cancer Institute (NCI) toxicity criteria (supplemental Table 2).
Selection of polymorphisms for genotyping
Genetic variants were selected based on the strength of evidence of published association studies. For instance, there is a huge body of work on the nonsynonymous variants rs1801131 and rs1801133 in the MTHFR gene, which were associated with a lower probability of EFS or hepatic toxicity.18-22 rs1051266 in SLC19A1, resulting in a less efficient transporter, has been associated with more adverse events and worse overall prognosis.23-26 A polymorphic tandem repeat in the 5′UTR of the TYMS enhancer region (rs34743033) was associated with a significantly greater chance of response to treatment in 205 ALL patients treated with MTX.27 A genome-wide association study implicated the intronic SNP rs11045879 in the SLCO1B1 transporter gene, which was in linkage disequilibrium (LD) with the functional SLCO1B1 SNP rs4149056, with MTX clearance and gastrointestinal toxicity in 640 ALL patients.28 The nonsynonymous SNP rs2306283 in SLCO1B1 was also recognized as a predictor of MTX clearance in 434 children with ALL.15 Our own results suggest an association of the nonsynonymous SNP rs717620 in ABCC2 with MTX kinetics,16 which demanded replication in a large cohort.
Sample size calculation
The sample size depends on the primary endpoint (MTX kinetics, eg, MTX clearance), the method of calculating the test statistic (multiple linear regression analysis), and the proposed effect size of the predictive variables (R2, ie, the amount of variance in the dependent variable that can be explained by the predictive variables). The following predictive variables were suggested: SLCO1B1 (rs4149056, rs2306283, and rs11045879), SLC19A1 (rs1051266), ABCC2 (rs717620), MTHFR (rs1801131 and rs1801133), and TYMS (rs34743033) genotypes, age, sex, and MRD class. For the a priori analysis, we used an estimate of the effect size from previous research. According to Treviño et al15 the 2 most powerful predictors of MTX clearance were the treatment regimen (R2 = 17.9%) and the SLCO1B1 genotypes (R2 = 9.3%). Because the treatment regimen was similar for all patients in our ALL-BFM 2000 study population (and thus was not considered as a predictive variable), we defined R2 = 9.3% as the anticipated effect size of our study. With these preconditions the minimum required sample size at a nominal type I error rate of 0.01 and a power level of 90% was 312. We exceeded the required sample size by inclusion of N = 415 patients.
During analysis of the data we recognized that the white blood cell (WBC) count significantly differed between our subcohort and the total ALL-BFM 2000 cohort. To avoid any bias, we considered WBC count as an additional predictive variable. Reestimation of the statistical power with WBC count as an additional predictive variable revealed that the multiple regression analysis provided a power of 98% for detecting the prespecified effect of R2 = 9.3% of the predictive variables on MTX clearance (at a nominal type I error rate of 0.01) in our study population (N = 415).
Genotyping
DNA was extracted from remission BM samples by using the QIAamp DNA Blood Midi Kit (Qiagen GmbH, Hilden, Germany) and stored at −20°C until use. Genotyping for rs4149056, rs11045879, and rs2306283 in SLCO1B1; rs717620 in ABCC2; rs1801131 and rs1801133 in MTHFR was carried out using the TaqMan Pre-Developed Assay Reagents for Allelic Discrimination (assay ID: C__30633906_10, C__31106904_10, C___1901697_20, C___2814642_10, C____850486_20, C___1202883_20; Applied Biosystems, Foster City, CA). SLC19A1 rs1051266 was genotyped with the following primers and probes: (forward) 5′-GCCTGACCCCGAGCT-3′, (reverse) 5′-CATGAAGCCGTAGAAGCAAAGGTA-3′, (probe 1) 5′VIC-ACACGAGGTGCCGCC-3′, (probe 2) 5′FAM-ACGAGGCGCCGCC-3′. Amplification was performed in a final volume of 6 μL containing 5 ng DNA, 4.5 pmol of each primer, 1.0 pmol of each probe, 3 μL of 2× Type-It Fast Genotyping Master Mix (contains buffer, passive reference dye ROX, deoxynucleotides, and Taq DNA polymerase; Qiagen, Hilden, Germany), and 1.2 µL of Q-Solution (Qiagen, Hilden, Germany) by use of the ABI Prism Sequence Detector 7900 (Applied Biosystems). Cycle parameters were as follows: initial denaturation at 95°C for 5 minutes and then 45 cycles at 95°C for 15 seconds and at 60°C for 30 seconds. After PCR, fluorescence yield for the 2 dyes was measured. SDS 2.1 software (Applied Biosystems) was used to plot and automatically call genotypes on the basis of a 2-parameter plot with fluorescence intensities of FAM and VIC. The 28 bp tandem repeat polymorphism in TYMS (rs34743033) was genotyped according to the method of Horie et al29 using the sense primer 5′-GTGGCTCCTGCGTTTCCCCC-3′ and the reverse primer 5′-GCTCCGAGCCGGCCACAGGCATGGCGCGG-3′. PCR amplicons were analyzed using 3% agarose gel electrophoresis. The expected PCR product sizes were 214 bp (2 repeats), 242 bp (3 repeats), and 270 bp (4 repeats).
Each SNP was tested for conformance of genotype frequencies to those expected under Hardy-Weinberg equilibrium with a χ² goodness-of-fit test. LD was calculated with Haploview (version 4.2; Broad Institute, Cambridge, MA).30
Pharmacokinetics
The MTX area under the concentration time curve (AUC)0-48h, peak MTX plasma concentration at the end of infusion (C24h), and MTX clearance were estimated based on at least 4 MTX plasma concentrations per course up to 48 hours from the start of infusion. Individual pharmacokinetic data were analyzed using the software package Phoenix WinNonlin/NLME (version 6.2.1, Pharsight Corp, St. Louis, MO). Plasma concentration data were fit to a 2-compartment infusion model with first-order elimination. Both dosing intervals (0–0.5 hours and 0.5–24 hours) with individual dosing rates were included in the model. Initial estimates (for apparent volume of distribution and rate constants between compartments) were derived from previous publications and set to V = 10 L, ke = 0.5 h−1, k12 = 0.07 h−1, and k21 = 0.12 h−1.13 Clearance was calculated from primary estimates, and AUC values were obtained from model-predicted concentration time courses. In rare cases when patients did not receive the standard MTX dose of 5000 mg/m2, for the purpose of comparison, AUC and C24h values were dose normalized. We assumed that MTX kinetics are approximately linear over the dose range, that is, the plasma concentration that results from the dose is proportional to that dose and the rate of elimination of the drug is proportional to the concentration. Individual parameters were modeled for each of the 4 high-dose methothrexate courses, and corresponding results were transferred to statistical analysis.
Genetic component rGC
The repeated drug application methodology can be used to estimate heritability without using a twin study design.31,32 This method relies on data obtained from studies in which a group of individuals receives an identical dose of a drug on 2 or more occasions. Also, it allows for the calculation of variance (ie, SD2 [standard deviation]) in a pharmacokinetic parameter within an individual and between individuals. The genetic component (rGC) as a measure of heritability of MTX kinetics was calculated according to the following equation: rGC = (SDb2 − SDw2)/SDb2, where SDb2 is the SD between patients (influenced mainly by variation in environmental factors, genetic factors, and measurement errors) and SDw is the SD within patients who received MTX at 4 cycles (influenced only by environmental factors and measurement errors).
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics 20 software (SPSS, Chicago, IL). Linear multiple regression models for the effect on pharmacokinetic parameters were calculated, with genotypes, age, sex, WBC, and MRD class as independent variables. All polymorphisms were included except SLCO1B1 rs11045879, which was in LD with SLCO1B1 rs4149056 and thus was excluded from the regression models for reasons of collinearity. Investigation of secondary endpoints was exploratory, that is, P values were not corrected for multiple endpoints. The association between SNPs and sum toxicity scores was calculated using multiple linear regression analysis, with age, sex, MRD class, WBC, AUC0-48h, and genotypes as independent variables. The overall toxicity score for each patient was calculated by adding up the grading of all adverse events that occurred in 17 toxicity categories during 4 courses of MTX. Similarly, the stomatitis score was calculated by adding up the NCI stomatitis grading of the 4 MTX courses. Associations between SNPs and EFS were determined by multiple analysis using the Cox proportional hazards model with age, DNA index, TEL/AML1, MRD class, MTX AUC0-48h, and effect of the SNPs. Fisher exact test and χ2 test were used to compare frequency distributions, and log-rank test was used to compare survival data between 2 patient populations. Bivariate correlations were tested with Pearson correlation test. Statistical significance was accepted for values of P < .05. Nominal P values are shown throughout.
Results
Patient characteristics and genotyping
Patient characteristics are summarized in Figure 1. Study endpoints, such as survival rates (Table 2) and MTX toxicity grading (not shown), did not significantly differ between ALL-BFM 2000 patients with genotype data included in our study and those without genotype data. Notably, our study cohort consisted of significantly more male patients (P = .025) and more patients with WBC count ≥ 50 000 at diagnosis (P < .001). Thus, our subcohort was not fully reflective of the overall ALL-BFM 2000 cohort.
Comparison of the investigated ALL-BFM 2000 subcohort and the remaining (not analyzed) ALL-BFM 2000 patients
| . | ALL-BFM 2000 study participants with SR and IR risk disease not analyzed . | ALL-BFM 2000 subcohortanalyzed . | P . |
|---|---|---|---|
| Sex, N (%) | |||
| Male | 753 (53.3) | 295 (59.1) | |
| Female | 659 (46.7) | 204 (40.9) | .025* |
| Age N (%) | |||
| <10 y | 1097 (77.7) | 395 (79.2) | |
| ≥10 y | 315 (22.3) | 104 (20.8) | .496* |
| WBC, N (%) | |||
| <10 000 | 800 (56.7) | 214 (42.9) | |
| 10 000≤50 000 | 432 (30.6) | 180 (36.1) | |
| 50 000≤100 000 | 101 (7.2) | 61 (12.2) | |
| ≥100 000 | 79 (5.6) | 44 (8.8) | <.001* |
| Immunophenotype, N (%) | |||
| Precursor B-ALL | 1230 (87.1) | 433 (86.8) | |
| T-ALL | 121 (8.6) | 59 (11.8) | |
| Other | 6 (0.4) | 1 (0.2) | |
| Unknown | 55 (3.9) | 6 (1.2) | .115* |
| DNA index, N (%) | |||
| <1.16 | 702 (49.7) | 305 (61.1) | |
| ≥1.16 | 211 (14.9) | 82 (16.4) | |
| Unknown | 499 (35.3) | 112 (22.4) | .448* |
| TEL/AML1, N (%) | |||
| Positive | 332 (23.5) | 128 (25.7) | |
| Negative | 908 (64.3) | 347 (69.5) | |
| Unknown | 172 (12.2) | 24 (4.8) | .942* |
| Treatment protocol, N (%) | |||
| SR | 532 (37.7) | 212 (42.5) | |
| intermediate risk | 880 (62.3) | 287 (57.5) | .058* |
| MRD class | |||
| MRD-SR | 533 (48.6) | 212 (50.4) | |
| MRD-MR | 504 (45.9) | 195 (46.3) | |
| MRD-SER | 60 (5.5) | 14 (3.3) | .215* |
| 5-year survival rate | |||
| % (95% CI) | 95.7% (94.6–96.8) | 96.9% (95.4–98.5) | .087† |
| EFS (5 y), % (95% CI) | 88.1% (86.4–86.8) | 90.8 (88.2–93.4) | .182† |
| . | ALL-BFM 2000 study participants with SR and IR risk disease not analyzed . | ALL-BFM 2000 subcohortanalyzed . | P . |
|---|---|---|---|
| Sex, N (%) | |||
| Male | 753 (53.3) | 295 (59.1) | |
| Female | 659 (46.7) | 204 (40.9) | .025* |
| Age N (%) | |||
| <10 y | 1097 (77.7) | 395 (79.2) | |
| ≥10 y | 315 (22.3) | 104 (20.8) | .496* |
| WBC, N (%) | |||
| <10 000 | 800 (56.7) | 214 (42.9) | |
| 10 000≤50 000 | 432 (30.6) | 180 (36.1) | |
| 50 000≤100 000 | 101 (7.2) | 61 (12.2) | |
| ≥100 000 | 79 (5.6) | 44 (8.8) | <.001* |
| Immunophenotype, N (%) | |||
| Precursor B-ALL | 1230 (87.1) | 433 (86.8) | |
| T-ALL | 121 (8.6) | 59 (11.8) | |
| Other | 6 (0.4) | 1 (0.2) | |
| Unknown | 55 (3.9) | 6 (1.2) | .115* |
| DNA index, N (%) | |||
| <1.16 | 702 (49.7) | 305 (61.1) | |
| ≥1.16 | 211 (14.9) | 82 (16.4) | |
| Unknown | 499 (35.3) | 112 (22.4) | .448* |
| TEL/AML1, N (%) | |||
| Positive | 332 (23.5) | 128 (25.7) | |
| Negative | 908 (64.3) | 347 (69.5) | |
| Unknown | 172 (12.2) | 24 (4.8) | .942* |
| Treatment protocol, N (%) | |||
| SR | 532 (37.7) | 212 (42.5) | |
| intermediate risk | 880 (62.3) | 287 (57.5) | .058* |
| MRD class | |||
| MRD-SR | 533 (48.6) | 212 (50.4) | |
| MRD-MR | 504 (45.9) | 195 (46.3) | |
| MRD-SER | 60 (5.5) | 14 (3.3) | .215* |
| 5-year survival rate | |||
| % (95% CI) | 95.7% (94.6–96.8) | 96.9% (95.4–98.5) | .087† |
| EFS (5 y), % (95% CI) | 88.1% (86.4–86.8) | 90.8 (88.2–93.4) | .182† |
DNA index, ratio of DNA content of leukemic G0/G1 cells to normal diploid lymphocytes; precursor B-ALL, precursor B-cell acute lymphoblastic leukemia; T-ALL, T-cell acute lymphoblastic leukemia.
Fisher exact test for 2 × 2 comparisons, all others χ2 test.
Log-rank test.
We genotyped 8 SNPs in SLCO1B1, SLC19A1, ABCC2, MTHFR, and TYMS genes (details are displayed in supplemental Table 3) with an average call rate of 99.998%. Allele frequencies did not significantly deviate from Hardy-Weinberg equilibrium. rs4149056 and rs11045879 SNPs in SLCO1B1 were in significant LD (D′= 0.956, r2 = 0.857).
Genetic component of MTX pharmacokinetics
Substantial interpatient variability was observed in C24h (coefficient of variation [CV] = 45.5%), AUC0-48h (CV = 44.5%), and MTX clearance (CV = 49.4%). Variation in MTX pharmacokinetics is substantially influenced by heredity as revealed by rGC. The estimated genetic component contributing to variation in MTX C24h, AUC0-48h, and clearance was 0.75, 0.62, and 0.68, respectively, suggesting that genetic factors contribute to MTX pharmacokinetics.
Association of SNPs in candidate genes with MTX pharmacokinetics
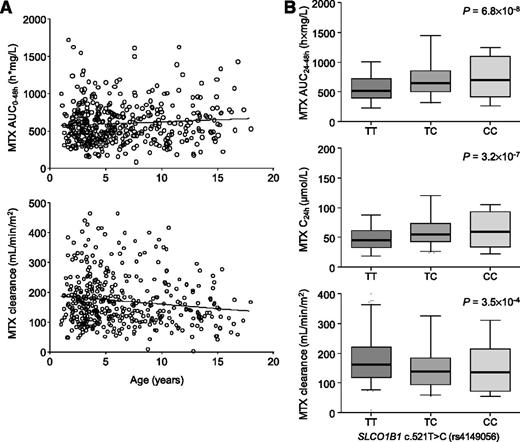
MTX clearance decreased with increasing age (Figure 2A; P = 4.7 × 10−3). Moreover, an influence of sex on MTX kinetics was observed previously.16 Therefore, age and sex were included as independent variables into the regression analyses. To exclude any bias, we also considered WBC and MRD as predictive variables. When genotypes were allowed to compete in the regression model (adjusted for sex, age, WBC, and MRD class), SLCO1B1 rs4149056 and rs2306283 remained significant predictors of AUC0-48h (Table 3). Consistent with the mathematical relationship between the pharmacokinetic parameters (AUC and the steady-state concentration at the end of infusion are inversely proportional to the clearance), the SLCO1B1 genotypes were also significantly associated with MTX C24h and MTX clearance (Table 3). In these multiple regression models, SLCO1B1 genotypes explained 6.8%, 7.0%, and 3.4% of the interindividual variability in MTX C24h, AUC0-48h, and clearance, respectively. Both genotypes at the SLCO1B1 locus were more powerful predictors of MTX kinetics than age. Supplemental Figure 1 shows MTX AUC0-48h levels stratified according to the SLCO1B1 rs2306283/rs4149056 diplotype. Per dysfunctional rs4149056 C allele, MTX AUC0-48h increased by 26%, MTX C24h increased by 24%, and MTX clearance decreased by 18%. A significant association was also found between SLCO1B1 rs11045879, which is in strong LD with SLCO1B1 rs4149056, and the pharmacokinetic parameters. Pharmacokinetic parameters significantly changed in a gene dose-dependent manner (Figure 2B), suggesting a per allele effect consistent with a codominant model of association.
Influence of age and genetic polymorphisms on pharmacokinetic parameters. (A) Correlation between age and the average AUC0-48h (upper panel) and between age and the average MTX clearance (lower panel) of each patient. (B) Association of the SLCO1B1 rs4149056 SNP with pharmacokinetic parameters. Genotype-dependent change in the area under the concentration time curve (AUC)0-48h, the peak MTX plasma concentration at the end of infusion (C24h), and the MTX clearance. Error bars show 5% to 95% percentiles. Shown are P values obtained from the multiple regression models with age, sex, MRD class, WBC, and genotypes as independent variables. Jonckheere trend test assuming an a priori ordering consistent with an additive allelic effect: P < .0001 (AUC0-48h and C24h), P < .001 (clearance).
Influence of age and genetic polymorphisms on pharmacokinetic parameters. (A) Correlation between age and the average AUC0-48h (upper panel) and between age and the average MTX clearance (lower panel) of each patient. (B) Association of the SLCO1B1 rs4149056 SNP with pharmacokinetic parameters. Genotype-dependent change in the area under the concentration time curve (AUC)0-48h, the peak MTX plasma concentration at the end of infusion (C24h), and the MTX clearance. Error bars show 5% to 95% percentiles. Shown are P values obtained from the multiple regression models with age, sex, MRD class, WBC, and genotypes as independent variables. Jonckheere trend test assuming an a priori ordering consistent with an additive allelic effect: P < .0001 (AUC0-48h and C24h), P < .001 (clearance).
Identification of predictive variables for MTX C24h, MTX AUC0-48h, and MTX clearance by regression analysis
| . | . | MTX AUC0-48h . | MTX C24h . | MTX clearance . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Included variables . | B . | (95% CI) . | P . | R2 (%) . | B . | (95% CI) . | P . | R2 (%) . | B . | (95% CI) . | P . | R2 (%) . | |
| SLCO1B1 | rs4149056 | 146.9 | (94.4, 199.4) | 6.8 × 10−8 | 5.4 | 12.6 | (7.9, 17.4) | 3.2 × 10−7 | 4.9 | −30.1 | (−46.6, −13.7) | 3.5 × 10−4 | 2.2 |
| SLCO1B1 | rs2306283 | −56.7 | (−98.3, −15.1) | 7.7 × 10−3 | 1.6 | −5.3 | (-9.1, −1.6) | 5.5 × 10−3 | 1.9 | 14.8 | (1.8, 27.8) | .026 | 1.2 |
| Age | — | — | — | — | — | — | — | — | −2.8 | (−4.8, −0.8) | 7.1 × 10−3 | 1.7 | |
| . | . | MTX AUC0-48h . | MTX C24h . | MTX clearance . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Included variables . | B . | (95% CI) . | P . | R2 (%) . | B . | (95% CI) . | P . | R2 (%) . | B . | (95% CI) . | P . | R2 (%) . | |
| SLCO1B1 | rs4149056 | 146.9 | (94.4, 199.4) | 6.8 × 10−8 | 5.4 | 12.6 | (7.9, 17.4) | 3.2 × 10−7 | 4.9 | −30.1 | (−46.6, −13.7) | 3.5 × 10−4 | 2.2 |
| SLCO1B1 | rs2306283 | −56.7 | (−98.3, −15.1) | 7.7 × 10−3 | 1.6 | −5.3 | (-9.1, −1.6) | 5.5 × 10−3 | 1.9 | 14.8 | (1.8, 27.8) | .026 | 1.2 |
| Age | — | — | — | — | — | — | — | — | −2.8 | (−4.8, −0.8) | 7.1 × 10−3 | 1.7 | |
Age, sex, WBC count, MRD class, and SLCO1B1 rs4149056, SLCO1B1 rs2306283, SLC19A1 rs1051266, ABCC2 rs717620, MTHFR rs1801131, MTHFR rs1801133, and TYMS rs34743033 genotypes were considered as independent variables in the regression analyses. Because SLCO1B1 rs11045879 was in LD with SLCO1B1 rs4149056, SLCO1B1 rs11045879 was not included in the regression models for reasons of collinearity. This table shows significant predictor variables included in the stepwise regression models. Age was a significant predictor variable of MTX clearance only.
B, regression coefficient; P, nominal P value of the regression coefficient; R2, proportion of variation in the dependent variable that is explained by the model.
Association of SNPs in candidate genes with MTX toxicity
Overall toxicity was evaluated by applying a sum score that integrates both frequency and severity of adverse events during protocol M. Regression analysis with sex, age, MRD class, WBC, MTX AUC0-48h, and genotypes as independent variables revealed that MTX AUC0-48h was the most significant predictor of high overall toxicity scores (regression coefficient B = 0.013; 95% confidence interval [CI], 0.007, 0.019; R2 = 0.043; P = 2.9 × 10−5).
Stomatitis, a common side effect of chemotherapy in children with ALL, is closely attributed to the use of (high-dose) MTX and therefore has been considered to be relatively specific for MTX. Surprisingly, MTX AUC0-48h was not related to the stomatitis sum toxicity score. However, the TYMS rs34743033 tandem repeat polymorphism was the only significant predictor of stomatitis in a multiple regression model considering sex, age, MRD class, WBC, MTX AUC0-48h, and genotypes as independent variables (B = −0.48; 95% CI, −0.84, −0.12; R2 = 0.018; P = .009). The stomatitis toxicity score decreased with increasing number of TYMS *3 alleles. Figure 3 shows the incidence of stomatitis stratified according to the NCI grade and TYMS rs34743033 genotypes.
Association of the rs34743033 genetic polymorphism in TYMS with frequency and severity of stomatitis in ALL patients during protocol M. Bar graph shows TYMS rs34743033 genotype-dependent incidence of stomatitis stratified according to the NCI toxicity grading for stomatitis.
Association of the rs34743033 genetic polymorphism in TYMS with frequency and severity of stomatitis in ALL patients during protocol M. Bar graph shows TYMS rs34743033 genotype-dependent incidence of stomatitis stratified according to the NCI toxicity grading for stomatitis.
Association of SNPs in candidate genes with EFS
In a multiple Cox regression analysis, which included age, WBC, DNA index, TEL/AML1, AUC0-48h, MRD class, and genotypes as independent variables, MRD class and MTHFR rs1801131 SNP were significantly associated with EFS (Figure 4). The adjusted hazard ratios were 7.3 (95% CI = 2.5 − 21.3; P = 3.2 × 10−4) for MRD-SER vs MRD SR patients and 3.1 (95% CI = 1.2 − 7.7; P = .015) for homozygous carriers of the MTHFR rs1801131 C allele vs patients with the AA reference genotype.
Association of the MTHFR rs1801131 genotype (A) and MRD status (B) with EFS. HR, hazard ratio.
Association of the MTHFR rs1801131 genotype (A) and MRD status (B) with EFS. HR, hazard ratio.
Discussion
By investigating key genes involved in the MTX pathway, our study aimed to detect genetic polymorphisms associated with MTX pharmacokinetics, toxicity, and outcome. The strongest association was observed between the SLCO1B1 rs4149056 SNP and MTX pharmacokinetic parameters such as C24h, AUC0-48h, and clearance.
The SLCO1B1 carrier is mainly expressed in the liver where it is located on the sinusoidal membrane of human hepatocytes.33,34 The carrier acts as an influx transporter for endogenous compounds (eg, bile acids and thyroid hormones)33 and for many xenobiotics and drugs.7 Several findings support the notion that SLCO is important for hepatic uptake and thus for the pharmacokinetics of MTX. First, independent studies have shown that SLCO1B1 can transport MTX in vitro.28,35 Second, in transgenic mice with functional and specific expression of human SLCO1B1 in the liver, the AUC for intravenous MTX was 1.5-fold decreased compared with wild-type mice, demonstrating a marked and possibly rate-limiting role for human SLCO1B1 in MTX elimination in vivo.36 Third, using a genome-wide association study approach, the tagging SNP rs11045879 in SLCO1B1, which is in LD with the rs4149056 loss-of-function SNP, was associated with reduced MTX clearance in patients with ALL.15 The latter study demonstrated the clinical significance of SLCO1B1 for MTX clearance for the first time and highlighted the role of SLCO1B1’s polymorphic expression for the interindividual variability in MTX pharmacokinetics.
A subsequent study, however, failed to show a univocally statistically significant association between SLCO1B1 rs11045879, which is in LD with SLCO1B1 rs4149056, and MTX plasma concentrations in ALL patients (corrected P = .08).14 Our study, which was performed in a cohort that provides sufficient statistical power, clearly replicates the association found by Treviño et al.15 A study by Ramsey et al, which was published during preparation of our manuscript, confirmed the association of SLCO1B1 rs4149056 with MTX clearance in a genome-wide association study.37
In contrast to the loss-of-function phenotype of the SLCO1B1 rs4149056 variant,7 the rs2306283 variant, another common coding SNP in SLCO1B1, increased the transport activity of OATP1B1 for MTX in vitro.28 In accordance with enhanced cellular uptake of MTX in vitro, presence of the mutant rs2306283 A allele was related to increased MTX clearance and decreased MTX AUC0-48h in our patients. The SLCO1B1 rs2306283 G allele, thus, in part counterbalances the effects of the mutant rs4149056 C allele on MTX kinetics.
Because altered MTX pharmacokinetics may affect treatment efficacy and adverse drug reactions, survival and MTX toxicity were investigated as secondary endpoints in an explorative analysis. We used an overall toxicity sum score that integrated both frequency and severity of 17 toxicity items reported during protocol M. Among the parameters and genotypes tested in the regression model, MTX AUC0-48h was a significant predictor of adverse events. The rather weak association of MTX AUC0-48h with adverse events—the regression model explained only 4.3% of variance—might be due, in part, to leucovorin rescue and unclear attribution of some types of adverse events to MTX.
In fact, causality assessment of adverse drug reactions is a general problem that also holds true for suspected toxicity of MTX in our study. In the ALL BFM-2000 study causality assessment scales were not applied. Thus, reported adverse events are most likely due to MTX but may also originate from cytostatic comedication, such as 6-mercaptopurine. Moreover, early symptomatic treatments for pain, diarrhea, nausea, and vomiting can cause adverse events or modify MTX toxicity.
Stomatitis is an adverse event that has been attributed specifically to high-dose MTX, as applied in protocol M. Surprisingly, in the multiple regression analysis, stomatitis was not associated with MTX AUC0-48h but with the TYMS tandem repeat polymorphism. Stomatitis was significantly less frequent and/or less severe in patients carrying at least 1 TYMS triple repeat (TYMS *3) allele compared with homozygous *2 carriers. It is known that an increased number of tandem repeats increases TYMS mRNA and protein expression and that overexpression of TYMS might be a mechanism by which individuals with at least 1 *3 allele develop resistance, that is, tolerate high-dose MTX with fewer side effects. A previous study reported that homozygosity for the TYMS triple repeat was associated with poorer outcome of ALL patients treated with high-dose MTX27 ; however, this was not confirmed in our study.
Conter et al showed that among patients presenting high MRD levels at day 33 (≥10E-3), those with no MRD detectable at day 78 had a favorable outcome, whereas those with MRD still present at day 78 (MRD-SER) had a marked increase in the risk of relapse.17 Our data confirmed the previous results by the demonstration that MRD-SER patients had significantly worse EFS than MRD-SR or MRD-MR patients. After adjustment for age, WBC, TEL/AML1, DNA index, MRD class, and AUC0-48h, our association analysis of individual polymorphisms showed a significant association of the MTHFR rs1801131 SNP with EFS. Variants in folate pathway genes have been found to be associated with relapse-free survival and EFS of pediatric ALL patients in previous studies.22,38,39 Some studies failed to replicate significant associations. Similarly, our study could not confirm associations of folate pathway gene polymorphisms with EFS, except for the association with the MTHFR rs1801131 variant, suggesting that the overall contribution to outcome is small and cannot be explained by this variant alone. Whether variants in a specific gene affect EFS rates may depend on the specific treatment protocol. Remission rates, relapse, or secondary leukemia depend on the respective protocol applied in a study. Thus, the association between MTHFR rs1801131 and outcome may be specific for the ALL-BFM 2000 protocol and must not apply to other protocols for treatment of pediatric ALL.
Our results may help to guide and optimize MTX treatment of individual patients based on genetic markers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Johannes und Frieda Marohn-Stiftung and the Kind-Philipp-Stiftung für Leukämieforschung.
Authorship
Contribution: S.R. conceived and designed the study, collected and assembled data, did data analysis and interpretation, wrote the manuscript, and gave final approval of the manuscript; O.Z. conceived and designed the study, collected and assembled data, did data analysis and interpretation, wrote the manuscript, gave final approval of the manuscript, and provided administrative support; B.R. did data analysis and interpretation, wrote the manuscript, and gave final approval of the manuscript; M.P. conceived and designed the study, wrote the manuscript, and gave final approval of the manuscript; M.Z. collected and assembled data, did data analysis and interpretation, wrote the manuscript, gave final approval of the manuscript, and provided study material or patients; A.M. collected and assembled data, did data analysis and interpretation, wrote the manuscript, gave final approval of the manuscript, and provided study material or patients; M. Stanulla collected and assembled data, did data analysis and interpretation, wrote the manuscript, gave final approval of the manuscript, and provided study material or patients; M. Schrappe collected and assembled data, did data analysis and interpretation, wrote the manuscript, gave final approval of the manuscript, and provided study material or patients; T.L. conceived and designed the study, collected and assembled data, did data analysis and interpretation, wrote the manuscript, gave final approval of the manuscript, and provided financial and administrative support.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: T. Langer, University Hospital for Children and Adolescents, Loschgestrasse 15, 91054 Erlangen, Germany; e-mail: thorsten.langer@uk-erlangen.de.
References
Author notes
S.R. and O.Z. contributed equally to this work.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal