Key Points
Banked third-party virus-specific T cells can safely and rapidly treat severe or intractable viral infections after HSCT.
Abstract
Virus-specific T cell (VST) lines could provide useful antiviral prophylaxis and treatment of immune-deficient patients if it were possible to avoid the necessity of generating a separate line for each patient, often on an emergency basis. We prepared a bank of 32 virus-specific lines from individuals with common HLA polymorphisms who were immune to Epstein-Barr virus (EBV), cytomegalovirus, or adenovirus. A total of 18 lines were administered to 50 patients with severe, refractory illness because of infection with one of these viruses after hematopoietic stem cell transplant. The cumulative rates of complete or partial responses at 6 weeks postinfusion were 74.0% (95% CI, 58.5%-89.5%) for the entire group (n = 50), 73.9% (95% CI, 51.2% -96.6%) for cytomegalovirus (n = 23), 77.8% for adenovirus (n = 18), and 66.7% (95% CI, 36.9%-96.5%) for EBV (n = 9). Only 4 responders had a recurrence or progression. There were no immediate infusion-related adverse events, and de novo graft-versus-host disease developed in only 2 patients. Despite the disparity between the lines and their recipients, the mean frequency of VSTs increased significantly postinfusion, coincident with striking decreases in viral DNA and resolution of clinical symptoms. The use of banked third-party VSTs is a feasible and safe approach to rapidly treat severe or intractable viral infections after stem cell transplantation. This study is registered at www.clinicaltrials.gov as NCT00711035.
Introduction
Viral illness is a major cause of morbidity and mortality in immunocompromised patients.1-5 After allogeneic hematopoietic stem cell transplantation (HSCT), up to a third of deaths may be related to viral infection.6 Pharmacologic treatments are available for patients with some types of viral infections, but these often have limited efficacy and significant adverse effects.7-9 One solution is to reconstitute the antiviral immunity of the recipient with virus-specific T cells (VSTs) derived from the stem cell donor. This approach appears effective as prophylaxis and therapy for refractory Epstein-Barr virus (EBV), cytomegalovirus (CMV), and adenovirus (AdV) infections, which are 3 common causes of viral morbidity and mortality after HSCT.10-15 Unfortunately, the need to generate specific T-cell lines for each individual patient renders this approach impractical for widespread or urgent use, and it is not an option when the donor lacks viral immunity (eg, after cord blood transplantation).
The current limitations of T-cell therapy for viral infections could be overcome if it were possible to prepare “off-the-shelf” reagents; that is, a bank of VSTs that were generated from virus-immune individuals with common HLA polymorphisms and frozen and stored. Promising results have been obtained with this approach in the treatment of EBV infections after solid organ transplants.16 Although the strategy may also be beneficial after HSCT,13 there remains the risk that these third-party, HLA-mismatched VSTs might also cause graft-versus-host-disease (GVHD) or graft rejection. It is also unknown whether such T cells would be effective against CMV and AdV infections, which occur more commonly than EBV in immunocompromised patients, and it is not known whether the banked T cells would have sufficient persistence for sustained protection. Therefore, we generated and stored a bank of T-cell lines specific for EBV, CMV, and AdV that were ready for immediate use and, in a multicenter study, administered them to 50 patients with severe, refractory CMV, AdV, or EBV infections.
Methods
Third-party T-cell bank
We generated a bank of VSTs that were either retained from our recipient-specific clinical study10 or were newly generated from donors with known antiviral activity, including HLA homozygous donors identified by the National Marrow Donor Program. To generate VSTs, we transduced up to 5 × 107 donor peripheral blood mononuclear cells (PBMCs) with a clinical grade Ad5f35pp65 vector at a multiplicity of infection of 10 viral particles (vp) per cell after an overnight adherence step.17 Starting on day 10 posttransduction, the cells were restimulated weekly with irradiated EBV-transformed lymphoblastoid cell lines transduced at an multiplicity of infection of 100 vp with the same Ad5f35pp65 vector at a responder:stimulator (R:S) ratio of 4:1, if expansion was performed in a 24-well plate, or at an R:S ratio of 1:5 if expanded in the G-Rex.17 After 2 to 4 stimulations, the VSTs were cryopreserved. All donors gave informed consent and met eligibility requirements. At the time of cryopreservation, each line was microbiologically screened, immunophenotyped by flow cytometry, and tested for virus specificity by interferon-gamma (IFN-γ) enzyme-linked immunospot (ELIspot) assay. A total of 32 lines were produced and characterized, 18 of which were administered to the 50 study patients. The selection of lines for infusion was based on the specificity of the line for the target virus through a shared HLA allele, as well as the overall level of HLA match. Thus, a line that only matched at 1 allele but with confirmed antiviral activity against the infecting virus through that shared allele would be preferred to a line that matched at 2 or 3 alleles but did not have confirmed antiviral activity through these shared alleles. Cells were transported cryopreserved in a dry shipper fitted with a data logger to participating sites using methods validated by National Heart, Lung and Blood Institute (NHLBI) Production Assistance for Cellular Therapy. All sites received an investigational brochure, standard operating procedures for thawing and infusing cells, and a DVD prepared by Baylor College of Medicine investigators showing the thaw-and-infusion procedure per the standard operating procedure.
Clinical trial design
The study was a multicenter trial (NCT00711035) sponsored by NHLBI, and after local and federal approval, it was opened to allogeneic HSCT recipients with CMV, AdV, or EBV infection that had persisted for at least 7 days despite standard therapy (defined as ganciclovir, foscarnet or cidofovir for CMV, rituximab for EBV, and cidofovir for AdV), receiving less than 0.5mg/kg/day of prednisone. Patients who received ATG, Campath, other T-cell immunosuppressive monoclonal antibodies, or a donor lymphocyte infusion within 28 days of the proposed administration date were excluded from participating. Additional exclusion criteria included the presence of other uncontrolled infections or active, acute GVHD grades II to IV. The clinical protocol in the supplemental Data provides full study details. Consenting patients who had a suitable VST and who met entry or exclusion criteria (see protocol) received an intravenous infusion of up to 2 × 107 VST/m2 and were eligible for additional infusions at intervals of at least 2 weeks, in the event of a partial response (PR). Patients could continue other antiviral therapy at the treating physician’s discretion. This study was conducted in accordance with the Declaration of Helsinki.
Clinical and virological end points
Toxicities were graded by the NCI Common Terminology Criteria for Adverse Events, version 3.0, and were reviewed by the NHLBI Data Safety Monitoring Committee. Safety end points were acute GVHD grades III to IV developing within 45 days of the last VST dose; grades III to V infusion-related GVHD; or grades IV to V nonhematological adverse events developing within 30 days of the last VST dose and not attributable to the preexisting infection, the original malignancy, or preexisting morbidities. Weekly organ-stage scores for GVHD, biopsy information, and relevant differential diagnoses were recorded, and a weekly grade was calculated from National Institutes of Health consensus criteria.18 Chronic GVHD was graded according to standard definitions.19
CMV, EBV, and AdV loads were monitored at each center by quantitative polymerase chain reaction in laboratories approved by the Clinical Laboratory Improvement Amendments. A complete response (CR) of the virus to treatment was defined as a decrease in viral load to below limits of assay detection, with resolution of clinical signs and symptoms. A PR was defined as a decrease in viral load of at least 50% from baseline with alleviation of any symptoms. For patients with EBV-associated posttransplant lymphoma posttransplant lymphoproliferative disease (PTLD), responses were monitored by ILWG criteria.20 Secondary graft failure was defined as initial neutrophil engraftment followed by a decline in the absolute neutrophil count to less than 500/mm3 for 3 consecutive measurements. A study end point committee reviewed all GVHD assessments and response data.
Immune monitoring
We used ELISpot assays according to published methods21 to determine the frequency of T cells that secreted IFN-γ in response to CMV, AdV, and EBV antigens or peptides. Clinical samples collected before and after treatment at days 14, 28, and 90 were cryopreserved and batched to minimize interassay variability. PBMCs stimulated with staphylococcal enterotoxin B (1 μg/mL) (Sigma-Aldrich) served as positive controls.
To track the persistence of donor-derived VSTs, we used high-throughput deep sequencing of TCRvβ CDR3 regions (Adaptive TCR Corp) to analyze the T-cell repertoire of the infused lines and of peripheral blood samples collected from 6 patients (4 responders and 2 nonresponders) before and after infusion.22
Statistical analysis
The primary end point was the safety of the VST infusion as determined by the frequency of severe toxicity not caused by the preexisting infection or by the original malignancy. None of the prespecified safety guidelines, including toxicity and secondary graft failure, were crossed, allowing accrual to continue until its planned completion. Cumulative rates of acute and chronic GVHD, PR, and CR were calculated by the cumulative incidence function, with death considered a competing risk.23 All data were analyzed with the SAS system (Cary, NC) version 9.2 and R version 2.15.0.
Results
Patients
We screened 82 patients who had undergone HSCT with bone marrow (n = 24), peripheral blood (n = 33), or cord blood (n = 12 single units and 13 double units) and identified a suitable line for 74 patients, 50 of whom met eligibility criteria and subsequently received VSTs. The lines matched at 1 to 4 of the recipients’ HLA antigens. Of these 50 patients who received VST infusions, 36 had a single infusion, 7 patients had 2 infusions, 6 patients had 3 infusions, and 1 patient had 6 infusions. Clinical details of these patients are summarized in Table 1. Of the 50 patients, 23 received VSTs for persistent CMV, 18 for persistent Adv, and 9 for refractory EBV-PTLD. Five of the 50 treated patients withdrew from the study or died of their underlying disease or of an intercurrent infection within 7 days of infusion (supplemental Figure 1 for flow diagram) but were nonetheless included in the outcome analysis.
Patient details
| . | Infection cohort . | |||
|---|---|---|---|---|
| Characteristics . | AdV (n = 18) . | CMV (n = 23) . | EBV (n = 9) . | Total (n = 50) . |
| Sex (n, %) | ||||
| Male | 12 (66.7) | 17 (73.9) | 4 (44.4) | 33 (66.0) |
| Ethnicity (n, %) | ||||
| Hispanic or Latino | 6 (33.3) | 4 (17.4) | 2 (22.2) | 12 (24.0) |
| Race (n, %) | ||||
| Black or African American | 3 (16.7) | 4 (17.4) | 0 (0.0) | 7 (14.0) |
| White | 13 (72.2) | 16 (69.6) | 9 (100.0) | 38 (76.0) |
| Age at infusion (y) | ||||
| Mean (SD) | 18.0 (17.5) | 36.6 (25.6) | 34.5 (17.9) | 29.5 (23.0) |
| Transplant type (n, %) | ||||
| Bone marrow | 8 (44.4) | 5 (21.7) | 1 (11.1) | 14 (28.0) |
| Peripheral blood stem cell | 5 (27.8) | 14 (60.9) | 2 (22.2) | 21 (42.0) |
| Single cord blood | 3 (16.7) | 2 (8.7) | 0 (0.0) | 5 (10.0) |
| Double cord blood | 2 (11.1) | 2 (8.7) | 6 (66.7) | 10 (20.0) |
| HLA match (recipient to first VST line) (n, %)* | ||||
| 1/6 | 5 (27.8) | 4 (17.4) | 3 (33.3) | 12 (24.0) |
| 2/6 | 9 (50.0) | 12 (52.2) | 3 (33.3) | 24 (48.0) |
| 3/6 | 3 (16.7) | 6 (26.1) | 3 (33.3) | 12 (24.0) |
| 4/6 | 1 (5.6) | 1 (4.3) | 0 (0.0) | 2 (4.0) |
| Number of infusions (n, %) | ||||
| 1 | 15 (83.3) | 17 (73.9) | 4 (44.4) | 36 (72.0) |
| 2 | 2 (11.1) | 4 (17.4) | 1 (11.1) | 7 (14.0) |
| 3 | 1 (5.6) | 2 (8.7) | 3 (33.3) | 6 (12.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 1 (11.1) | 1 (2.0) |
| . | Infection cohort . | |||
|---|---|---|---|---|
| Characteristics . | AdV (n = 18) . | CMV (n = 23) . | EBV (n = 9) . | Total (n = 50) . |
| Sex (n, %) | ||||
| Male | 12 (66.7) | 17 (73.9) | 4 (44.4) | 33 (66.0) |
| Ethnicity (n, %) | ||||
| Hispanic or Latino | 6 (33.3) | 4 (17.4) | 2 (22.2) | 12 (24.0) |
| Race (n, %) | ||||
| Black or African American | 3 (16.7) | 4 (17.4) | 0 (0.0) | 7 (14.0) |
| White | 13 (72.2) | 16 (69.6) | 9 (100.0) | 38 (76.0) |
| Age at infusion (y) | ||||
| Mean (SD) | 18.0 (17.5) | 36.6 (25.6) | 34.5 (17.9) | 29.5 (23.0) |
| Transplant type (n, %) | ||||
| Bone marrow | 8 (44.4) | 5 (21.7) | 1 (11.1) | 14 (28.0) |
| Peripheral blood stem cell | 5 (27.8) | 14 (60.9) | 2 (22.2) | 21 (42.0) |
| Single cord blood | 3 (16.7) | 2 (8.7) | 0 (0.0) | 5 (10.0) |
| Double cord blood | 2 (11.1) | 2 (8.7) | 6 (66.7) | 10 (20.0) |
| HLA match (recipient to first VST line) (n, %)* | ||||
| 1/6 | 5 (27.8) | 4 (17.4) | 3 (33.3) | 12 (24.0) |
| 2/6 | 9 (50.0) | 12 (52.2) | 3 (33.3) | 24 (48.0) |
| 3/6 | 3 (16.7) | 6 (26.1) | 3 (33.3) | 12 (24.0) |
| 4/6 | 1 (5.6) | 1 (4.3) | 0 (0.0) | 2 (4.0) |
| Number of infusions (n, %) | ||||
| 1 | 15 (83.3) | 17 (73.9) | 4 (44.4) | 36 (72.0) |
| 2 | 2 (11.1) | 4 (17.4) | 1 (11.1) | 7 (14.0) |
| 3 | 1 (5.6) | 2 (8.7) | 3 (33.3) | 6 (12.0) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 1 (11.1) | 1 (2.0) |
Patients were accrued and treated at Baylor College of Medicine (n = 18, of which 14 were enrolled at Texas Children’s Hospital and 4 at The Methodist Hospital), MD Anderson Cancer Center (n = 14), Duke University (n = 8), Dana-Farber Cancer Center (n = 4), Children’s Hospital of Los Angeles (n = 3), Hackensack University Medical Center (n = 2), and Massachusetts General Hospital (n = 1).
Matching by low-resolution typing.28
Phenotype and specificity of VSTs
Eighteen T-cell lines were administered, with each given to 1 to 6 of the 50 recipients. Phenotypes of the infused lines are shown in supplemental Table 1. There was a mixture of helper CD4+ (median, 10.1%; range, 1.5%-99.1%) and CD8+ (median, 85.2%; range, 0.7%-98.8%) subsets, with expression of CD62L and CD45RO memory markers and significant numbers of T cells reactive against the specified CMV, AdV, or EBV antigens.
Clinical and antiviral efficacy
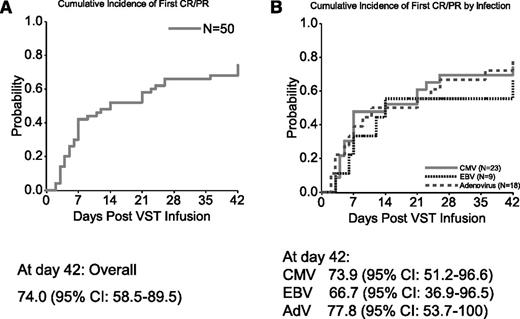
The cumulative rate of CRs or PRs at 6 weeks of all 50 patients who received VSTs is 74.0% (95% CI, 58.5%-89.5%) (Figure 1A).
Cumulative response rates at 6 weeks postinfusion of VSTs. (A) Results based on the first CR/PR in all 50 patients, regardless of the type of infection. (B) Results by specific viral infection: CMV, AdV, and EBV. The curves were constructed with use of the cumulative incidence function. Further details are given in “Statistical analysis.”
Cumulative response rates at 6 weeks postinfusion of VSTs. (A) Results based on the first CR/PR in all 50 patients, regardless of the type of infection. (B) Results by specific viral infection: CMV, AdV, and EBV. The curves were constructed with use of the cumulative incidence function. Further details are given in “Statistical analysis.”
Cytomegalovirus.
Twenty-three patients received VSTs for persistent CMV infection, and 19 were evaluable for response (Table 2). Seventeen responded to VSTs, a 6-week cumulative response rate of 73.9% (95% CI, 51.2%-96.6%) (Figure 1B). Nine patients had a CR and 8 a PR; 2 had no response (Figure 2A and Table 2). Of the 17 responders, 10 had a concomitant increase in CMV-specific T cells. The number of spot-forming cells (SFCs) increased from a mean (±SEM) of 91.1 ± 50.2 preinfusion to 168 ± 106.0 postinfusion per 4 × 105 input cells (P = .0150) (supplemental Figure 2).
Viral responses to CMV-specific T cells
| Pt. no. . | Viral disease . | Duration of previous standard therapy (defined as ganciclovir, foscarnet, or cidofovir) . | Tissue disease . | Line infused . | HLA match (low-resolution) with recipient . | No. of infusions . | Best response by 6 wk . | Viral outcome . |
|---|---|---|---|---|---|---|---|---|
| 20 | CMV | 16 d | Yes | C2274 | 2/6 | 1 | CR | Resolution of pneumonia and CMV antigenemia |
| 22 | CMV/AdV | 14 d | No | C2420 | 2/6 | 1 | PR | CR/CMV after 42 d, also CR/AdV |
| 23 | CMV | 4 mo | Yes | C2420 | 2/6 | 1 | N/E | Died day 3 post-VST infusion of HHV-7 pneumonia |
| 27 | CMV | 22 d | No | C3366 | 3/6 | 1 | PR | CR after day 42 |
| 30 | CMV | 2 mo | Yes | C3365 | 3/6 | 1 | PR | PR by viral load but response not sustained. |
| 41 | CMV | 32 d | No | C2353 | 3/6 | 2 | PR | CR after day 42 |
| 49 | CMV | 4 mo | Yes | C3366 | 3/6 | 1 | PR | Retinitis PR at day 42, CR later |
| 50 | CMV | 9 mo | No | C3889 | 2/6 | 1 | CR | Sustained CR |
| 56 | CMV | 18 d | Yes | C3629 | 2/6 | 2 | CR | Sustained CR |
| 57 | CMV | 4 mo | Yes | C3528 | 2/6 | 3 | CR | CR on viral load, also retinitis |
| 58 | CMV | 4 mo | Yes | C2339 | 2/6 | 2 | CR | CR after day 42 |
| 61 | CMV | 25 d | No | C2420 | 3/6 | 1 | CR | Sustained CR |
| 62 | CMV | 5 mo | Yes | C3365 | 2/6 | 1 | PR | CR after day 42 |
| 67 | CMV | 36 d | Yes | C3466 | 2/6 | 1 | N/E | No follow-up data; withdrew from study at 3 d |
| 71 | CMV | 37 d | No | C3889 | 1/6 | 3 | NR | Transient responses |
| 73 | CMV | 3 mo | No | C3874 | 3/6 | 2 | CR | Sustained CR |
| 77 | CMV | 17 d | Yes | C3921 | 1/6 | 1 | N/E | Died day 5 post-VST infusion of persistent CMV pneumonia |
| 83 | CMV | 14 d | No | C3889 | 1/6 | 1 | CR | Sustained CR |
| 84 | CMV | 3 mo | No | C3889 | 1/6 | 1 | NR | No response |
| 85 | CMV | 34 d | Yes | C3629 | 2/6 | 1 | N/E | Died day 1 post-VST infusion of CMV pneumonia |
| 87 | CMV | 14 d | Yes | C3921 | 2/6 | 1 | PR | PR of CMV, then died of relapsed ALL |
| 91 | CMV | 2 mo | Yes | C3921 | 4/6 | 1 | PR | CR after day 42 |
| 93 | CMV | 21 d | No | C2353 | 2/6 | 1 | CR | Sustained CR |
| Pt. no. . | Viral disease . | Duration of previous standard therapy (defined as ganciclovir, foscarnet, or cidofovir) . | Tissue disease . | Line infused . | HLA match (low-resolution) with recipient . | No. of infusions . | Best response by 6 wk . | Viral outcome . |
|---|---|---|---|---|---|---|---|---|
| 20 | CMV | 16 d | Yes | C2274 | 2/6 | 1 | CR | Resolution of pneumonia and CMV antigenemia |
| 22 | CMV/AdV | 14 d | No | C2420 | 2/6 | 1 | PR | CR/CMV after 42 d, also CR/AdV |
| 23 | CMV | 4 mo | Yes | C2420 | 2/6 | 1 | N/E | Died day 3 post-VST infusion of HHV-7 pneumonia |
| 27 | CMV | 22 d | No | C3366 | 3/6 | 1 | PR | CR after day 42 |
| 30 | CMV | 2 mo | Yes | C3365 | 3/6 | 1 | PR | PR by viral load but response not sustained. |
| 41 | CMV | 32 d | No | C2353 | 3/6 | 2 | PR | CR after day 42 |
| 49 | CMV | 4 mo | Yes | C3366 | 3/6 | 1 | PR | Retinitis PR at day 42, CR later |
| 50 | CMV | 9 mo | No | C3889 | 2/6 | 1 | CR | Sustained CR |
| 56 | CMV | 18 d | Yes | C3629 | 2/6 | 2 | CR | Sustained CR |
| 57 | CMV | 4 mo | Yes | C3528 | 2/6 | 3 | CR | CR on viral load, also retinitis |
| 58 | CMV | 4 mo | Yes | C2339 | 2/6 | 2 | CR | CR after day 42 |
| 61 | CMV | 25 d | No | C2420 | 3/6 | 1 | CR | Sustained CR |
| 62 | CMV | 5 mo | Yes | C3365 | 2/6 | 1 | PR | CR after day 42 |
| 67 | CMV | 36 d | Yes | C3466 | 2/6 | 1 | N/E | No follow-up data; withdrew from study at 3 d |
| 71 | CMV | 37 d | No | C3889 | 1/6 | 3 | NR | Transient responses |
| 73 | CMV | 3 mo | No | C3874 | 3/6 | 2 | CR | Sustained CR |
| 77 | CMV | 17 d | Yes | C3921 | 1/6 | 1 | N/E | Died day 5 post-VST infusion of persistent CMV pneumonia |
| 83 | CMV | 14 d | No | C3889 | 1/6 | 1 | CR | Sustained CR |
| 84 | CMV | 3 mo | No | C3889 | 1/6 | 1 | NR | No response |
| 85 | CMV | 34 d | Yes | C3629 | 2/6 | 1 | N/E | Died day 1 post-VST infusion of CMV pneumonia |
| 87 | CMV | 14 d | Yes | C3921 | 2/6 | 1 | PR | PR of CMV, then died of relapsed ALL |
| 91 | CMV | 2 mo | Yes | C3921 | 4/6 | 1 | PR | CR after day 42 |
| 93 | CMV | 21 d | No | C2353 | 2/6 | 1 | CR | Sustained CR |
HHV-7, human herpesvirus-7; N/E, not evaluable; NR, no response; SD, stable disease.
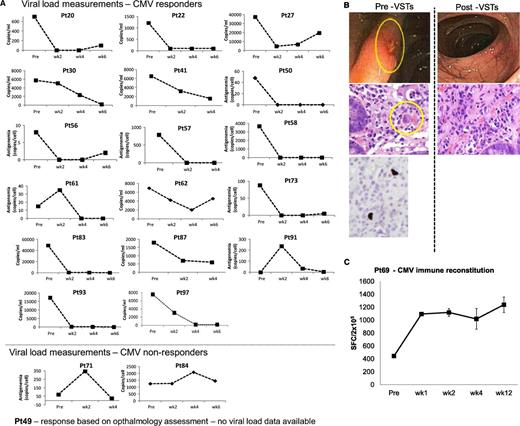
Outcomes after treatment with banked VSTs: CMV-infected patients. (A) Depiction of viral load measurements before and during 6 weeks postinfusion of VSTs in 19 evaluable patients (responders and nonresponders) treated for persistent CMV infection. Results are presented as copies per milliliter (CMV detected in blood) or as copies per cell in patients with CMV antigenemia. Because viral loads were measured by different assays at different institutions, interpatient comparisons should not be made. (B) An example of a patient with CMV colitis with evidence of viral inclusions on H&E staining that contained CMV by immunostaining (left panel). After VST infusion, there was a complete resolution of CMV colitis and no evidence of viral inclusions by H&E staining (right panel). (C) Frequency of CMVpp65–directed T cells in peripheral blood before and at weeks 1, 2, 4, and 12 postinfusion, as measured with an IFN-γ ELIspot assay after overnight stimulation of PBMCs with CMVpp65 pepmix. Results are expressed as mean (±SD) spot-forming cells (SFCs) per 2 × 105 input cells.
Outcomes after treatment with banked VSTs: CMV-infected patients. (A) Depiction of viral load measurements before and during 6 weeks postinfusion of VSTs in 19 evaluable patients (responders and nonresponders) treated for persistent CMV infection. Results are presented as copies per milliliter (CMV detected in blood) or as copies per cell in patients with CMV antigenemia. Because viral loads were measured by different assays at different institutions, interpatient comparisons should not be made. (B) An example of a patient with CMV colitis with evidence of viral inclusions on H&E staining that contained CMV by immunostaining (left panel). After VST infusion, there was a complete resolution of CMV colitis and no evidence of viral inclusions by H&E staining (right panel). (C) Frequency of CMVpp65–directed T cells in peripheral blood before and at weeks 1, 2, 4, and 12 postinfusion, as measured with an IFN-γ ELIspot assay after overnight stimulation of PBMCs with CMVpp65 pepmix. Results are expressed as mean (±SD) spot-forming cells (SFCs) per 2 × 105 input cells.
Responders included patients with established organ disease as well as those with persistent viremia alone. For example, patient #69 presented with a 3-week history of CMV antigenemia in peripheral blood and CMV colitis, despite treatment with cidofovir (Figure 2B). He was infused with VST line C3629, matching at HLA-A24, B14, DR01, and DR03, and with confirmed activity against CMVpp65 (supplemental Table 1). By week 3 postinfusion, there was complete resolution of antigenemia and CMV colitis, as determined by symptom resolution, endoscopy, and biopsy results (Figure 2B). This clinical response was associated with an increased frequency of CMV-specific T cells in peripheral blood (Figure 2C).
Adenovirus.
Of the 18 patients with AdV infections who received VSTs, 17 were evaluable for response (Table 3). Fourteen responded to the infusions with a 6-week cumulative response rate of 77.8% (95% CI, 53.7%-100%) (Figure 1B). Seven patients had a CR and 7 a PR; 3 had no response (Figure 3A and Table 3). Of the 14 responding patients, 7 had a concomitant increase in AdV-specific T cells with an increase in the number of SFCs from a mean (±SEM) of 6.6 ± 3.9 preinfusion to 145.5 ± 123.5 postinfusion per 4 × 105 cells (P = .0391) (supplemental Figure 3).
Viral responses to AdV-specific T cells
| Pt. no. . | Viral disease . | Duration of previous standard therapy . | Line infused . | HLA match (low-resolution) to recipient . | No. of infusions . | Best response by 6 wk . | Comments . |
|---|---|---|---|---|---|---|---|
| 18 | AdV | Cidofovir 33 d | C2582 | 1/6 | 1 | PR | PR based on AdV in stool Resolution of elevated liver enzymes |
| 21 | AdV | Cidofovir 40 d | C2339 | 2/6 | 1 | CR | Sustained CR |
| 25 | AdV | Cidofovir 38 d | C3466 | 2/6 | 1 | PR | CR after day 42 |
| 26 | AdV | Renal function precluded use of cidofovir | C3528 | 1/6 | 1 | NR | Died at 14 d postinfusion of progressive AdV infection |
| 38 | AdV | Cidofovir 36 d | C3005 | 2/6 | 2 | PR | CR after day 42 |
| 40 | AdV | Renal function precluded use of cidofovir | C2339 | 2/6 | 1 | NR | Died day 9 post-VST infusion of progressive AdV infection |
| 52 | AdV | Renal function precluded use of cidofovir | C3889 | 1/6 | 1 | PR | CR after day 42 |
| 59 | AdV | Cidofovir 29 d | C2457 | 1/6 | 1 | CR | Sustained CR |
| 68 | AdV | Cidofovir 20 d | C3889 | 1/6 | 3 | PR | PRs but recurrences |
| 69 | AdV/CMV | Cidofovir 5 d - unable to tolerate because of renal function | C3629 | 4/6 | 1 | CR | Sustained CR/AdV; also CR/CMV and resolution CMV colitis |
| 70 | AdV | Cidofovir 3 mo | C2270 | 2/6 | 1 | N/E | Died at 6 d postinfusion of progressive AdV infection |
| 76 | AdV | Cidofovir 9 d | C2457 | 3/6 | 1 | CR | Sustained CR |
| 81 | AdV | Cidofovir 3 wk | C3005 | 3/6 | 1 | NR | Died day 19 post-VST infusion of persistent AdV infection |
| 86 | AdV | Cidofovir 20 d | C2339 | 3/6 | 2 | PR | PR but recurrence, died day 18 post-VST of AdV and paraflu |
| 88 | AdV | Cidofovir 7 d | C4378 | 2/6 | 1 | PR | Sustained CR |
| 94 | AdV | Cidofovir 8 d | C3617 | 2/6 | 1 | CR | Sustained CR |
| 96 | AdV | Cidofovir 14 d | C2353 | 2/6 | 1 | CR | Sustained CR |
| 97 | AdV/CMV | Cidofovir 31 d | C3629 | 2/6 | 1 | CR | Sustained CR; Also CR of CMV |
| Pt. no. . | Viral disease . | Duration of previous standard therapy . | Line infused . | HLA match (low-resolution) to recipient . | No. of infusions . | Best response by 6 wk . | Comments . |
|---|---|---|---|---|---|---|---|
| 18 | AdV | Cidofovir 33 d | C2582 | 1/6 | 1 | PR | PR based on AdV in stool Resolution of elevated liver enzymes |
| 21 | AdV | Cidofovir 40 d | C2339 | 2/6 | 1 | CR | Sustained CR |
| 25 | AdV | Cidofovir 38 d | C3466 | 2/6 | 1 | PR | CR after day 42 |
| 26 | AdV | Renal function precluded use of cidofovir | C3528 | 1/6 | 1 | NR | Died at 14 d postinfusion of progressive AdV infection |
| 38 | AdV | Cidofovir 36 d | C3005 | 2/6 | 2 | PR | CR after day 42 |
| 40 | AdV | Renal function precluded use of cidofovir | C2339 | 2/6 | 1 | NR | Died day 9 post-VST infusion of progressive AdV infection |
| 52 | AdV | Renal function precluded use of cidofovir | C3889 | 1/6 | 1 | PR | CR after day 42 |
| 59 | AdV | Cidofovir 29 d | C2457 | 1/6 | 1 | CR | Sustained CR |
| 68 | AdV | Cidofovir 20 d | C3889 | 1/6 | 3 | PR | PRs but recurrences |
| 69 | AdV/CMV | Cidofovir 5 d - unable to tolerate because of renal function | C3629 | 4/6 | 1 | CR | Sustained CR/AdV; also CR/CMV and resolution CMV colitis |
| 70 | AdV | Cidofovir 3 mo | C2270 | 2/6 | 1 | N/E | Died at 6 d postinfusion of progressive AdV infection |
| 76 | AdV | Cidofovir 9 d | C2457 | 3/6 | 1 | CR | Sustained CR |
| 81 | AdV | Cidofovir 3 wk | C3005 | 3/6 | 1 | NR | Died day 19 post-VST infusion of persistent AdV infection |
| 86 | AdV | Cidofovir 20 d | C2339 | 3/6 | 2 | PR | PR but recurrence, died day 18 post-VST of AdV and paraflu |
| 88 | AdV | Cidofovir 7 d | C4378 | 2/6 | 1 | PR | Sustained CR |
| 94 | AdV | Cidofovir 8 d | C3617 | 2/6 | 1 | CR | Sustained CR |
| 96 | AdV | Cidofovir 14 d | C2353 | 2/6 | 1 | CR | Sustained CR |
| 97 | AdV/CMV | Cidofovir 31 d | C3629 | 2/6 | 1 | CR | Sustained CR; Also CR of CMV |
Abbreviations are explained in Table 2.
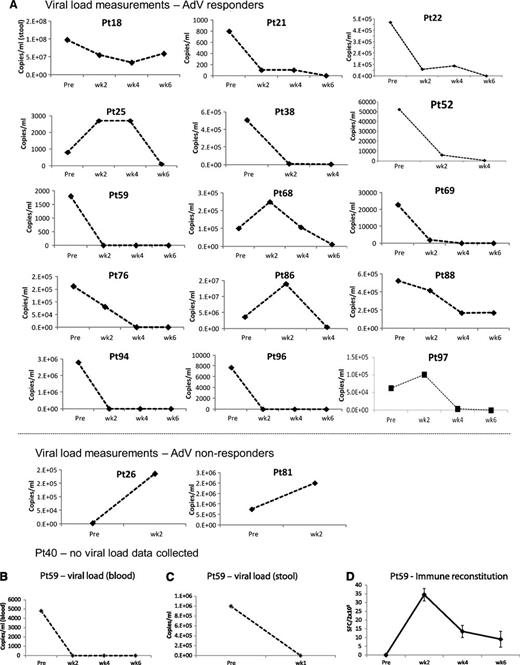
Outcomes after treatment with banked VSTs: AdV-infected patients. (A) Serial viral load measurements before and during 6 weeks post-VST infusion in 17 evaluable patients (responders and nonresponders) treated for persistent AdV. Results are presented as copies per milliliter, where Adv was detected either in blood or in the stool. Because viral loads were measured by different assays at different institutions, interpatient comparisons should not be made. In (B) and (C), elevated AdV loads are detected in both blood (B) and stool (C) in a patient (#59) whose infection resolved after VST infusion. Results are expressed as copies per milliliter. (D) Frequency of AdV-directed T cells in peripheral blood before and at weeks 1, 2, 4, and 6 postinfusion, as measured with an IFN-γ ELIspot assay after overnight stimulation of PBMCs with Adv pepmixes (Hexon and Penton). Results are expressed as mean (±SD) SFCs per 2 × 105 input cells.
Outcomes after treatment with banked VSTs: AdV-infected patients. (A) Serial viral load measurements before and during 6 weeks post-VST infusion in 17 evaluable patients (responders and nonresponders) treated for persistent AdV. Results are presented as copies per milliliter, where Adv was detected either in blood or in the stool. Because viral loads were measured by different assays at different institutions, interpatient comparisons should not be made. In (B) and (C), elevated AdV loads are detected in both blood (B) and stool (C) in a patient (#59) whose infection resolved after VST infusion. Results are expressed as copies per milliliter. (D) Frequency of AdV-directed T cells in peripheral blood before and at weeks 1, 2, 4, and 6 postinfusion, as measured with an IFN-γ ELIspot assay after overnight stimulation of PBMCs with Adv pepmixes (Hexon and Penton). Results are expressed as mean (±SD) SFCs per 2 × 105 input cells.
A representative antiviral response is shown in Figure 3B-D. This patient (#59) presented with fever, diarrhea, abdominal pain, and persistently elevated levels of AdV in the stool and blood (1 million and 4800 copies/mL before infusion) and in the duodenal tissue. At 1 week after VST infusion (supplemental Table 1; line C2457, matched at HLA-B35 with activity against AdV), adenoviral copies in the stool were reduced to 100 copies/mL, and AdV viremia and clinical symptoms had resolved (Figure 3B-C). The virological response in this recipient was, again, associated with a persistent increase in AdV-reactive T cells (Figure 3D).
EBV.
Nine patients received VSTs for EBV-associated disease that was refractory to rituximab treatment. Eight had PTLD, and 1 had persistent EBV viremia. The 6-week cumulative response rate for this group (Figure 1B) was 66.7% (95% CI, 36.9%-96.5%), including 2 CRs and 4 PRs. Figure 4A shows the response of viremia in patients #16, #19, #37, #43, #47, and #79. Of these 6 responding patients (Table 4), 4 had a concomitant increase in EBV-specific T cells with the number of SFCs increasing from a mean (±SEM) of 45.7 ± 21.4 preinfusion to 104.5 ± 43.0 postinfusion per 4 × 105 input cells (P = .0156) (supplemental Figure 4).
Outcomes after treatment with banked VSTs: EBV-infected patients. (A) Serial viral load measurements before and during 6 weeks post-VST infusion in patients (responders and nonresponders) with EBV-LPD. Results are presented as copies per milliliter. (B, left) Positron emission tomography scan of a patient with extensive and progressive LPD despite sirolimus therapy, with a second scan (right) showing complete resolution of all lesions after VST infusion. (C) Frequency of EBV-directed T cells in peripheral blood before and at weeks 1, 2, and 4 postinfusion, as measured with an IFN-γ ELIspot assay after overnight stimulation of PBMCs with EBV lymphoblastoid cell lines. Results are expressed as mean (±SD) SFC per 2 × 105 input cells.
Outcomes after treatment with banked VSTs: EBV-infected patients. (A) Serial viral load measurements before and during 6 weeks post-VST infusion in patients (responders and nonresponders) with EBV-LPD. Results are presented as copies per milliliter. (B, left) Positron emission tomography scan of a patient with extensive and progressive LPD despite sirolimus therapy, with a second scan (right) showing complete resolution of all lesions after VST infusion. (C) Frequency of EBV-directed T cells in peripheral blood before and at weeks 1, 2, and 4 postinfusion, as measured with an IFN-γ ELIspot assay after overnight stimulation of PBMCs with EBV lymphoblastoid cell lines. Results are expressed as mean (±SD) SFC per 2 × 105 input cells.
Viral responses to EBV-specific T cells
| Pt. no. . | Viral disease . | Previous standard therapy . | Line infused . | HLA match (low-resolution) to recipient . | No. of infusions . | Best response by 6 wk . | Comments . |
|---|---|---|---|---|---|---|---|
| 16 | EBV | Rituximab (tumor later found to be CD20 negative) | C2274 | 2/6 | 3 | CR | CR EBV-PTLD based on imaging |
| 19 | EBV | Rituximab | C3366 C2270 | 2/6 | 3 | PR | CR based on viral load PR peripheral disease and stable CNS disease at 5 wk based on imaging when removed from study because of relapse of AML |
| 1/6 | |||||||
| 24 | EBV | Rituximab | C3629 | 1/6 | 1 | NR | Decrease in EBV-DNA followed by a chronic GVHD flare treated with steroids. Died of EBV lymphoma |
| 37 | EBV | Rituximab | C3617 | 3/6 | 1 | CR | CR EBV-PTLD based on imaging |
| 43 | EBV | Rituximab | C3005 | 1/6 | 1 | PR | Very good PR based on imaging |
| 47* | EBV (AdV) | Rituximab | C2339 (C2654) | 1/6 | 5 (1) | PR (CR) | Five infusions for EBV-PTLD with CR after 6 wk based on imaging and viral loads (subsequent emergency use of VST infusion for AdV followed by CR) |
| (1/6) | |||||||
| 74 | EBV | Rituximab | C3365 C2582 | 3/6 | 2 | NR | Died of EBV lymphoma. Second line administered after confirming EBV-PTLD origin was in second, not first, CBU |
| 1/6 | |||||||
| 79 | EBV | Rituximab | C3617 | 2/6 | 3 | PR | Transient PRs then recurrences, subsequent CR by viral load after infusion of donor-derived EBV-specific T cells |
| 90 | EBV | Rituximab | C2339 | 3/6 | 1 | NR | Died of EBV lymphoma |
| Pt. no. . | Viral disease . | Previous standard therapy . | Line infused . | HLA match (low-resolution) to recipient . | No. of infusions . | Best response by 6 wk . | Comments . |
|---|---|---|---|---|---|---|---|
| 16 | EBV | Rituximab (tumor later found to be CD20 negative) | C2274 | 2/6 | 3 | CR | CR EBV-PTLD based on imaging |
| 19 | EBV | Rituximab | C3366 C2270 | 2/6 | 3 | PR | CR based on viral load PR peripheral disease and stable CNS disease at 5 wk based on imaging when removed from study because of relapse of AML |
| 1/6 | |||||||
| 24 | EBV | Rituximab | C3629 | 1/6 | 1 | NR | Decrease in EBV-DNA followed by a chronic GVHD flare treated with steroids. Died of EBV lymphoma |
| 37 | EBV | Rituximab | C3617 | 3/6 | 1 | CR | CR EBV-PTLD based on imaging |
| 43 | EBV | Rituximab | C3005 | 1/6 | 1 | PR | Very good PR based on imaging |
| 47* | EBV (AdV) | Rituximab | C2339 (C2654) | 1/6 | 5 (1) | PR (CR) | Five infusions for EBV-PTLD with CR after 6 wk based on imaging and viral loads (subsequent emergency use of VST infusion for AdV followed by CR) |
| (1/6) | |||||||
| 74 | EBV | Rituximab | C3365 C2582 | 3/6 | 2 | NR | Died of EBV lymphoma. Second line administered after confirming EBV-PTLD origin was in second, not first, CBU |
| 1/6 | |||||||
| 79 | EBV | Rituximab | C3617 | 2/6 | 3 | PR | Transient PRs then recurrences, subsequent CR by viral load after infusion of donor-derived EBV-specific T cells |
| 90 | EBV | Rituximab | C2339 | 3/6 | 1 | NR | Died of EBV lymphoma |
AML, acute myeloid leukemia; CBU, cord blood unit; CNS, central nervous system. Other previously used abbreviations are explained in Table 2.
Subsequently went on to have an AdV infection and received a different VST line as emergency treatment after approval by the US Food and Drug Administration.
Figure 4B-C illustrates the antiviral response in patient #37, who presented with extensive and progressive PTLD despite rituximab therapy. At 1 month after infusion of VST line C3617 with confirmed activity against EBV, matched at HLA-A33, B15, DR03 (supplemental Table 1), the second positron emission tomography scan showed that all lesions had resolved (Figure 4B). This clinical and virological response was associated with an increase in virus-reactive T cells (Figure 4C).
Factors influencing response.
Given the heterogeneity of the patients, the range of viruses treated, and the diversity in the phenotype of lines infused (with respect to CD4 and CD8 content), we could not identify correlations between the characteristics of the lines and the likelihood of antiviral response. In particular, no correlation was observed between CD4 number or percentage in the infused line and the clinical response. Lines that were almost exclusively CD4+ produced clinical benefit, as illustrated by patient #37, who received line C3617 as treatment of EBV lymphoma that was refractory after 6 doses of rituximab. Although the line was matched at 3 antigens, with HLA-A33, B15, and DR03 shared, the line was 99% CD4+ but nevertheless produced a complete clinical response. Conversely, line C2274 was composed of 96% of CD8+ T cells and produced clinical responses both in patient #16 (treated for EBV) and patient #20 (treated for CMV). These observations match those made in individuals receiving donor-derived virus-specific CTLs in which lines that are predominantly CD4 or CD8 can both be protective.12
Clinical safety
All of the infusions were well tolerated. There were no immediate adverse effects, and despite the HLA disparity between VSTs and recipients, de novo GVHD occurred in only 2 patients (grade I in each case) (Table 5). In the 8 patients in whom acute GVHD developed within 45 days of the first infusion (grade I in 6 patients, grade II in 1 patient, and grade III in 1 patient), 6 had a history of GVHD before they received VSTs. An additional patient had a flare of chronic skin GVHD. Two patients experienced transplant-associated microangiopathy, a complication that occurs in up to 10% of HSCT recipients, particularly in those receiving sirolimus, as were both of our patients.24 Only 1 patient had secondary graft failure, concomitant with leukemic relapse (Table 6).
Maximal acute GVHD grade within 45 days of first infusion
| . | Maximum acute GVHD grade . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | I . | II . | III . | IV . | ||||||
| Virus . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . |
| CMV (n = 23) | 19 | (82.6) | 3 | (13.0) | 1 | (4.4) | 0 | (0.0) | 0 | (0.0) |
| EBV (n = 9) | 8 | (88.9) | 1 | (11.1) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| AdV (n = 18) | 15 | (83.3) | 2 | (11.1) | 0 | (0.0) | 1 | (5.6) | 0 | (0.0) |
| Total (n = 50) | 42 | (84.0) | 6 | (12.0) | 1 | (2.0) | 1 | (2.0) | 0 | (0.0) |
| . | Maximum acute GVHD grade . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 . | I . | II . | III . | IV . | ||||||
| Virus . | n . | % . | n . | % . | n . | % . | n . | % . | n . | % . |
| CMV (n = 23) | 19 | (82.6) | 3 | (13.0) | 1 | (4.4) | 0 | (0.0) | 0 | (0.0) |
| EBV (n = 9) | 8 | (88.9) | 1 | (11.1) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| AdV (n = 18) | 15 | (83.3) | 2 | (11.1) | 0 | (0.0) | 1 | (5.6) | 0 | (0.0) |
| Total (n = 50) | 42 | (84.0) | 6 | (12.0) | 1 | (2.0) | 1 | (2.0) | 0 | (0.0) |
Summary of unexpected adverse events occurring within 30 days of T-cell infusion
| Pt. no. . | Infection cohort . | Days since first infusion . | Description of adverse event . | Severity . | Relationship to VST therapy . | Center expected . | Medical monitor- determined, unexpected grade 3-5 adverse events . |
|---|---|---|---|---|---|---|---|
| 16 | EBV | 40 | TMA | 3: Severe | 3 - Possible | 2 - No | Yes |
| 24 | EBV | 19 | Chronic cough | 2: Moderate | 3 - Possible | 2 - No | No |
| 24 | EBV | 28 | TMA/hypoxia | 4: Life threatening | 3 - Possible | 2 - No | Yes |
| 47 | EBV | 1 | Elevated lipase | 3: Severe | 3 - Possible | 2 - No | Yes |
| 47 | EBV | 1 | Hemorrhage, gastrointestinal | 3: Severe | 3 - Possible | 2 - No | Yes |
| 61 | CMV | 28 | Grade 4 neutropenia | 3: Severe | 1 - Unrelated | 2 - No | Yes |
| 76 | AdV | 6 | Hemorrhagic cystitis | 3: Severe | 1 - Unrelated | 2 - No | Yes |
| 86 | AdV | 0 | Anaphylaxis | 4: Life threatening | 2 - Unlikely | 2 - No | Yes |
| Pt. no. . | Infection cohort . | Days since first infusion . | Description of adverse event . | Severity . | Relationship to VST therapy . | Center expected . | Medical monitor- determined, unexpected grade 3-5 adverse events . |
|---|---|---|---|---|---|---|---|
| 16 | EBV | 40 | TMA | 3: Severe | 3 - Possible | 2 - No | Yes |
| 24 | EBV | 19 | Chronic cough | 2: Moderate | 3 - Possible | 2 - No | No |
| 24 | EBV | 28 | TMA/hypoxia | 4: Life threatening | 3 - Possible | 2 - No | Yes |
| 47 | EBV | 1 | Elevated lipase | 3: Severe | 3 - Possible | 2 - No | Yes |
| 47 | EBV | 1 | Hemorrhage, gastrointestinal | 3: Severe | 3 - Possible | 2 - No | Yes |
| 61 | CMV | 28 | Grade 4 neutropenia | 3: Severe | 1 - Unrelated | 2 - No | Yes |
| 76 | AdV | 6 | Hemorrhagic cystitis | 3: Severe | 1 - Unrelated | 2 - No | Yes |
| 86 | AdV | 0 | Anaphylaxis | 4: Life threatening | 2 - Unlikely | 2 - No | Yes |
TMA, thrombotic microangiopathy.
T-cell persistence
To obtain further evidence that the VST infusions had contributed to beneficial immune reconstitution, we used deep-sequencing analysis of peripheral blood T-cell TCR vβ chains in 6 treated patients (4 responders and 2 nonresponders) to track T-cell clones that were present in the infused line but absent in the patients before treatment. In each of 4 responders, donor VST-derived TCR sequences became apparent concomitantly with the reduction in viral titers (supplemental Figure 5), which, in 1 patient, persisted through 12 weeks after infusion. Finally, the benefits associated with VST infusions appeared to be durable. Of the 37 responding patients, only 4 (11%) experienced later progression or recurrence of the original virus infection (Tables 2-4).
Discussion
In this multicenter trial, we administered banked VST lines to 50 recipients of allogeneic stem cell transplants who had intractable EBV, CMV, or AdV infection or disease. From a bank of just 32 VSTs, we were able to identify a line for 90% of the screened patients within 24 hours. Of the 50 patients who were treated with these VSTs, 74.0% had a CR or PR (73.9% for CMV, 77.8% for AdV, and 66.7% for EBV), and most of these responses (89%) were durable. By contrast, patients with intractable disease while receiving standard therapy have had a consistently dismal outcome,25,26 a record confirmed by the outcome in the 8 patients in the current study for whom a line was not available and who continued with “standard therapy.” They had a 13% response rate, and 6 (75%) died of viral disease (supplemental Table 2). Therefore, this use of banked cells overcomes a major impediment to the broader use of VST therapy to treat intractable viral illness after HSCT, namely the lengthy time and cost or complexity of generating individual cell lines for individual donor-recipient pairs, as well as the difficulty of preparing such cells from nonimmune sources, such as cord blood donors.
The small bank size we used reflects the efficacy of VSTs, even when matched at only a single relevant HLA allele with the recipient, provided that this allele could present relevant virus-derived antigens. This advantage results from the ability of a single multispecific VST line to provide effective treatment to individuals simultaneously infected with more than one of the targeted viruses, such as patient #22 (CMV+AdV), patient #69 (CMV+AdV), and patient #97 (CMV+AdV) (Tables 2 and 3). Despite a high degree of HLA disparity between VSTs and the recipients, the mean number of VSTs increased after infusion, coinciding with a decrease in viral DNA levels and resolution of clinical symptoms. These benefits were apparent regardless of the level of HLA matching. Deep-sequencing analysis of TCRvβ CDR3 in the peripheral blood of 4 responding patients (2 with CMV, 1 with EBV, and 1 with AdV) detected the presence of donor-derived VST cells, which persisted for up to 12 weeks after infusion in 1 patient. The capacity of VSTs with such limited matching to survive and function in vivo likely reflects the depth of suppressed immunity in these transplant recipients. However, it is notable that the immune activity increased far less than that observed in patients who had received VSTs derived from their own (HLA-matched) stem cell donor.10 Moreover, in almost half of the responders, there was no measurable increase in the frequency of VSTs. There are several possible explanations for this observation. The most obvious is that the infusion in these patients contributed no benefit, and that disease resolution was associated with alternative therapy. We believe this is unlikely, given the extensive prior therapies these patients received (Tables 2-4) and the poor outcome of patients whose posttransplant viral infections fail to respond to conventional therapy.25,26 An alternative explanation is that many infused lines had antiviral activity only recognized in the context of a single HLA allele. Because the infused product was multispecific, the activity against any single epitope may be too low for detection postinfusion: a problem of “signal-to-noise” ratio. It is also possible that the majority of virus-specific cells may be at the site of disease rather than at elevated frequencies in the peripheral blood, the compartment we sampled. Moreover, samples were batched and analyzed after freeze and thaw, and we have shown that such manipulation can lead to underestimation of virus-reactive T cells. Finally, in this study, our first postinfusion follow-up time point was day 14 after the infusion, and this may have been too delayed to detect early, but nonetheless significant, activity in some patients.
It is notable that the same line produced antiviral activity in some patients and not in others. For example, C3889 was used to treat 6 patients (4 with CMV and 2 with AdV). Four of the 6 patients (2 with CMV and 2 with AdV) had antiviral responses, whereas in the other 2 patients (#71 and #84), the line infused should have had potent CMV-directed activity in the context of the single-shared (HLA-A24) allele. Both of these patients had a history of GVHD. We do not yet have sufficient information to know whether failure was a consequence of patient infection with a strain of CMV mutated across the targeted epitope, making it resistant to the infused T cells, or if the recipient more immediately rejected the third-party cells by a more sensitized host immune system.
In the majority of patients, we saw expansion of HLA-mismatched VSTs, which was not associated with any evidence for destructive alloreactivity. There was no significant de novo GVHD and only 1 episode of secondary stem-cell graft failure (in a patient with relapse). Although recrudescence of GVHD occurred in 6 patients, steroid therapy had already been tapered for each of these individuals because of the viral infection. The low incidence of de novo GvHD may reflect the lineage of the VSTs, which are largely central or effector memory-derived T cells that are less likely than naïve T cells to be alloreactive.27 Two patients did experience transplant-associated microangiopathy, attributable to concurrent sirolimus or to an unusual manifestation of alloreactivity.
The banked-cell approach we describe circumvents a major obstacle to the wider use of VSTs to treat severe viral infections after HSCT. It avoids the lengthy time and cost or complexity of making individual cell lines for individual donor-recipient pairs without encountering the potential problems of “off-the-shelf” third-party VSTs that include rapid rejection by a mismatched host, the induction of GVHD or rejection because of alloreactivity of the infused line, or the need for a vast panel of VSTs if close HLA matching is required for safety and therapeutic benefit. Although a randomized trial will be required to definitively assess the value of banked VSTs for the treatment of these viral infections in HSCT recipients as well as in other groups of immunocompromised patients, the current results from a multicenter study support the feasibility and effectiveness of even small banks of third-party VSTs for severe CMV, AdV, and EBV infections after allogeneic transplantation with either adult or cord blood-derived stem cells.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the enrolling physicians and study coordinators at each site, Yu-Feng Lin for study coordination for searches, Kate Christensen for data management, Deborah Lyon for QC testing, and Oumar Diouf for assistance with GMP production. The authors also thank the National Marrow Donor Program for identifying homozygous normal donors.
The clinical trial was supported by National Institutes of Health-NHLBI grant U54HL08100, Specialized Centers for Cellular Therapy, and the manufacture of VSTs by NHLBI Production Assistance for Cellular Therapy. The authors also appreciate the support of shared resources by cancer center support grant P30CA125123.
Authorship
Contribution: A.M.L., M.K.B., C.M.R., and H.E.H. participated in the study design; H.E.H. was the study chair and wrote the clinical protocol; C.M.B., E.J.S., P.S., J.H.A., N.K., S.-Y.P., S.D.R., B.R.D., and P.K. were site lead investigators and reviewed the clinical protocol; H.E.H., B.J.G., and M.K.B. were the IND holders; A.M.M. was the study statistician; A.M.L. and C.M.R. developed manufacturing methodology, and A.P.G. provided QA of manufactured lines; A.M.L., C.M.B., and H.E.H. selected CTL lines; A.M.L. undertook immune monitoring and analysis; C.M.B., A.M.M., E.J.S., P.S., J.H.A., N.K., S.-Y.P., S.D.R., P.K., and H.E.H. were on the clinical end points review committee; A.M.L., M.K.B., C.M.R. and H.E.H. analyzed the laboratory data and wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helen Heslop, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1640, Houston, TX 77030; e-mail: hheslop@bcm.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal