Key Points
Mast cells contribute to early neutrophil recruitment.
Mast cells and macrophages both make CXCL1 and CXCL2.
Abstract
Neutrophil recruitment is an important early step in controlling tissue infections or injury. Here, we report that this influx depends on both tissue-resident mast cells and macrophages. Mice with mast cell deficiency recruit reduced numbers of neutrophils in the first few hours of intraperitoneal lipopolysaccharide (LPS) stimulation. Conversely, in mice with clodronate-ablated macrophages, neutrophils extravasate, but have limited ability to reach the peritoneal fluid. Tissue macrophages synthesize neutrophil chemoattractants CXCL1/CXCL2 (CXC chemokine ligands 1/2) in response to LPS. Mast cells also produce these chemokines of which a proportion are preformed in granules. Release of the granules and new CXCL1/CXCL2 synthesis is Toll-like receptor 4–dependent. Both in vivo studies with blocking monoclonal antibodies and in vitro chemotaxis experiments show the neutrophil response to mast cells and macrophages to be CXCL1/CXCL2-dependent. The data are in keeping with the model that mast cells, optimally positioned in close proximity to the vasculature, initiate an early phase of neutrophil recruitment by releasing the chemoattractants CXCL1/CXCL2. Having arrived within the stimulated tissue, neutrophils penetrate further in a macrophage-dependent manner. Therefore, we demonstrate a positive role for mast cells in tissue inflammation and define how this comes about with contribution from a second tissue cell, the macrophage.
Introduction
A major stimulus for activation of the innate arm of the immune response is recognition of invading microorganisms and their products by the Toll-like receptors (TLRs).1-3 A result of this stimulation is the early arrival of neutrophils at sites of tissue infection by pathogens such as bacteria. Neutrophils play a critical initial role in controlling infections, first by phagocytosing the microorganisms and second by releasing mediators that draw other leukocytes into the injured tissue.4,5 It is therefore important to understand how these cells are recruited. The CXC chemokine receptor 2 (CXCR2)–binding chemokines, CXC chemokine ligand 1 (CXCL1; KC), CXCL2 (macrophage inflammatory protein–2 [MIP-2]), and, in some settings, CXCL5 and CXCL7, are potent chemoattractants to which neutrophils respond.6-8 Previously, we reported that TLR4-expressing tissue macrophages release CXCL1 and CXCL2 following stimulation with lipopolysaccharide (LPS), a major membrane component of gram-negative bacteria.9
Mast cells have been best analyzed in terms of their role in allergic reactions induced by crosslinking their surface immunoglobulin E.10,11 They are, however, becoming increasingly associated with innate immunity and with neutrophil recruitment following bacterial infection.12-15 As mast cells express TLRs, including TLR4, direct activation by bacterial products might be possible.16,17 Although sparsely represented in tissues, they are strategically located in close proximity to vessels and thus in a position to directly influence circulating leukocytes.14,15,18,19
Mast cells and macrophages are both long-lived tissue cell types with the capacity to make a wide array of biological mediators capable of modifying immune responses. For mast cells, preformed products like histamine, proinflammatory cytokines including tumor necrosis factor (TNF) and proteases, chymase, tryptase, and carboxypeptidase A are released in granule form within seconds after stimulation, whereas lipid mediators such as leukotrienes, cyclo-oxygenase and many cytokines are newly synthesized at sites of infection.18 TNF, leukotrienes, chymases, and tryptases are all reported to be important for neutrophil recruitment.14,20,21
In this study, we show that mast cells and macrophages both contribute to the early stages of recruiting neutrophils into tissues. Mast cells release neutrophil chemoattractants CXCL1 and CXCL2 in both granular and newly synthesized forms following LPS stimulation. As previously demonstrated, macrophages release only synthesized CXCL1/CXCL2 under these circumstances. Mast cells that are closely associated with the vasculature are therefore in an advantageous position to initiate the recruitment of neutrophils from the circulation. This is followed by their migration further into tissue that is regulated by scattered tissue macrophages, with potentially further mast cell contribution.
Material and methods
Mice
C57BL6/JCrl mice were used for the majority of experiments; TLR4−/−, MyD88−/−, and TRIF−/− mice (all C57BL6/JCrl background) were obtained from Dr Caetano Reis e Sousa (Cancer Research UK London Research Institute) with the kind permission of Dr Shizuo Akira (Osaka University, Osaka, Japan). Mcpt5-Cre iDTR mice and Mcpt5-Cre R-DTA mice (C57BL6/J background) were generated as described.19 Cre− littermates were used as control mice. To induce selective depletion of mast cells, both Mcpt5-Cre+iDTR+/− and Cre−iDTR+/− littermate control mice received 2 consecutive weekly injections IV of 25 ng of diphtheria toxin (DT) per gram of body weight and were used 1 week after the last DT injection. All experiments made use of sex-matched 8- to 12-week-old mice and were conducted in accordance with the regulations of the UK Home Office, Landesdirektion Dresden and the Landesverwaltungsamt Sachsen-Anhalt (reference number 203.h-42502-2-879-UniMD).
In vivo peritonitis model
Peritonitis was induced by intraperitoneal (IP) injection of 10 ng of Ultra Pure Salmonella minnesota LPS (Alexis Biochemicals) in 500 μL of phosphate-buffered saline (PBS). In some experiments, mice were injected IV 15 minutes before LPS with rat monoclonal antibodies (mAbs) specific for CXCL1, CXCL2, or IgG2a and IgG2b isotype controls (total of 100 μg per mouse; R&D Systems Europe Ltd) as described previously.22 To ablate macrophages, mice were IP injected twice (day −4 and day −1) with 200 μL of a suspension of clodronate liposomes containing ∼1 mg of encapsulated clodronate (Roche Diagnostics GmbH) or control PBS liposomes.23 Peritoneal cells were collected by washing with 5 mL of cold PBS containing 5mM EDTA, and macrophages and neutrophils were quantified as described in “Flow cytometry” in the supplemental Methods.
Preparation of purified mast cell population
Peritoneal leukocytes (2 × 108/mL) were fractionated on a preformed continuous gradient generated from 70% isotonic Percoll in 0.15M NaCl (Amersham Pharmacia Biotech). The gradient was calibrated between 1.018 and 1.138 g/mL with density marker beads (Amersham Biosciences). Mast cells were collected at band density of 1.088 g/mL and their purity was >95% positive when tested by flow cytometry using CD117–fluorescein isothiocyanate mAb (BD Biosciences).
In vitro stimulation of mast cells
Purified peritoneal mast cells at 0.5 × 105 cells per mL were stimulated in 24-well plates (Falcon) with the indicated amount of S minnesota LPS (Alexis Biochemicals) or medium alone at 37°C for various time periods. Cycloheximide (CHX) (5 μg/mL; Sigma-Aldrich) was added to selected samples to inhibit protein synthesis. The cell-culture supernatants were removed, spun, and analyzed by enzyme-linked immunosorbent assay (ELISA) for the presence of chemokines (R&D Systems Europe Ltd).
Neutrophil chemotaxis in vitro
To generate supernatants, purified peritoneal mast cells were adhered to 24-well plates (Falcon) at 0.5 × 105 cells per mL and stimulated with 1 μg/mL S minnesota LPS or medium alone at 37°C for 4 hours. Spun and filtered supernatants were placed in the Transwell inserts at 600 μL per well (3-μm Polycarbonate membrane; Costar). Recombinant mouse CXCL1 protein at 10 ng/mL (R&D Systems Europe Ltd) was used as positive control.
Bone marrow leukocytes (5 × 106) in 100 μL, with/without preincubation for 15 minutes at 37°C with anti-mouse CXCR2 mAb (10 or 20 μg per sample) or rat IgG2a (20 μg per sample) (both R&D Systems Europe Ltd), were added to the Transwell inserts. Neutrophils in the harvested cell population were identified and quantified using 1A8–fluorescein isothiocyanate (Ly-6G) and analyzed as described in “Flow cytometry” in the supplemental Methods.
Additional methods
Flow cytometry, immunohistochemistry, confocal microscopy, and statistical analysis are found in supplemental Methods (available on the Blood website).
Results
Mast cells express neutrophil-attracting chemokines, CXCL1 and CXCL2
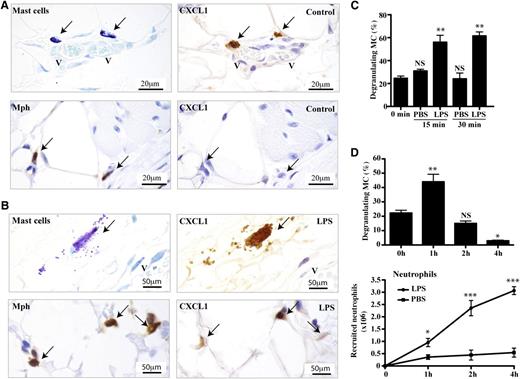
As mast cells have not been fully characterized as chemokine-producing cells in vivo, we investigated their ability to produce neutrophil-attracting chemokines. The first observation was that they express CXCL1 constitutively, whereas macrophages do not (Figure 1A). Following IP stimulation with LPS, mast cells in the peritoneal wall released CXCL1-containing granules within their immediate environment and the macrophages also now expressed CXCL1 as previously demonstrated (Figure 1B).9 Control immunostaining as performed with either isotype-specific mAb (data not shown) or no primary mAb demonstrated that the detection of CXCL1 was specific (supplemental Figure 1A).24,25 Mast cells also expressed CXCL2 (MIP-2), the close homolog of CXCL1 (supplemental Figure 1B). The release of the mast cell CXCL1 granules in vivo was transient as it was detectable as early as 15 minutes and up to 1 hour before diminishing over the subsequent 4 hours (Figure 1C-D). The mast cells did not release their total content of CXCL1-expressing granules (data not shown). Neutrophil recruitment into peritoneal fluid was significant at 1 hour and associated in timing with mast cell degranulation, at least at this early stage (Figure 1D).
Release of CXCL1-containing granules by mast cells in vivo after LPS stimulation. Consecutive sections of peritoneal wall tissue obtained 1 hour after IP injection of (A) PBS or (B) LPS into WT mice. The sections were stained with toluidine blue (mast cells), with MAC-2 (Mph) or anti-CXCL1 mAbs. Arrows indicate mast cells or macrophages overlapping with CXCL1-expressing cells. The immunostaining is representative of tissues from n = 4 mice. (C) Percentage of mast cells displaying CXCR1 granules in peritoneal wall sections of mice treated with either PBS or LPS over time. Mean ± SEM of 1 experiment of 2 is shown with each group representing 3 mice. (D) Percentage of mast cells displaying CXCR1 granules in peritoneal tissue (top panel) and recruited neutrophils in peritoneal lavage (bottom panel) after IP injection of LPS or PBS over 4 hours. Data represent mean ± SEM from n = 3 experiments with each data point representing n = 8 mice. *P < .05; **P < .01; and ***P < .001. Mph, macrophage; V, vessel.
Release of CXCL1-containing granules by mast cells in vivo after LPS stimulation. Consecutive sections of peritoneal wall tissue obtained 1 hour after IP injection of (A) PBS or (B) LPS into WT mice. The sections were stained with toluidine blue (mast cells), with MAC-2 (Mph) or anti-CXCL1 mAbs. Arrows indicate mast cells or macrophages overlapping with CXCL1-expressing cells. The immunostaining is representative of tissues from n = 4 mice. (C) Percentage of mast cells displaying CXCR1 granules in peritoneal wall sections of mice treated with either PBS or LPS over time. Mean ± SEM of 1 experiment of 2 is shown with each group representing 3 mice. (D) Percentage of mast cells displaying CXCR1 granules in peritoneal tissue (top panel) and recruited neutrophils in peritoneal lavage (bottom panel) after IP injection of LPS or PBS over 4 hours. Data represent mean ± SEM from n = 3 experiments with each data point representing n = 8 mice. *P < .05; **P < .01; and ***P < .001. Mph, macrophage; V, vessel.
Release of CXCL1-positive mast cell granules is dependent on TLR4 and the MyD88 pathway
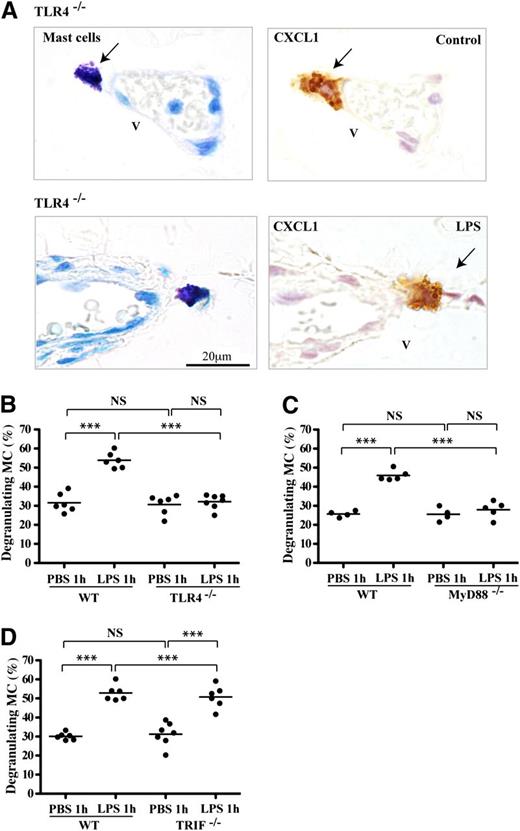
LPS stimulates cells via TLR4 by signaling through 2 distinct pathways characterized by the MyD88 and TRIF adaptor proteins.1-3 We found previously that neutrophils were recruited via TLR4 and the MyD88 pathway.9 We now asked whether TLR4 was involved in the production of CXCL1/CXCL2-containing mast cell granules and/or their release. Mast cells in the peritoneal wall tissue of TLR4−/− mice contained abundant CXCL1, suggesting that TLR4 stimulation was not required for synthesis of this preformed chemokine (Figure 2A). However, following LPS stimulation, there was no release of the CXCL1-containing granules, indicating a dependence on TLR4 triggering for this step. Quantification of the proportion of degranulating mast cells revealed that the LPS-stimulated wild-type (WT) mast cells showed substantial degranulation, whereas the TLR4−/− mast cells displayed background levels of CXCL1 release (Figure 2B). The findings for CXCL2 were similar (data not shown).
CXCL1 in mast cells lacking TLR4 activity. (A) Consecutive peritoneal wall tissue sections of TLR4mut mice immunostained for toluidine blue (mast cells) and chemokine CXCL1 following 1 hour of PBS or LPS stimulation. Arrows indicate mast cells overlapping with CXCL1-expressing cells. Tissue sections are representative of 2 experiments with n = 4 mice per group. (B-D) Numbers of mast cells releasing CXCR1-expressing granules of mice treated with LPS or PBS for 1 hour as a percentage of total mast cells in peritoneal wall sections of (B) TLR4−/−, (C) MyD88−/−, or (D) TRIF−/− mice compared with control C57BL/6 mice. Data are shown as mean ± SEM (n = 4-7 mice/group). *P < .05; **P < .01; and ***P < .001.
CXCL1 in mast cells lacking TLR4 activity. (A) Consecutive peritoneal wall tissue sections of TLR4mut mice immunostained for toluidine blue (mast cells) and chemokine CXCL1 following 1 hour of PBS or LPS stimulation. Arrows indicate mast cells overlapping with CXCL1-expressing cells. Tissue sections are representative of 2 experiments with n = 4 mice per group. (B-D) Numbers of mast cells releasing CXCR1-expressing granules of mice treated with LPS or PBS for 1 hour as a percentage of total mast cells in peritoneal wall sections of (B) TLR4−/−, (C) MyD88−/−, or (D) TRIF−/− mice compared with control C57BL/6 mice. Data are shown as mean ± SEM (n = 4-7 mice/group). *P < .05; **P < .01; and ***P < .001.
To determine which of the 2 signaling TLR4 pathways was active in causing CXCL1-expressing granule release, both MyD88−/− and TRIF−/− mice were LPS-stimulated. The result was that the MyD88−/− mast cells were unable to release CXCL1 granules (Figure 2C), but the TRIF−/− mice resembled WT mice (Figure 2D). Thus, either direct or indirect stimulation of TLR4 signaling through the MyD88 pathway caused prechemokine-loaded mast cells to release the CXCL1 chemokine in granule form.
Mast cell chemokines cause neutrophil chemoattraction
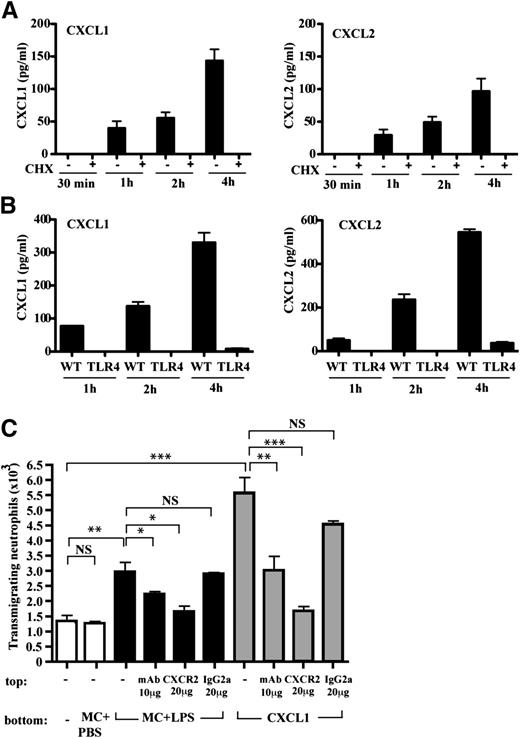
It is well described in the literature that mast cells do not degranulate in vitro in response to LPS26-30 and we confirmed this finding with respect to CXCL1/CXCL2 (data not shown). Another issue was whether new synthesis of CXCL1/CXCL2 occurred and whether this was TLR4-dependent. An investigation of chemokine production using CHX to block protein synthesis demonstrated that mast cells were able to newly synthesize both CXCL1 and CXCL2 in measurable amounts after 1 hour of LPS stimulation (Figure 3A). In contrast, cultured TLR4−/− mast cells synthesized neither CXCL1 nor CXCL2 under identical conditions of LPS exposure providing evidence that the stimulus was direct (Figure 3B).
Mast cells release CXCR2-binding neutrophil chemoattractants. (A) Time course of CXCL1 and CXCL2 ± CHX synthesis by purified mast cells; mean ± SEM of 4 experiments. (B) Time course of CXCL1 and CXCL2 synthesis by purified WT and TLR4−/− mast cells; mean ± SEM of 2 experiments. (C) Supernatants from purified mast cells stimulated with PBS (MC + PBS) or LPS (MC + LPS) for 4 hours tested for neutrophil chemoattractant activity and compared with recombinant CXCL1. Neutrophils were preincubated with anti-CXCR2 or control IgG2a mAbs; mean ± SEM of 3 experiments is shown.
Mast cells release CXCR2-binding neutrophil chemoattractants. (A) Time course of CXCL1 and CXCL2 ± CHX synthesis by purified mast cells; mean ± SEM of 4 experiments. (B) Time course of CXCL1 and CXCL2 synthesis by purified WT and TLR4−/− mast cells; mean ± SEM of 2 experiments. (C) Supernatants from purified mast cells stimulated with PBS (MC + PBS) or LPS (MC + LPS) for 4 hours tested for neutrophil chemoattractant activity and compared with recombinant CXCL1. Neutrophils were preincubated with anti-CXCR2 or control IgG2a mAbs; mean ± SEM of 3 experiments is shown.
Although mast cells could produce CXCR2-binding chemokines, a key issue was whether these chemokines or another mast cell product was active in neutrophil recruitment. We collected supernatant from freshly isolated mast cells stimulated for 4 hours with PBS or LPS and asked whether it was chemotactic for neutrophils compared with recombinant CXCL1. Importantly, the LPS-stimulated, but not control, mast cell supernatant had chemotactic activity (Figure 3C). The next question was whether the chemoattractant was a CXCR2-binding chemokine. Anti-CXCR2 mAb blocked chemotaxis to recombinant CXCL1 and also the chemotactic effect of the LPS-stimulated mast cell supernatant (Figure 3C). Thus, this mast cell supernatant contained CXCR2-binding chemokines that were active in inducing neutrophil chemotaxis at least in vitro.
In addition to CXCL1 and CXCL2, other CXCR2-binding ligands such as CXCL5 (Lix) and CXCL7 (NAP-2) can act as neutrophil chemoattractants.31 CXCL1 was the major chemokine synthesized by cultured peritoneal mast cells over 24 hours followed by lower levels of CXCL2, but no CXCL5 or CXCL7 was detected (supplemental Figure 2A). By comparison, bone marrow–derived tissue macrophages also produced CXCL1 and CXCL2 as well as detectable levels of CXCL5 and CXCL7 but only after exposure to LPS up to 24 hours (supplemental Figure 2B).
In summary, these results indicate that cultured mast cells released newly synthesized chemokines CXCL1 and CXCL2, but not CXCL5 and CXCL7, in response to LPS in vitro. Although other mast cell products may also be involved in neutrophil recruitment, the CXCR2 chemokines present in cultured mast supernatant stimulate neutrophil chemotaxis.
A role for CXCL1/CXCL2 in recruiting neutrophils in vivo
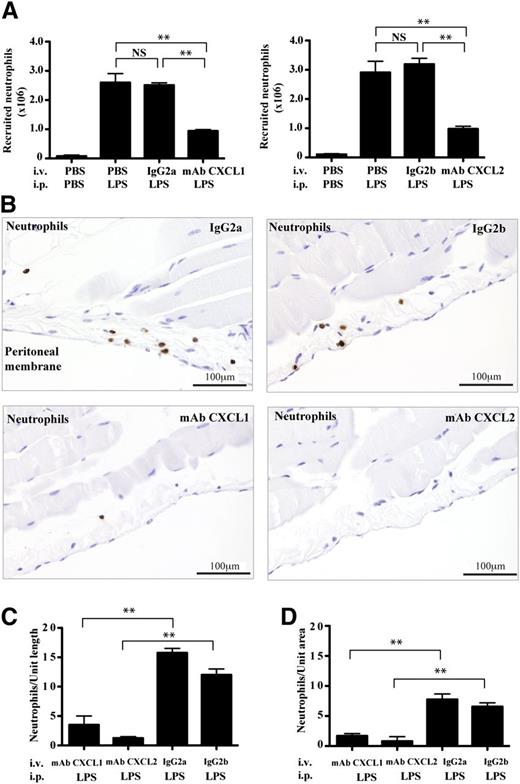
The experiments so far show that both mast cells and macrophages release CXCR2-binding chemokines CXCL1 and CXCL2 and, specifically, that the mast cell chemokines can chemoattract neutrophils in vitro. It was, however, important to test whether blocking the activity of the CXCL1/CXCL2 in vivo would affect neutrophil recruitment into tissue. We therefore injected mice IV with anti-CXCL1, anti-CXCL2, or isotype control mAbs and, after 2 hours of exposure to LPS, found that neutrophil recruitment into the peritoneal cavity was significantly suppressed by the chemokine mAbs (Figure 4A). Blocking CXCL1 and CXCL2 together yielded no additional reduction in neutrophil recruitment, further suggesting that they functioned cooperatively (supplemental Figure 3A). The use of the mAbs did not alter the level of circulating neutrophils, showing that they were not having their effect indirectly by causing neutrophil depletion (supplemental Figure 3B).
The effect of blocking CXCL1/CXCL2 in vivo on the pattern of neutrophil recruitment. (A) Numbers of recruited neutrophils in peritoneal fluid of mice at 2 hours that were injected IV with anti-CXCL1, anti-CXCL2, or isotype control mAbs 15 minutes before IP injection either with LPS or PBS; mean ± SEM of n = 2 experiments with n = 4 mice per group. (B) Peritoneal wall sections immunostained for neutrophils (brown) from LPS-stimulated mice that were treated as in panel A. Representative images from n = 2 experiments with n = 4 mice per group. Quantification of neutrophils either (C) lining the peritoneal membrane as in panel B or (D) within the peritoneal wall tissue; n = 4 tissue sections.
The effect of blocking CXCL1/CXCL2 in vivo on the pattern of neutrophil recruitment. (A) Numbers of recruited neutrophils in peritoneal fluid of mice at 2 hours that were injected IV with anti-CXCL1, anti-CXCL2, or isotype control mAbs 15 minutes before IP injection either with LPS or PBS; mean ± SEM of n = 2 experiments with n = 4 mice per group. (B) Peritoneal wall sections immunostained for neutrophils (brown) from LPS-stimulated mice that were treated as in panel A. Representative images from n = 2 experiments with n = 4 mice per group. Quantification of neutrophils either (C) lining the peritoneal membrane as in panel B or (D) within the peritoneal wall tissue; n = 4 tissue sections.
In terms of the distribution of neutrophils within the tissue, treatment with both isotype control mAbs revealed a typical pattern with highest cell concentration at the peritoneal membrane surrounding the cavity, in contrast to both anti-CXCL1– and anti-CXCL2–injected mice that had severely reduced numbers of neutrophils in this location (Figure 4B). When the neutrophils were quantified, the mAb-induced reduction in neutrophil numbers was observed not only at the peritoneal membrane (Figure 4C), but also throughout the tissue (Figure 4D). The conclusion was that these 2 CXCR2-binding chemokines were essential contributors to the recruitment of neutrophils from the first stage of their uptake from the circulation. As both anti-CXCL1 and -CXCL2 mAbs blocked neutrophil entry, the implication was that these chemokines have different roles but operated in the same pathway or sequence of events.
The effect of macrophage depletion on neutrophil recruitment
The data so far have highlighted the essential role of both CXCL1 and CXCL2 in neutrophil recruitment during LPS-mediated peritonitis. We previously described tissue macrophages as a source of these chemoattractants.9 Given that mast cells can also produce CXCL1/CXCL2, a key question was whether these cells and their products had impact in vivo in terms of eliciting neutrophils and, if so, whether there was a specific tissue pattern associated with the recruitment.
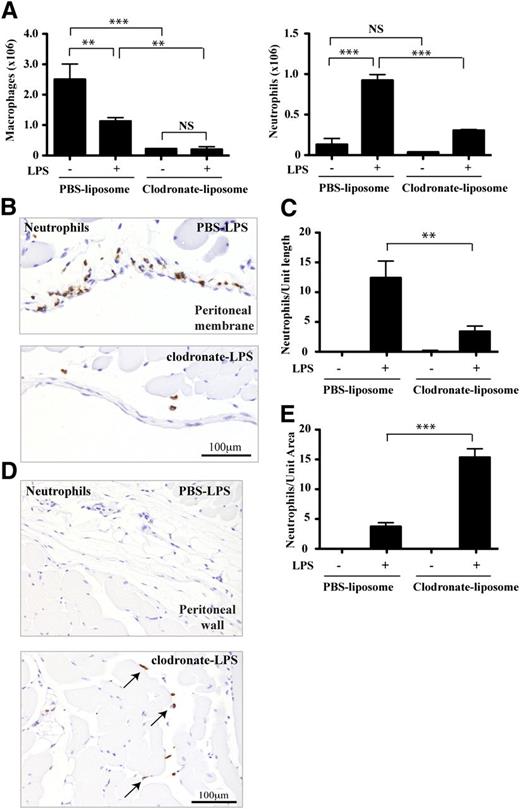
To investigate any role of mast cells following LPS stimulation, and to distinguish mast cell from macrophage activity, we ablated the peritoneal macrophages using clodronate liposomes.23 Following this treatment, the macrophage numbers in the peritoneal cavity were reduced by 92% (Figure 5A). After 2 hours of LPS stimulation, the macrophages of PBS liposome-treated mice were reduced as expected.32 There was, however, no further alteration to the severe reduction in macrophages observed in the clodronate liposome-treated mice. Quantification of tissue mast cells showed that they were unchanged by liposome treatment (0 hours: PBS liposomes vs clodronate liposomes; 171.7 ± 2.1 vs 175.7 ± 1.5 per unit tissue length; n = 4 mice).
The effect of macrophage depletion on neutrophil recuitment. (A) The effect of PBS- vs clodronate-liposome treatment on peritoneal macrophage and neutrophil numbers with/without LPS stimulation for 2 hours; n = 4 experiments with 4 to 8 mice per group in each experiment. (B) Representative tissue sections focusing on the peritoneal membrane and immunostained for neutrophils (brown) from LPS-stimulated mice that have received either PBS- or clodronate-containing liposomes. (C) Quantification of neutrophils lining the peritoneal membrane as in panel B. (D) Typical views of peritoneal wall tissue sections of PBS- and clodronate-liposome treated mice showing neutrophil distribution (brown). (E) Quantification of neutrophils within the peritoneal wall tissue as illustrated in panel D. Data from n = 3 experiments with 4 to 8 mice per group; A,C, and E are plotted as mean ± SEM.
The effect of macrophage depletion on neutrophil recuitment. (A) The effect of PBS- vs clodronate-liposome treatment on peritoneal macrophage and neutrophil numbers with/without LPS stimulation for 2 hours; n = 4 experiments with 4 to 8 mice per group in each experiment. (B) Representative tissue sections focusing on the peritoneal membrane and immunostained for neutrophils (brown) from LPS-stimulated mice that have received either PBS- or clodronate-containing liposomes. (C) Quantification of neutrophils lining the peritoneal membrane as in panel B. (D) Typical views of peritoneal wall tissue sections of PBS- and clodronate-liposome treated mice showing neutrophil distribution (brown). (E) Quantification of neutrophils within the peritoneal wall tissue as illustrated in panel D. Data from n = 3 experiments with 4 to 8 mice per group; A,C, and E are plotted as mean ± SEM.
In terms of neutrophils, they were recruited effectively into the peritoneal fluid of PBS liposome-treated mice following an LPS stimulus, whereas their numbers were dramatically reduced in the clodronate liposome-treated mice (Figure 5A). This negative effect on neutrophil numbers was confirmed immunohistochemically by examining the peritoneal membrane. At 2 hours after LPS stimulation, the membrane from the control PBS liposome-treated mice was lined with neutrophils, whereas in clodronate liposome-treated mice, they were much reduced (Figure 5B) and this was reflected in their quantification (Figure 5C). In contrast in the clodronate liposome-treated mice, unlike control mice, neutrophils were present within the peritoneal wall tissue itself with a distribution pattern ranging from the vicinity of blood vessels through to the stromal network intersecting the muscle layers (Figure 5D-E).
Thus, macrophages are key effectors of neutrophil presence at the peritoneal membrane and within the cavity. However, our observations indicated that, although reduced, there was still neutrophil recruitment into the peritoneal wall tissue in the absence of macrophages. The range of these neutrophils was restricted as they were apparently unable to penetrate as far as the peritoneal membrane and cavity.
Reduced neutrophil extravasation in mice with induced mast cell deficiency
As mast cells were the only tissue cells other than macrophages in which CXCL1 and CXCL2 were easily detected, we asked whether these cells were active in LPS-induced inflammation. We investigated neutrophil recruitment using a novel mouse model of mast cell deficiency19 generated by crossing the mast cell-specific Cre transgenic line, Mcpt5-Cre,33 with the iDTR line.34 In Mcpt5-Cre+iDTR+ offspring, a loxP-flanked stop element is deleted selectively in mast cells by Cre-mediated recombination allowing expression of a simian DT receptor (DTR) and rendering the mast cells sensitive to DT. Following this treatment, mast cell numbers were reduced by 99% in peritoneal fluid of Mcpt5-Cre+iDTR+ mice compared with Cre− control mice (supplemental Figure 4A) and as previously reported.19 Also as previously reported, the numbers of peritoneal macrophages remained unaffected by the mast cell depletion regime.19 In the tissues of Cre-littermate controls, 36.2% of the mast cells were associated with vessels with the remainder scattered within the peritoneal wall tissue (supplemental Figure 4B-C). In contrast in the DT-treated Mcpt5-Cre+iDTR+ mice, mast cells were absent in the peritoneal wall and cavity membrane (supplemental Figure 4A,C).
Following LPS stimulation, neutrophils were recruited to the peritoneal fluid over 4 hours in Cre-control littermate mice. However, the numbers of neutrophils able to reach the peritoneal fluid in Mcpt5-Cre+iDTR+ mice at 1 hour were reduced to 29.4% of Cre-control mice (Figure 6A). After an additional 2 and 4 hours, neutrophil numbers were reduced to 59.1% and 50.0%.
Neutrophil recruitment in Mcpt5-Cre+iDTR+ mast cell–depleted mice. (A) Neutrophils recruited into the peritoneal fluid of Mcpt5-Cre+iDTR+ mice (black) compared with Cre-control mice (gray) post-LPS treatment or PBS treatment of Cre-control mice (white) at 1, 2, and 4 hours; mean ± SEM of n = 2 experiments, n = 5-7 mice per group, is shown. (B) Mast cells in peritoneal fluid in the same experiments as panel A. (C) Representative peritoneal wall tissue sections from Mcpt5-Cre+iDTR+ mice compared with Cre-control littermate mice highlighting neutrophil distribution (brown) 2 hours post-LPS treatment (representative of n = 5 samples for each tissue type). (D) Time course over 1, 2, and 4 hours of neutrophils recruited into peritoneal wall tissue after LPS stimulation of Mcpt5-Cre+iDTR+ mice (black) and Cre-control control mice (gray) or PBS treatment of Cre-control mice (white); n = 3 experiments with 3 to 6 mice per group. (E) Mast cells in peritoneal wall tissue in same experiments as panel D.
Neutrophil recruitment in Mcpt5-Cre+iDTR+ mast cell–depleted mice. (A) Neutrophils recruited into the peritoneal fluid of Mcpt5-Cre+iDTR+ mice (black) compared with Cre-control mice (gray) post-LPS treatment or PBS treatment of Cre-control mice (white) at 1, 2, and 4 hours; mean ± SEM of n = 2 experiments, n = 5-7 mice per group, is shown. (B) Mast cells in peritoneal fluid in the same experiments as panel A. (C) Representative peritoneal wall tissue sections from Mcpt5-Cre+iDTR+ mice compared with Cre-control littermate mice highlighting neutrophil distribution (brown) 2 hours post-LPS treatment (representative of n = 5 samples for each tissue type). (D) Time course over 1, 2, and 4 hours of neutrophils recruited into peritoneal wall tissue after LPS stimulation of Mcpt5-Cre+iDTR+ mice (black) and Cre-control control mice (gray) or PBS treatment of Cre-control mice (white); n = 3 experiments with 3 to 6 mice per group. (E) Mast cells in peritoneal wall tissue in same experiments as panel D.
As a control for the LPS stimulation, PBS-injected Cre-control mice showed no neutrophil recruitment over the 4-hour period. Moreover, the mast cell number in the peritoneal fluid was not altered in either PBS- or LPS-treated Cre-control mice, while it was severely depleted in Mcpt5-Cre+iDTR+ mice (Figure 6B). Macrophage numbers were similar between the different groups of mice and independent of mast cell depletion (for example, at 1 hour of treatment: PBS, Cre-control mice, 26.4 ± 3.7; LPS, Cre-control, 20.45 ± 4.2; LPS, Mcpt5-Cre+iDTR+, 24.1 ± 3.8; mean × 104 ± SD).
In terms of the peritoneal wall tissue, neutrophils were essentially absent after 1 hour of LPS stimulation in Mcpt5-Cre+iDTR+ mice, while they began to accumulate in the tissue of Cre-control littermates (Mcpt5-Cre+iDTR+, 0.5 ± 0.5 vs Cre−, 23.7 ± 1.3 (Mcpt5-Cre+iDTR+/Cre− = 2.0%) (Figure 6C-D). There was some neutrophil recruitment at 2 hours post-LPS in the mast cell-depleted mice (Mcpt5-Cre+iDTR+/Cre− = 17.8%) but this differential with control mice had disappeared by 4 h (Mcpt5-Cre+iDTR+/Cre− = 88.0%). Again, PBS-injected Cre-control mice showed no tissue neutrophil recruitment. The difference between Mcpt5-Cre+iDTR+ mice and Cre-control mice is illustrated immunohistochemically in typical tissue sections at 2 hours post-LPS stimulation where neutrophils were observed to localize at the peritoneal membrane area. Moreover, the tissue mast cell numbers remained similar in both PBS- and LPS-injected Cre-control mice with a major deficiency in Mcpt5-Cre+iDTR+ mice (Figure 6E).
In an additional experiment, we used mice with a constitutive mast cell deficiency that were created by crossing Mcpt5-Cre–expressing mice with the RDTA line.19,35 After 1 hour of LPS treatment, neutrophil recruitment into peritoneal fluid was reduced to 47% of Cre-RDTA littermate mice (1.26.3 ± 0.22 × 106 neutrophils in Cre-RDTA mice vs 0.59 ± 0.17 × 106 neutrophils in Mcpt-Cre+RDTA mice; mean ± SEM, n = 5-6 mice per group). Moreover, these mast cell–deficient mice displayed a 91.6% decrease in neutrophil recruitment into the peritoneal wall tissue after 1 hour of LPS treatment compared with Cre− littermates (Mcpt5-Cre+RDTA+/−, 6.0 ± 4.0 vs Cre−, 71.8 ± 11.6 (mean neutrophil number per unit tissue length ± SEM); Cre+/Cre− = 8.4%).
Therefore, mast cells have an essential role in neutrophil recruitment to an inflammatory agent such as LPS for at least 4 hours poststimulus because in the absence of mast cells, neutrophil migration into the inflamed tissue is much reduced.
Discussion
There is growing interest in understanding how mast cells participate in positive immunoregulation particularly in the innate aspects of an immune response.15,18,36 They have been implicated in recruiting neutrophils that are frequently the first immune cells to enter an inflamed or infected tissue site.4,5 Published data on the in vivo activities of mast cells in neutrophil recruitment and defense against bacterial infection are largely based on experiments in mast cell–deficient kit mutant mouse lines and the reconstitution of these mice with in vitro–differentiated mast cells.12-15,37,38 As these mice display deficiencies beyond the mast cell lineage, in particular in altered neutrophil numbers,39 it seemed worthwhile to reevaluate questions concerning neutrophil recruitment and responsiveness in a mouse model of mast cell deficiency that is independent of kit mutations.40 In this study, we have made use of Mcpt5-Cre+iDTR+ mice with DT-inducible mast cell deficiency that specifically targets the tissue-resident subpopulation of connective tissue-type mast cells without affecting other cells of the leukocyte lineage, and confirmed findings using the constitutive mast cell–deficient Mcpt5-Cre+RDTA mice.19 After injection of both these strains of mice with LPS to mimic a response to gram-negative bacteria, we show that mast cells are essential for neutrophil recruitment into tissue, particularly at an early stage, but that a complete response also requires tissue macrophages. Similarly to macrophages, mast cells release the major neutrophil chemoattractants CXCL1 and CXCL2 in vitro and in vivo. We propose that, together, these 2 tissue-resident cell types contribute to neutrophil recruitment in a 2-step process involving first the capture of neutrophils from the circulation and then, second, their penetration deeper into the injured tissue.
Our previous work highlighted the importance of tissue macrophages in neutrophil recruitment.9 However, in this study, the involvement of a second nonmacrophage cell type was revealed by ablating macrophages. In this situation, neutrophils entered peritoneal tissue, but remained in the vicinity of vessels and within the peritoneal wall, but failed to migrate to the peritoneal membrane and fluid as observed in controls. An important and nonredundant role for mast cells was directly demonstrated using mast cell–depleted mice.19 In these mice, there was a reduction in numbers of neutrophils entering the tissue in spite of the presence of macrophages. This effect was most prominent at the early stage of up to 4 hours of inflammatory stimulus, suggesting that mast cells were responsible for the initial attraction of neutrophils from the circulation and that, in their absence, macrophages were not sufficient. This mast cell effect is in keeping with their proximity to the vasculature where neutrophils first enter tissue from the circulation. Mast cells are also found away from the vessels and in the peritoneal fluid, although without macrophages they are limited in their ability to attract neutrophils toward these tissue locations. Thus, mast cells appear to have a key role as gatekeepers during the first few hours of a response, at least in this model of peritoneal inflammation.
Our data also suggested how mast cells fulfill this function. Granules containing preformed chemokines CXCL1 and CXCL2 were released by mast cells within 15 minutes of in vivo stimulation, constituting an ideal mechanism to stimulate neutrophil entry from the circulation at an early stage following the inflammatory signal. In terms of release of the CXCL1/CXCL2-expressing granules in vivo, this occurred in a TLR4- and TIRAP/MyD88-dependent manner with no dependence on the second TLR4-controlled TRAM/TRIF signaling pathway that is accessed by endocytosed TLR4 receptors.2 Involvement of the MyD88 signaling pathway is compatible with our previous study showing that neutrophil recruitment in response to LPS is MyD88- but not TRIF-dependent.9 These findings contrast with reports stating that mast cells do not degranulate in response to LPS27-30 although they express the TLR4 receptor.16,17 These studies have been performed in vitro and indeed we were also unable to observe LPS-induced granule release from peritoneal mast cells in vitro. It is possible that the in vivo release does not occur directly via TLR4 triggering or that there is a requirement for other accessory factors such as, potentially, the matrix components in which the mast cells are embedded.
In addition, mast cells, like macrophages, also had the capacity to newly synthesize CXCL1 and CXCL2, making detectable amounts within 1 hour of LPS treatment. The fact that synthesis of CXCL1/2 in vitro was curtailed in LPS-treated TLR4−/− mast cells provided evidence that the new synthesis involved direct stimulation through TLR4. In support of our findings are reports that human intestinal mast cells constitutively express IL-8, the homolog of mouse CXCL141 and that human skin connective tissue-type mast cells make IL-8 in granular form.26 It remains possible that these events are indirectly controlled in vivo, but the implication is that TLR4-mediated signaling at the membrane level is sufficient for CXCL1/L2 granule release and new synthesis.
Within peritoneal wall tissue, we identified only mast cells and macrophages to be CXCL1/CXCL2 expressors with these cell types occupying distinct but overlapping tissue spheres. The pattern of complete blockade in neutrophil recruitment in the presence of anti-CXCL1 and anti-CXCL2 implicates these chemokines in early mast cell responses. Interestingly, blocking either chemokine was effective, suggesting that both are necessary for this process in vivo and that they have distinct and nonredundant roles. As CXCL2 binds substantially more strongly to glycosaminoglycans than CXCL1, it might serve a separate function potentially in tethering and tissue-associated gradient formation.42 Other major mast cell products such as TNF, tryptases, and, particularly for mucosal mast cells, the chymases, are reported to have neutrophil chemoattracting activities.43-45 How they compare with CXCL1/CXCL2 in terms of neutrophil recruitment, whether they operate directly or indirectly in vivo or under particular circumstances of mast cell stimulation are all issues of relevance for future investigation. It should also be stated that other tissue cell types such as intestinal epithelial cells also express TLR4 and thus could be expected to participate in the tissue phase of such a response.46,47
In summary, we previously described the key role of tissue macrophages and their synthesis of CXCL1 and CXCL2 in neutrophil recruitment.9 A question arising from that study was how the chemoattractants released by widely scattered tissue macrophages were able to disseminate sufficiently to efficiently capture neutrophils from the circulation. In the current study, we now show that the CXCR2-binding chemokines are indeed central to the process, that neutrophil recruitment requires both mast cells and macrophages and that each cell type has a distinctive role in the process. Their specialized positioning in the affected tissue ensures that a spatially controlled release of CXCR2 ligands is achieved which establishes an effective chemokine presence from the blood vessel deep into the inflamed site. Future work will reveal whether this double role of mast cells and macrophages constitutes a general pattern of responsiveness to tissue infection and injury.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. Watkins and S. Lighterness (CRUK LRI) for excellent animal husbandry. The expert assistance by N. Schubert, C. Hiller, and T. Häring (Dresden) is gratefully acknowledged.
This work was supported by Cancer Research UK (K.D.F., E.N., N.H.) and by grants from the German Research Council: DFG grants SFB 854 and Priority Program 1468 (M.G.), DFG grants Ro2133/2 and Ro2133/3 (A.R.), DFG grants Ro2133/2 and SFB832 (project A14) (K.H.), Ro2133/4 (Priority Program 1394) (A.R.), and Du1172/1, Du1172/2 (Priority Program 1468), and Du1172/3 (Priority Program 1394) (A.D.).
Authorship
Contribution: K.D.F. and A.D. performed experiments, interpreted results, and assisted with writing the manuscript; M.H. performed experiments; A.R. and M.G. interpreted results and commented on the manuscript; E.N. performed immunohistochemistry experiments; N.v.R. supplied an important reagent; K.H. and A.R. collaborated on the mast cell deletion model; and N.H. directed the project and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.H. and M.G. is University Hospital Essen, University Duisburg-Essen, Institute of Experimental Immunology and Imaging, Hufelandstraße 55, D- 45122 Essen, Germany.
Correspondence: Nancy Hogg, Leukocyte Adhesion Laboratory, 44 Lincoln’s Inn Fields, Cancer Research UK London Research Institute, London WC2A 3LY, United Kingdom: e-mail: nancy.hogg@cancer.org.uk.
References
Author notes
K.D.F. and A.D. are joint first authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal