Key Points
Hemostasis, thrombosis, and ischemic brain infarction efficiently occur at unexpectedly low platelet counts in mice.
The threshold platelet count required for occlusive thrombus formation differs between thrombosis models.
Abstract
Platelets are essential mediators of hemostasis and thrombosis. Platelet counts (PCs) in humans average 250 platelets/nL, but it is not entirely clear how platelet numbers affect hemostasis and occurrence of thrombotic events. Mice, displaying PCs of ∼1000 platelets/nL, are widely used to assess platelet function in (patho-)physiology, but also in this species, the significance of PC for hemostasis and thrombotic disease is not established. We reduced PCs in mice to defined ranges between 0 and 1000 platelets/nL by platelet-depleting antibodies and challenged them in different arterial thrombosis models: the transient middle cerebral artery occlusion (tMCAO) stroke model and tail bleeding experiments. We show that thrombotic occlusion of the injured aorta and the carotid artery were partially impaired when PCs were reduced by 70% or 80%, respectively. In contrast, tail bleeding times and thrombus formation in small arterioles were largely unaffected by reductions of PC up to 97.5%. Similarly, infarct growth and neurological deficits after tMCAO were unaffected by reductions of PCs up to 90%, whereas a further reduction was protective. These results reveal that arterial thrombosis, cerebral infarction, and hemostasis in mice efficiently occur at unexpectedly low PCs, which may have implications for humans at risk of thrombotic or hemorrhagic disease.
Introduction
Platelets are small anucleated cell fragments derived from the cytoplasm of bone marrow megakaryocytes. They circulate in the blood stream for ∼8 to 10 days and “survey” the integrity of the vascular system by discriminating between intact and injured vessel walls. On endothelial damage, platelets rapidly adhere to the vessel wall, become activated, and recruit additional platelets to form a stable thrombus that seals the site of injury. This process is essential to limit posttraumatic blood loss, but it is also a major pathomechanism underlying thrombotic vessel occlusion in the setting of myocardial infarction and stroke.1,2 The platelet count (PC) of healthy humans ranges from 150 to 450 platelets/nL blood, with an average of ∼250 platelets/nL, and thrombocytopenia is diagnosed at PCs <150 platelets/nL.3 Thrombocytopenia occurs relatively frequent in humans and can be caused by different mechanisms in the context of a large variety of pathologies or medical treatments. Immune thrombocytopenia (ITP) is an autoimmune disease where autoantibodies directed against platelet surface antigens trigger platelet destruction and may cause moderate to severe bleeding symptoms.4 Immune-mediated drug-induced thrombocytopenia induces peripheral platelet destruction by drug-dependent antibodies that bind to platelet surface glycoproteins only in the presence of the sensitizing drug.5,6 Other drugs induce thrombocytopenia as a major side effect by affecting platelet production in the bone marrow through direct myelosupression.7,8 Thrombocytopenia can also be associated with chronic infections such as HIV9 or hepatitis C10 or may be an accessory syndrome of hereditary diseases.11-13
Generally, medical treatment of thrombocytopenia with functional platelets is indicated in adult patients with a platelet count of <30 platelets/nL blood.14-17 However, it is widely accepted that the decision to treat thrombocytopenia should not entirely be based on the PC, but rather made in the context of clinical symptoms and disease progression.16,18 Indeed, studies in humans have shown that the bleeding risk at reduced PCs is influenced by the individual’s age,19,20 the occurrence of trauma,21 genetic susceptibility, environmental effects, medication, or comorbidity.22-25 Similarly, studies in mice have shown that low PCs alone do not cause spontaneous bleeding, but rather a combination of a low platelet count and additional factors, such as binding of antibodies to endothelial cells,26 inflammation,27 or the nature of the target antigen of antiplatelet antibodies28 determine the occurrence of hemorrhage.
On the other hand, the link between PCs and the occurrence of acute ischemic disease states, such as myocardial infarction or stroke, remains unclear. Two studies revealed an increased incidence of thromboembolic events in humans with ITP and proposed different mechanisms that might explain this thrombotic tendency.3,29 Mice, with an average PC of 1000 platelets/nL blood, are an important model system to study hemostasis and the pathomechanisms underlying ischemic diseases. Although platelet counts can efficiently be lowered in mice, no systematic study has been reported that assessed the significance of PCs for normal hemostasis and susceptibility to experimental ischemic disease states in these animals.
In our study, we induced different grades of thrombocytopenia in mice and subjected them to different models of occlusive thrombus formation and ischemic stroke. We show that (experimental) occlusive thrombus formation and ischemic brain infarction are not or only very moderately affected by a reduction of platelet counts by ≥70%. Similarly, tail bleeding times were unaltered over a wide range of platelet count reductions.
Materials and methods
Mice
Bleeding time and thrombosis model experiments were performed with C57/Bl-6 and 129/Sv mice. For the stroke experiments, 129/Sv/C57/Bl-6 mice were used. Animal studies were approved by the district government of Lower Franconia. Mice were between 6 and 12 weeks of age, if not indicated differently.
Chemicals and reagents
Medetomidine (Pfizer), midazolam (Roche Pharma AG), fentanyl (Janssen-Cilag GmbH), high-molecular-weight heparin (Sigma), adenosine 5′-diphosphate (ADP) (Sigma-Aldrich), thrombin (Roche Diagnostics), convulxin (Enzo Life Science), and rabbit anti-mouse platelet serum (Accurate Chemical) were purchased. The antibody against activated integrin αIIbβ3 (JON/A-PE), platelet-depleting anti-glycoprotein (GP) Ibα antibody, and IgG control were from Emfret Analytics. All other antibodies were generated and modified in our laboratory.28
Platelet depletion and calculation of the platelet count
For platelet depletion, mice received rat anti-mouse GPIbα antibodies or rabbit anti-mouse platelet serum intravenously in phosphate-buffered saline (PBS) 12 hours prior to the experiment. Control mice received the same volume of vehicle or irrelevant rat IgG. To measure the platelet count, mice were bled retro-orbitally, and the blood samples were collected into heparin-containing tubes. The blood sample (50 µL) was further diluted with modified Tyrode–N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES; 5 mM glucose, 0.35% bovine serum albumin, pH 7.4) to a final dilution of 1:20. Fifty microliters of the diluted sample was stained with conjugated anti-integrin αllbβ3-phycoerythrin antibody (clone JON228 ) and anti–GPV-fluorescein isothiocyanate (clone DOM128 ) for 15 minutes at room temperature to identify the platelet population in whole blood (supplemental Figure 1 on the Blood website). After addition of 500 µL PBS, fluorescent beads (AccuCount fluorescent particles, 5.2 µm; Spherotec) were added, and the sample was analyzed on a FACScalibur (Becton Dickinson). The platelet count for each mouse was determined prior to the experiment.
Flow cytometric analysis of glycoprotein expression and platelet activation
To determine the glycoprotein expression levels, 50 µL diluted blood (1:20) was incubated with fluorescein isothiocyanate (FITC)–labeled antibodies for 15 minutes at room temperature and directly analyzed. For platelet activation studies, 50 μL diluted blood was washed twice in Tyrode-HEPES buffer and diluted in Tyrode-HEPES buffer containing 2 mM CaCl2. Samples were activated with agonists and stained with antibodies against the activated form of integrin αIIbβ3 (JON/A-PE) and anti–P-selectin for 15 minutes at 37°C.
Tail bleeding time assay
Tail bleeding times were determined as described previously.30 In brief, 1 mm of the tail tip was amputated, and the tails were immersed in 0.9% isotonic saline at 37°C. The time to complete arrest of bleeding (no blood flow for 1 minute) was determined.
Intravital microscopy of thrombus formation in FeCl3-injured mesenteric arterioles
Intravital microscopy was performed as described previously.31 In brief, injury of mesenteric arterioles was induced by application of a filter paper saturated with 20% FeCl3. Adhesion and aggregation of fluorescently labeled platelets in arterioles were monitored for 40 minutes or until complete occlusion occurred (blood flow stopped for 1 minute). Mice were 4 weeks of age.
Mechanical injury of the abdominal aorta
The abdominal cavity of anesthetized mice was opened to expose the abdominal aorta. An ultrasonic flowprobe (0.5PSB699; Transonic Systems) was placed around the abdominal aorta, and thrombus formation was induced by a single firm compression with a forceps. Blood flow was monitored for 30 minutes.
Transient middle cerebral artery occlusion model of stroke
Transient middle cerebral artery occlusion (tMCAO) was performed as described previously.32 In brief, a thread was advanced through the carotid artery into the middle cerebral artery to reduce cerebral blood flow and removed after 1 hour to allow reperfusion. The extent of infarction was assessed 24 hours after reperfusion on 2,3,5-triphenyltetrazolium chloride–stained brain sections. The global neurologic status was scored as previously described.33 Motor function and coordination were graded using the grip test.34
FeCl3 injury of the carotid artery
The right carotid artery was exposed through a midline incision in the neck. An ultrasonic flowprobe was placed around the vessel, and thrombosis was induced by topical application of a 0.5-mm2 filter paper saturated with 10% FeCl3 for 90 seconds. Blood flow was monitored for 30 minutes or until full occlusion (>5 minutes) of the vessel occurred.35
Triple anesthesia
Mice were anesthetized intraperitoneally with a combination of midazolam/medetomidine/fentanyl (5/0.5/0.05 mg/kg body weight).
Statistics
For statistical analysis between experimental groups, we applied the one-way analysis of variance with Tukey as the post hoc test. All statistical evaluation was done with OriginPro 8.6 (Origin Laboratory Corporation). For a test of independence, the two-tailed Fisher’s test for control vs the respective group was used. P < .05 compared with control was considered statistically significant (*P < .05; **P < .01; ***P < .001).
Results
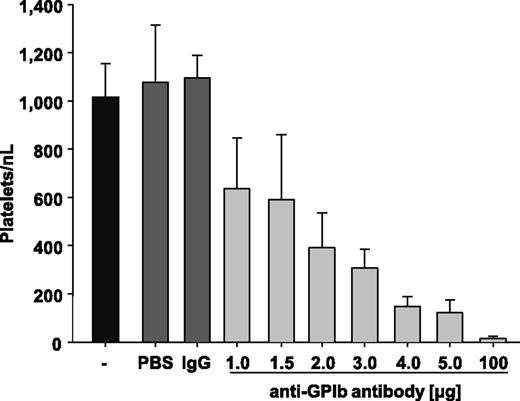
To study the impact of PCs on hemostasis and experimental thrombosis, thrombocytopenia was induced in mice by injection of polyclonal anti-glycoprotein (GP) Ibα antibodies, which deplete circulating platelets in mice independently of immune effector mechanisms.28,36 In a first set of experiments, we determined the antibody doses required to achieve defined PCs 12 hours after injection. Injection of vehicle (PBS) or rat IgG (100 µg/mouse) had no detectable effect on the PC in control mice, which averaged ∼1100 platelets/nL blood, similar to that previously reported by others.37,38 Injection of increasing amounts (1-5 µg) of the anti-GPIbα antibody dose-dependently reduced the PC (Figure 1). To induce virtually complete platelet depletion (<15 platelets/nL blood), mice received 100 µg of the anti-GPIbα antibodies (Figure 1). Importantly, we did not observe any signs of spontaneous bleeding in such severely thrombocytopenic animals during visual inspection of the skin and inner organs. However, on vessel injury during blood sampling or surgery, animals depleted to <15 platelets/nL blood had difficulties sealing the wound and showed increased and prolonged bleeding at the site of injury.
Injection of anti-GPIbα antibody induces thrombocytopenia. Mice were injected with PBS, IgG negative control, or 1.0, 1.5, 2.0, 3.0, 4.0, or 5.0 µg anti-GPIbα antibody, and platelet counts were determined after 12 hours. Untreated mice (-) are shown as a control.
Injection of anti-GPIbα antibody induces thrombocytopenia. Mice were injected with PBS, IgG negative control, or 1.0, 1.5, 2.0, 3.0, 4.0, or 5.0 µg anti-GPIbα antibody, and platelet counts were determined after 12 hours. Untreated mice (-) are shown as a control.
To study possible effects of the anti-GPIbα antibody treatment on the circulating platelet population, mice received 2.0 µg antibody, and platelets were analyzed after 12 hours by flow cytometry. At this time point, the PC was reduced to ∼30% (334 ± 86 platelets/nL; control was 1100 ± 99 platelets/nL), and a slight increase in the mean fluorescence intensity (MFI) of major glycoproteins compared with controls was detectable (Figure 2A). This increase could be attributed to the appearance of a small population of newly generated platelets displaying an increased size and, accordingly, increased surface expression levels of prominent glycoprotein receptors, which were visible as a shoulder in the histogram (Figure 2B, left). In contrast, the size and surface glycoprotein expression levels in the main platelet population were unaltered (Figure 2B, right). As young and large platelets might display altered function and have been associated with prothrombotic properties,29,39 we tested the reactivity of platelets in partially depleted mice and found normal integrin αIIbβ3 activation and P-selectin exposure in response to different agonists (ADP, thrombin, and convulxin; Figure 2C). These results confirmed that anti-GPIbα treatment efficiently reduces the PC in mice without affecting the function of the remaining platelet population.
Flow cytometric analysis of circulating platelets after induction of thrombocytopenia. Mice were injected with 2.0 µg of polyclonal anti-GPIbα antibody, resulting in a reduction of platelet counts to ∼30% of control after 12 hours (time point of analysis). (A) Expression levels of glycoproteins in platelets of control (shaded area) and depleted animals (gray line) are depicted. (B) A new population of platelets can be identified in the samples of platelet depleted mice (left). If this population is excluded from analysis [based on its forward scatter (FSC)/side scatter (SSC) characteristics], no differences in glycoprotein expression patterns between platelets from control and depleted animals were observed as exemplified here for GPIb (right). Histograms are representative for 8 animals from 2 independent experiments. (C) Flow cytometric analysis of (left) integrin αIIbβ3 activation and (right) degranulation-dependent P-selectin exposure in response to ADP (10 µM), thrombin (Thr; 0.01 U/mL), and convulxin (CVX; 1 µg/mL). Results are means ± standard deviation of 4-5 mice per group.
Flow cytometric analysis of circulating platelets after induction of thrombocytopenia. Mice were injected with 2.0 µg of polyclonal anti-GPIbα antibody, resulting in a reduction of platelet counts to ∼30% of control after 12 hours (time point of analysis). (A) Expression levels of glycoproteins in platelets of control (shaded area) and depleted animals (gray line) are depicted. (B) A new population of platelets can be identified in the samples of platelet depleted mice (left). If this population is excluded from analysis [based on its forward scatter (FSC)/side scatter (SSC) characteristics], no differences in glycoprotein expression patterns between platelets from control and depleted animals were observed as exemplified here for GPIb (right). Histograms are representative for 8 animals from 2 independent experiments. (C) Flow cytometric analysis of (left) integrin αIIbβ3 activation and (right) degranulation-dependent P-selectin exposure in response to ADP (10 µM), thrombin (Thr; 0.01 U/mL), and convulxin (CVX; 1 µg/mL). Results are means ± standard deviation of 4-5 mice per group.
Only very low platelet counts are required to maintain normal hemostasis in mice
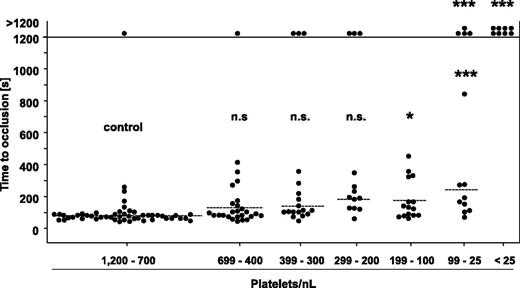
To test the significance of the PC for normal hemostasis, mice were subjected to a tail bleeding time assay 12 hours after induction of thrombocytopenia. In the control group, 48 of 49 mice arrested bleeding within the observation period, with a mean occlusion time of 80 ± 35 seconds, and similar results were obtained in mice with PCs reduced down to 200 platelets/nL (Figure 3). Remarkably, also in the groups of mice with PCs ranging from 199 to 100 platelets/nL and 99 to 25 platelets/nL, most of the animals arrested bleeding, although the mean bleeding times were significantly prolonged compared with the control. In sharp contrast, mice with a PC <25 platelets/nL (ie, <2.5% of control) were not able to arrest bleeding. Importantly, we obtained similar results with 129/Sv mice (supplemental Figure 3A), excluding that mouse strain-specific effects accounted for the unexpectedly low platelet count required to maintain normal hemostasis. To exclude that unrecognized side effects of the platelet-depleting anti-GPIb antibodies contributed to this effect, we analyzed bleeding times in mice in which thrombocytopenia had been induced by a rabbit anti-mouse platelet serum (supplemental Figure 2A). Interestingly, this treatment not only reduced the PC, but in addition partially compromised the reactivity of the remaining platelet population to thrombin and the GPVI agonist convulxin (supplemental Figure 2B). Despite this nonspecific side effect, we obtained very similar results in the bleeding time experiments as for anti-GPIb–treated mice (supplemental Figure 2C).
Tail bleeding time assay with control mice and mice with reduced platelet counts. One millimeter of the tail tip was cut off, and bleeding into 37°C prewarmed saline was monitored over a 20-minute time period. The horizontal line indicates the mean value to occlusive plug formation. Each symbol represents 1 animal (n.s., not significant; *P < .05; **P < .01; ***P < .001).
Tail bleeding time assay with control mice and mice with reduced platelet counts. One millimeter of the tail tip was cut off, and bleeding into 37°C prewarmed saline was monitored over a 20-minute time period. The horizontal line indicates the mean value to occlusive plug formation. Each symbol represents 1 animal (n.s., not significant; *P < .05; **P < .01; ***P < .001).
These results demonstrated that the arrest of bleeding at a wound site only requires very low PCs in mice, suggesting that hemostatic defects in thrombocytopenic mice may only occur when the low platelet counts are associated with altered platelet function, vascular defects, or other pathogenic factors.
Only platelet count reductions >70% significantly affect occlusive thrombus formation in the aorta
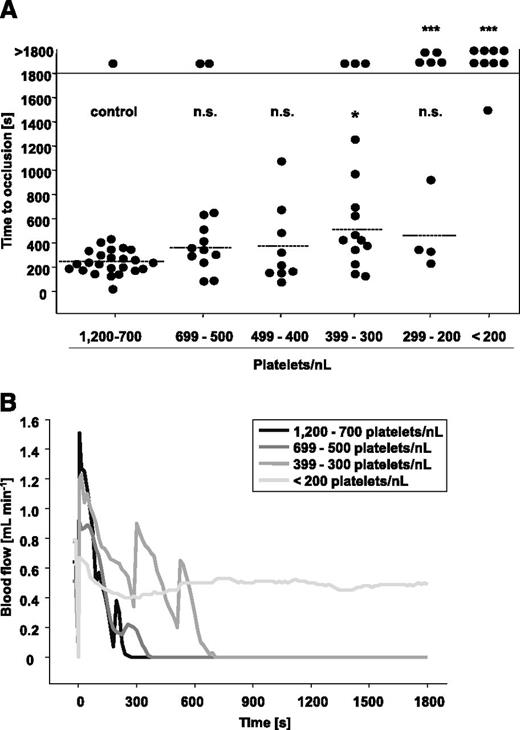
To test the significance of PCs for occlusive arterial thrombus formation, we subjected groups of mice with defined degrees of thrombocytopenia to three well-established thrombosis models. In the first model, the abdominal aorta is mechanically injured, and occlusive thrombus formation rapidly occurs through collagen-dependent mechanisms.35 The aorta represents the largest artery in mice, with rather low shear rates but high blood throughput. As shown in Figure 4A, 24 of 25 control mice formed occlusive thrombi in 247 ± 96 seconds, and very similar results were obtained in mice with platelet counts ranging from 699 to 400 platelets/nL. In contrast, when PCs were lowered to 399 to 300 platelets/nL, only 12 of 15 mice formed occlusive thrombi, and this was significantly delayed compared with the controls (511 ± 335 seconds, P = .04). At PCs between 299 and 200 platelets/nL blood, occlusive thrombus formation became severely impaired, and we observed two different outcomes: either occlusive thrombus formation took place (461 ± 313 seconds, 4 of 9 mice, P = .63) or occlusive thrombus formation was abolished (5 of 9 mice, P = .002). At PCs <200 platelets/nL blood, occlusion was only seen in 1 of 9 mice, and this occurred at a late time point (1502 seconds). Representative blood flow measurements are shown in Figure 4B. The medium gray line (group 399 to 300 platelets/nL) indicates that the embolization of large thrombus fragments partly contributed to the delayed occlusion times. These results revealed that experimental occlusive thrombus formation in a mechanically injured large vessel of mice can efficiently occur at PCs of 30% of control, whereas it is increasingly impaired on further reduction of platelet numbers.
Mechanical injury of the aorta in control mice and mice with reduced platelet counts. (A) The abdominal aorta was mechanically injured using a forceps (compression for 15 seconds), and then blood flow was monitored by an ultrasonic flow probe for 30 minutes. The horizontal line indicates the mean time to vessel occlusion. Each symbol represents 1 animal (n.s., not significant, *P < .05; **P < .01; ***P < .001). (B) Representative blood flow measurements are shown.
Mechanical injury of the aorta in control mice and mice with reduced platelet counts. (A) The abdominal aorta was mechanically injured using a forceps (compression for 15 seconds), and then blood flow was monitored by an ultrasonic flow probe for 30 minutes. The horizontal line indicates the mean time to vessel occlusion. Each symbol represents 1 animal (n.s., not significant, *P < .05; **P < .01; ***P < .001). (B) Representative blood flow measurements are shown.
Occlusive thrombus formation in the FeCl3-injured carotid artery is less affected by reduced platelet counts compared with the aorta injury model
Next, we subjected groups of mice with defined degrees of thrombocytopenia to a model where the carotid artery is chemically injured by the topical application of FeCl3 and thrombus formation occurs through collagen and thrombin-driven mechanisms (Figure 5).35 The carotid artery represents a medium-sized vessel where moderate shear rates prevail.
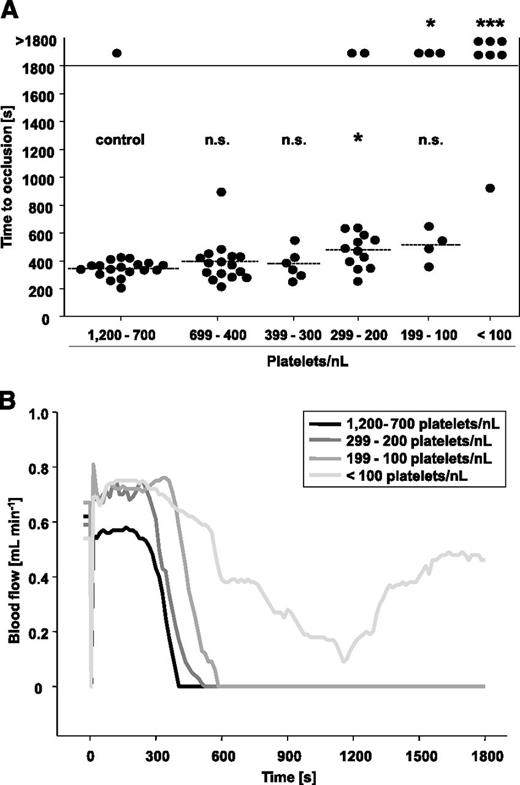
FeCl3-induced injury of the carotid artery in control mice and mice with reduced platelet count. (A) Carotid arteries were topically injured with filter paper saturated with 10% FeCl3 for 90 seconds, and then time to occlusion was determined by an ultrasonic flow probe. The horizontal line indicates the mean time to occlusion. Each symbol represents one animal (n.s., not significant; *P < .05; **P < .01; ***P < .001). (B) Representative blood flow measurements are shown.
FeCl3-induced injury of the carotid artery in control mice and mice with reduced platelet count. (A) Carotid arteries were topically injured with filter paper saturated with 10% FeCl3 for 90 seconds, and then time to occlusion was determined by an ultrasonic flow probe. The horizontal line indicates the mean time to occlusion. Each symbol represents one animal (n.s., not significant; *P < .05; **P < .01; ***P < .001). (B) Representative blood flow measurements are shown.
In the control group, 18 of 19 mice formed occlusive thrombi within 345 ± 58 seconds, and very similar results were obtained in mice with PCs reduced down to 300 platelets/nL (Figure 5A). At PCs of 299 to 200 platelets/nL, a significant increase in the mean occlusion time was observed (477 ± 122 seconds, P = .03), and 2 of 14 vessels failed to occlude within the observation period. A further delay and impairment of occlusive thrombus formation was detectable in mice with PCs of 199 to 100 platelet/nL, with only 4 of 7 mice occluding within 514 ± 121 seconds (P = .06). This was clearly different from the aorta injury model where occlusive thrombus formation was already abrogated at PCs <200 platelets/nL blood. At PCs <100 platelets/nL blood, 6 of 7 mice failed to occlude (P = .0002 compared with control), and the one remaining vessel occluded very late (928 seconds). In Figure 5B, representative blood flow measurements of mice with reduced PCs and respective controls are shown. These results revealed that occlusive thrombus formation in the chemically injured carotid artery is only severely impaired when PCs drop to <100 platelets/nL blood (ie, ∼10% of control).
Platelet counts of 2.5% of control are sufficient to efficiently occlude FeCl3-injured mesenteric arterioles
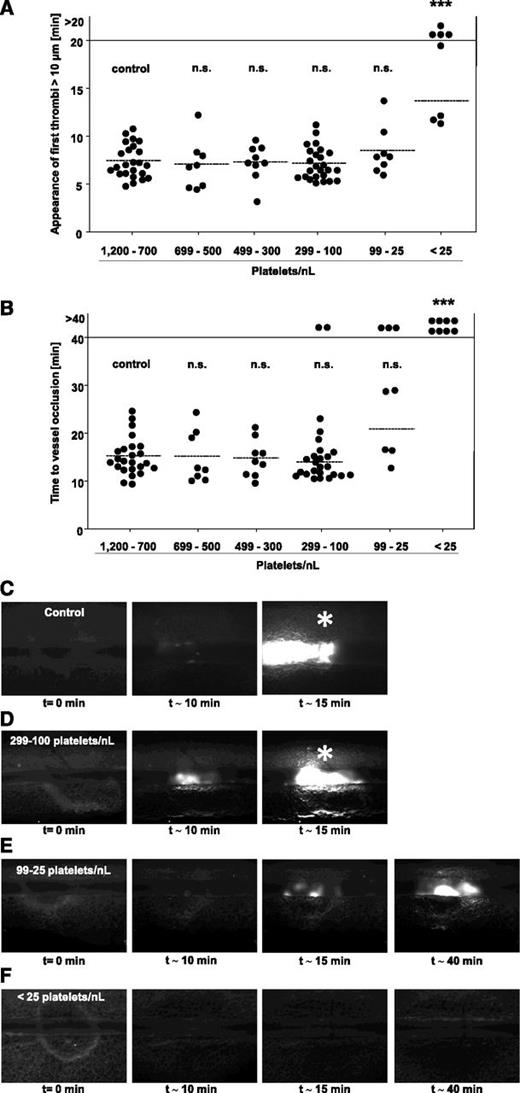
The above described data suggested that the vascular bed and/or the type of vessel injury influenced the threshold PC required to achieve vessel occlusion. To study this in more detail, we tested groups of mice with defined PCs in a third well-established thrombosis model, where a FeCl3 injury is induced in mesenteric arterioles, representing small vessels where high shear rates prevail. Injury was induced by topical application of 20% FeCl3, and thrombus formation of fluorescently labeled platelets was monitored by intravital fluorescence microscopy. In all mice, the time to the formation of the first thrombus with a diameter of >10 µm (Figure 6A) and the time to full vessel occlusion (Figure 6B) were determined. Remarkably, the time to first thrombus formation was not significantly altered in mice with PCs reduced down to 25 platelets/nL (Figure 6A). However, at lower PCs, we made 2 different observations: either appearance of thrombi >10 µm was delayed or abolished (Figure 6A; 4/8 vessels). Also remarkable, the time to vessel occlusion was not significantly altered compared with control down to a platelet count of 100 platelets/nL blood (Figure 6B). At PCs <100 platelets/nL, we observed 3 different outcomes: occlusive thrombus formation was comparable to control, delayed, or abolished (Figure 6B). At a PC <25 platelets/nL blood, occlusive thrombus formation was abolished in all vessels (P = .0001). Representative images of thrombus formation in the different groups are shown in Figure 6C-F. Importantly, similar results were obtained in anti-GPIb–treated 129/Sv mice and in C57/Bl-6 mice rendered thrombocytopenic by rabbit anti-mouse platelet serum, excluding mouse strain- or antibody-specific effects to be responsible for the unexpectedly low platelet counts required to achieve full vessel occlusion in this model (supplemental Figures 2D and 3B).
FeCl3-induced injury of mesenteric arterioles in control mice and mice with reduced platelet count. Mesenteric arterioles were injured by topical application of 20% FeCl3, and adhesion and thrombus formation of fluorescently labeled platelets were monitored by in vivo microscopy. Statistical evaluations of (A) time to appearance of first thrombi >10 µm and (B) time to occlusion are shown. The horizontal line indicates the mean value to vessel occlusion. Each symbol represents 1 arteriole (n.s., not significant; *P < .05; **P < .01; ***P < .001). (C-F) Representative images of control and platelet-depleted mice at different time points are depicted. Asterisk indicates stable occlusion of the vessel.
FeCl3-induced injury of mesenteric arterioles in control mice and mice with reduced platelet count. Mesenteric arterioles were injured by topical application of 20% FeCl3, and adhesion and thrombus formation of fluorescently labeled platelets were monitored by in vivo microscopy. Statistical evaluations of (A) time to appearance of first thrombi >10 µm and (B) time to occlusion are shown. The horizontal line indicates the mean value to vessel occlusion. Each symbol represents 1 arteriole (n.s., not significant; *P < .05; **P < .01; ***P < .001). (C-F) Representative images of control and platelet-depleted mice at different time points are depicted. Asterisk indicates stable occlusion of the vessel.
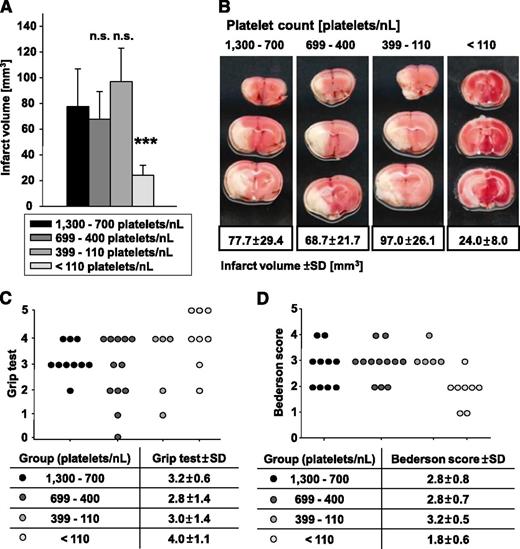
Only ∼10% of the normal platelet count is sufficient to induce full brain infarction following tMCAO
Ischemic stroke is caused by thromboembolic occlusion of a cerebral artery, leading to focal ischemia and infarction of the affected brain territory. We recently showed that blocking of distinct platelet surface receptors essential for platelet adhesion and activation protects from ischemic stroke after tMCAO in mice.34,40,41
To test the significance of the PC for infarct growth following focal cerebral ischemia, we subjected groups of mice with defined PCs to tMCAO (60 minutes) and assessed infarct size and neurological deficits after 24 hours. Four groups of mice were analyzed with platelet counts of 1300 to 700 (control), 699 to 400, 399 to 110, and <110 platelets/nL. Remarkably, a reduction of PCs down to 110 platelets/nL blood had no significant effect (P = .85 for group 699 to 400 platelets/nL and P = .56 for group 399 to 110 platelets/nL) on infarct size at 24 hours after tMCAO (Figure 7A-B) and also did not significantly alter the resulting neurological deficits compared with control as determined by the grip test (Figure 7C), assessing coordination and motor function, and the Bederson score (Figure 7D), assessing global neurological function. The critical platelet dependency of this pathology, however, became apparent when platelet counts were reduced to <110 platelets/nL blood. In these animals, the infarct size at 24 hours was significantly reduced (P = .0003) to one-third compared with control (Figure 7A-B), and this was associated with improved neurological function (Figure 7C-D). Interestingly, we did not observe major intracranial bleeding in any of these mice, suggesting that even very low platelet numbers are sufficient to prevent hemorrhage in the ischemic brain. These data revealed that ∼10% of the normal platelet count is sufficient to mediate full brain infarction following focal cerebral ischemia.
Formation of cerebral brain infarction and neurologic deficits were tested in the tMCAO model. (A) Brain infarct volumes in control and platelet-depleted mice 24 hours after 60-minute tMCAO are represented as mean ± standard deviation. n.s., not significant; *P < .05; **P < .01; ***P < .001. (B) Representative images of 4 corresponding coronal brain sections from control and platelet-depleted mice stained with 2,3,5-triphenyltetrazolium chloride 24 hours after tMCAO. (C) Grip test and (D) Bederson score were determined 24 hours after tMCAO.
Formation of cerebral brain infarction and neurologic deficits were tested in the tMCAO model. (A) Brain infarct volumes in control and platelet-depleted mice 24 hours after 60-minute tMCAO are represented as mean ± standard deviation. n.s., not significant; *P < .05; **P < .01; ***P < .001. (B) Representative images of 4 corresponding coronal brain sections from control and platelet-depleted mice stained with 2,3,5-triphenyltetrazolium chloride 24 hours after tMCAO. (C) Grip test and (D) Bederson score were determined 24 hours after tMCAO.
Discussion
We used a well-characterized murine model of thrombocytopenia to address the question of to what extent isolated thrombocytopenia affects hemostasis and acute thrombotic or thrombo-inflammatory disease states. We show that a reduction of 70% to 80% of the PC did not impair occlusive thrombus formation in large vessels, and even lower PCs were effective to maintain hemostasis and thrombotic occlusion of small arterioles. These data provide the first direct experimental evidence that only severe PC reductions have significant effects on hemostasis and thrombotic or thrombo-inflammatory pathologies.
Several studies in humans consistently found that thrombocytopenia with functional platelets generally induces no or only minor bleeding symptoms, with only rare cases of life-threatening hemorrhages.4,42 This is in line with our observations in mice demonstrating that isolated thrombocytopenia barely affects normal hemostasis as assessed in a tail bleeding assay. Although there is no clear correlation between bleeding time and bleeding risk,43 one may speculate on the grounds of these results that, in humans, the occurrence of bleeding episodes during thrombocytopenia might to a great extent be determined by other factors than the degree of thrombocytopenia. This has impressively been demonstrated in mice where only the combination of (severe) thrombocytopenia and inflammation resulted in spontaneous hemorrhage in different organs.27 In-depth clinical studies will be required to identify the factors that may trigger hemorrhagic complications in thrombocytopenic patients.
Although numerous patient studies assessed the causal relationship between thrombocytopenia and increased bleeding, only very limited and partially contradictory information is available on the effect of isolated thrombocytopenia on the risk of acute thrombotic disease in humans. Sarpatwari et al3 described an increased risk for arterial and venous thromboembolism in humans with ITP. Others reported that the risk for arterial thrombosis was decreased in male ITP patients but was twofold increased in female ITP patients compared with non-thrombocytopenic controls.44 Similarly, various case reports of thrombotic events such as ischemic stroke45 or myocardial infarction during ITP46 indicate that thrombocytopenia caused by autoantibodies does not generally protect humans from thrombotic events but in some cases may even increase the thrombotic risk. One possible explanation could be that autoantibodies directed against platelet surface proteins may affect the function and/or the activation state of the cells, thereby increasing their reactivity. This assumption is supported by observations in mice showing that the antigenic specificity of antiplatelet receptor antibodies to a great extent determines possible functional alterations in the cells and also whether they are cleared from the circulation.28,47,48
The anti-GPIbα antibodies used here are known to rapidly deplete circulating platelets independently of their Fc portion and thus immune effector mechanisms,28 thereby inducing only minimal side effects. In line with this, we found that the population of circulating platelets after anti-GPIbα antibody treatment was functionally intact (Figure 2). The appearance of a small population of newly generated platelets did not lead to a significantly different activation response of the overall platelet population. We can, however, not definitively rule out subtle differences in the reactivity of the newly generated platelets compared with the older platelets, but we consider it very unlikely that such differences, if existent, account for the unexpectedly robust hemostatic and thrombotic activity in the thrombocytopenic animals. Our model system therefore allows studies on the effect of a largely isolated thrombocytopenia on hemostasis and thrombosis. Using this model system, we observed no signs of spontaneous bleeding in severely thrombocytopenic mice, confirming that thrombocytopenia alone is not sufficient to induce spontaneous hemorrhage in mice.27 Furthermore, we found that even severe reductions of the PC (∼97.5%) had no major effect on hemostasis or occlusive thrombus formation in small arterioles (Figures 3 and 6). In contrast, occlusive thrombus formation in larger arteries required higher PCs (carotid artery: ∼20%; aorta: ∼30% of control), demonstrating that considerable differences in the sensitivity for PC reductions exist between these thrombosis models. This may at least partially be explained by the different diameters of the injured vessels, indicating that the formation of a large vessel occluding thrombus may require a higher PC to be accomplished than a thrombus that occludes a smaller vessel. Considering that in smaller vessels higher shear rates prevail, one may also speculate that the mechanisms promoting platelet recruitment and activation as well as thrombus stabilization under such conditions may be more stringent and therefore less dependent on the actual number of platelets per blood volume than under low or intermediate shear conditions. In line with this assumption, occlusive thrombus formation in the aorta of thrombocytopenic mice is not only delayed due to slower recruitment of platelets from the circulation, but also compromised by the embolization of large thrombus fragments, indicating impaired thrombus stability that might be a consequence of inefficient local accumulation of released or locally produced agonists, such as ADP, thromboxane A2, or thrombin.
Additionally, other factors such as blood pressure in the injured vessel or the nature of the injury likely influence the sensitivity of the different models to PC reductions, whereas the mechanism of platelet count reduction or mouse strain-specific effects could be largely excluded in our study (supplemental Figures 2 and 3). It may be difficult to completely dissect how these and possible other factors determine the “threshold PC” required for vessel occlusion in a certain vascular bed under given experimental conditions, and the situation in diseased humans may be even more complex. However, irrespective of this important question, our data reveal that vessel-occluding platelet-rich thrombi can form with virtually unaltered kinetics at remarkably low PCs in mice, indicating that thrombocytopenia might also not provide protection from thrombotic events in humans.
Genetic mouse models are increasingly used to study platelet function in vitro and in vivo.49 Some of these mouse lines display altered platelet function in association with reduced PCs.30,50 Based on our data, we speculate that bleeding abnormalities or impaired experimental thrombosis in such animals is to a great extent related to the functional platelet defect rather than reduced PCs, which may have important implications for the interpretation of the data obtained in these mouse models. On the basis of this assumption, one could also speculate that the bleeding phenotypes seen in humans suffering from inherited platelet disorders such as the gray platelet syndrome, Wiskott-Aldrich syndrome, or Berhard-Soulier syndrome are primarily caused by functional platelet defects but not by reduced PC.
Ischemic stroke is a leading cause of death or severe disability worldwide, and platelets are essential components of vessel-occluding emboli in this setting. On the basis of the data from our thrombosis models, we speculate that also the formation of large embolizing thrombi would not be strongly affected by moderate to even pronounced PC reductions. However, clearly, further studies in animals or in patients will be required to test this directly. A major challenge in stroke treatment is, however, that spontaneous or therapeutic recanalization of a previously occluded cerebral artery is often followed by a reperfusion injury of the affected brain territory leading to the development of large infarcts. The exact mechanisms by which platelets contribute to this pathology have not been established but integrin αIIbβ3-dependent thrombus formation was shown not to be essential.34 Rather, platelets appear to orchestrate a thrombo-inflammatory cascade involving T cells and the contact activation system finally leading to a dysregulation of the blood-brain barrier and neurologic damage.2,41 Experimental evidence suggests that the GPIb-von Willebrand factor (vWF)-GPVI signaling axis in platelets is of central importance in this setting, but the molecular interplay of the contributing pathogenic factors is currently unclear.34 In this study, we found that infarct growth following tMCAO was virtually unaffected by PC reductions up to 90%, whereas a further reduction was highly protective (Figure 7). This also indicates that thrombo-inflammatory pathologies, similar to occlusive thrombus formation, may not be significantly affected even by pronounced platelet count reductions.
Taken together, we showed that hemostasis, thrombosis, and ischemic brain infarction are largely unaffected over a wide range of PC reduction in mice. Although data obtained in experimental animals cannot be directly extrapolated to the situation in humans, we speculate that low PCs alone would not significantly reduce the risk of thrombotic events such as myocardial infarction or stroke. This may have important implications for the risk assessment in cardiovascular patients but also for the development of novel therapeutic strategies to prevent or treat acute ischemic events.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stefanie Hartmann for excellent technical assistance and Deya Cherpokova and Viola Lorenz for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 688, TPA1 and TPB1, to B.N. and G.S.).
Authorship
Contribution: M.M., T.V., and P.K. performed experiments, analyzed data, and contributed to the writing of the manuscript; G.S. and C.K. analyzed data and contributed to the writing of the manuscript; and B.N. designed research, analyzed data, and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Nieswandt, University Hospital Würzburg, Rudolf Virchow Center, Deutsche Forschungsgemeinschaft (DFG) Research Center for Experimental Biomedicine, Josef-Schneider-Strasse 2, 97080 Würzburg, Germany; e-mail: bernhard.nieswandt@virchow.uni-wuerzburg.de.

![Figure 2. Flow cytometric analysis of circulating platelets after induction of thrombocytopenia. Mice were injected with 2.0 µg of polyclonal anti-GPIbα antibody, resulting in a reduction of platelet counts to ∼30% of control after 12 hours (time point of analysis). (A) Expression levels of glycoproteins in platelets of control (shaded area) and depleted animals (gray line) are depicted. (B) A new population of platelets can be identified in the samples of platelet depleted mice (left). If this population is excluded from analysis [based on its forward scatter (FSC)/side scatter (SSC) characteristics], no differences in glycoprotein expression patterns between platelets from control and depleted animals were observed as exemplified here for GPIb (right). Histograms are representative for 8 animals from 2 independent experiments. (C) Flow cytometric analysis of (left) integrin αIIbβ3 activation and (right) degranulation-dependent P-selectin exposure in response to ADP (10 µM), thrombin (Thr; 0.01 U/mL), and convulxin (CVX; 1 µg/mL). Results are means ± standard deviation of 4-5 mice per group.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/24/10.1182_blood-2012-10-461459/4/m_4938f2.jpeg?Expires=1769838614&Signature=DpFXTCHNz6BMqNj0ovVwkzvz70TrsPx3BQ6lxX6J950V4fcAKETmkjgeaJDfdJ5hfKzkl~8k5r5DI54gUvuHVhPZwgregJdsUt7Zi7gSBC-g686TCLEJoAqp6YwMYJ6J6sKViBuoDYHlUpJBVPXEAAh8N85F7F-82gRT12oDVeluqqzepZx9ANpXul~pvqqCvCCaW1PaE0dwilAhCrEBk9Gix92JhkLjiKA44El4FYxvbwbDOLQ8JBd33nUPkVves2xqxKFZ2LwnTQ3MUya8SSFpHF2owb1tUv3ozr9QjKlNN2J958XcYQYqs1UMttVZErpEEAHF9FCOX~P1iVTdxA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)