Key Points
After splenectomy, patients with ITP have a higher risk of venous thrombosis and sepsis than patients with ITP who do not undergo splenectomy.
Abstract
Patients with immune thrombocytopenia (ITP) who relapse after an initial trial of corticosteroid treatment present a therapeutic challenge. Current guidelines recommend consideration of splenectomy, despite the known risks associated with surgery and the postsplenectomy state. To better define these risks, we identified a cohort of 9976 patients with ITP, 1762 of whom underwent splenectomy. The cumulative incidence of abdominal venous thromboembolism (AbVTE) was 1.6% compared with 1% in patients who did not undergo splenectomy; venous thromboembolism (VTE) (deep venous thrombosis and pulmonary embolus) after splenectomy was 4.3% compared with 1.7% in patients who did not undergo splenectomy. There was increased risk of AbVTE early (<90 days; hazard ratio [HR] 5.4 [confidence interval (CI), 2.3-12.5]), but not late (≥90 days; HR 1.5 [CI, 0.9-2.6]) after splenectomy. There was increased risk of VTE both early (HR 5.2 [CI, 3.2-8.5]) and late (HR 2.7 [CI, 1.9-3.8]) after splenectomy. The cumulative incidence of sepsis was 11.1% among the ITP patients who underwent splenectomy and 10.1% among the patients who did not. Splenectomy was associated with a higher adjusted risk of sepsis, both early (HR 3.3 [CI, 2.4-4.6]) and late (HR 1.6 or 3.1, depending on comorbidities). We conclude that ITP patients post splenectomy are at increased risk for AbVTE, VTE, and sepsis.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by the antibody and cell-mediated destruction of platelets in conjunction with impaired thrombopoiesis1 and an increased risk of bleeding. ITP can be associated with other autoimmune disorders such as systemic lupus erythematosus (SLE), lymphoproliferative disorders, or infections, or can develop without obvious underlying illness (ie, idiopathic).
Diagnosis is made on the basis of exclusion of other causes, with the typical clinical picture of isolated thrombocytopenia and relatively mild bleeding manifestations.2 Because serious spontaneous bleeding is rare with platelet counts >30 × 109L,3 the current treatment guidelines recommend maintaining the platelet count at >30 × 109/L to avoid bleeding complications, while trying to minimize the adverse effects of therapy.4
Initial therapy for ITP is corticosteroids or, for patients who are actively bleeding or who have a contraindication to steroids, intravenous immunoglobulin or anti-D globulin.4 Although corticosteroids induce an initial response in 70% to 90% of patients, the majority will relapse when steroids are tapered or stopped.5-9
Second-line therapies include rituximab, cyclophosphamide, azathioprine, and the newer thrombopoietic agents romiplostim and eltrombopag. Splenectomy has been considered a standard therapy in the management of patients who are refractory to corticosteroids and is highly efficacious, with approximately two-thirds of patients subsequently achieving a normal platelet count.10
Although the development of laparoscopic splenectomy has lowered the immediate postoperative risks,11 barriers to more frequent use of splenectomy include the unpredictability of response,12 reluctance to perform splenectomy on patients with thrombocytopenia, and the hesitancy of patients to undergo an invasive procedure. Hematologists may be less inclined to recommend this therapy because of the reported higher risk of both sepsis and venous thromboembolism (VTE).
However, the incidence of postsplenectomy sepsis and VTE in patients with ITP is not well-defined. Although patients with ITP have a greater risk of VTE compared with the general population,13 little data exist on the incidence of VTE after splenectomy for ITP. Likewise, postsplenectomy sepsis remains a significant concern, but the absolute incidence of postsplenectomy sepsis has been difficult to quantify.14 Larger series have reported the incidence of postsplenectomy sepsis or serious infection to be in the range of 2% to 8%.15-17 In examining the risk of infection after splenectomy in ITP patients, most studies are small series and have demonstrated either no or minimal increased risk of infection.6,18-25
The aim of this study was to better define the incidence and risks factors associated with AbVTE, VTE, and sepsis in adult patients with ITP after splenectomy compared with patients with ITP who did not undergo splenectomy.
Methods
Cohort definition
The State of California Office of Statewide Health Planning and Development (OSHPD) maintains records of all patients hospitalized in nonfederal hospitals in the state, called the Patient Discharge Database. Since July 1990, the State of California has required that these hospitals report the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes corresponding to medical diagnoses and procedures performed on every patient. Data are encrypted and linked to a master death registry. The State of California Committee for the Protection of Human Subjects and the University of California, Davis institutional review board approved this project.
To identify a cohort of adult cases with ITP, we identified all patients 18 years of age or older who had a diagnosis of ITP in the principal (first) position from January 1990 to November 2009. We excluded patients with SLE or chronic lymphocytic leukemia. In a separate step, all cases discharged with a principal procedure code for splenectomy were ascertained. The datasets were then merged using unique record linkage numbers that allow longitudinal tracking of serial admissions for individual cases.
To compare ITP cases that underwent or did not undergo splenectomy, we first identified and excluded those ITP cases that underwent splenectomy during the index admission or during any prior hospitalization back to 1990. Because ITP is known to have a variable clinical phenotype and this database does not have laboratory data, we reasoned that including cases that required hospital admission specifically for ITP would generate a more homogeneous cohort of cases that had similar disease severity. Therefore, we excluded patients with less severe disease who did not require hospital admission before splenectomy to enrich for a population for whom the disease was severe enough to require hospitalization for ITP rather than for splenectomy alone. Using this cohort definition, we then identified the ITP cases that did or did not undergo splenectomy at some time after the index hospitalization.
Outcomes
Incident VTE was defined as cases admitted with a diagnosis of lower extremity acute deep vein thrombosis or pulmonary embolism (PE) using 14 specific ICD-9-CM codes for deep vein thrombosis and 3 for PE in the principal or second position. Incident AbVTE was defined as thrombosis occurring in the mesenteric, portal, or hepatic veins using one of 5 specific ICD-9-CM codes. Previous studies have shown high positive predictive value for VTE (>87%) for cases with these codes in the first or second position.26 Sepsis was defined by one of 25 specific ICD-9-CM codes, which code for sepsis, septicemia, and systemic inflammatory response syndrome. We selected cases with any of these sepsis codes in only the principal, second, or third position.
Comorbidity
Twenty-nine potential chronic comorbidities were determined with Elixhauser comorbidity index,27 using comorbidities listed for the index “ITP admission.”
Data analysis
The characteristics of the cases that underwent or did not undergo splenectomy were compared using bivariate statistics. The risks of AbVTE, VTE, and sepsis were compared using the Cox proportional hazard analysis, with splenectomy as a time-dependent covariate. A risk factor was considered significant if P < .01.
The cumulative incidences of AbVTE, VTE, and sepsis were determined for the entire ITP cohort. To illustrate the comparison between splenectomy cases and nonsplenectomy cases for the incidence and time course of AbVTE, VTE, and sepsis, a matched Kaplan-Meier time-to-event analysis was generated. Nonsplenectomy cases were matched 2:1 with splenectomy cases based on: age (within 2 years), sex, number of comorbidities, race/ethnicity, and year of index admission. Each nonsplenectomy case selected required duration of follow-up equal to or greater than the time between the index ITP admission and the date of splenectomy. Incidence of AbVTE, VTE, or sepsis was then compared only during the comparable follow-up period after the index hospitalization. On the Kaplan-Meier plots, the origin was the day of splenectomy or the equal follow-up time for each matched nonsplenectomy case.
Survival estimates were determined using the Kaplan-Meier method and compared using log-rank. Logistic regression analysis was used to analyze predictors of death associated with incident AbVTE, VTE, or sepsis, adjusting for age (<60 or ≥60 years), sex, comorbidities, race/ethnicity, and splenectomy.
All analyses were done using SAS analysis software, version 9.3 (SPSS Institute, Cary, NC).
Results
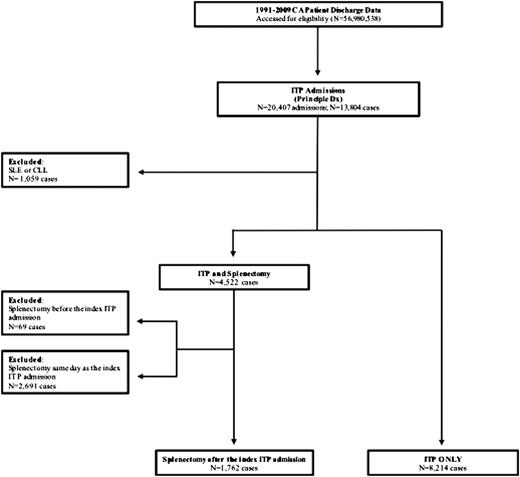
A total of 13 804 cases were identified with a principal diagnosis code for ITP. We excluded 1059 cases with SLE or chronic lymphocytic leukemia, and cases that underwent splenectomy before (69) or during (2691) the index ITP admission. The analysis cohort included 9976 ITP cases (Figure 1). The frequency of ITP as the principal diagnosis was 0.03% among the 36 099 198 unique cases hospitalized in California during the study period. The median age was 55 years.
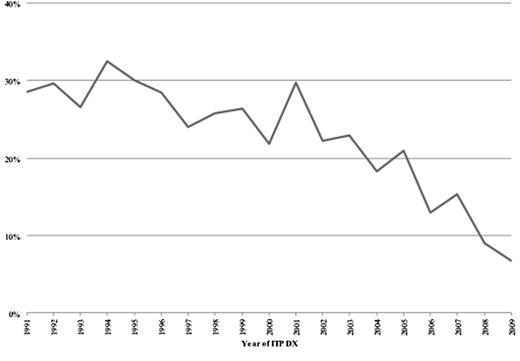
The majority of cases were female (58.2%) and Caucasian (58.4%) (Table 1). Reflecting the demography of California, significant proportions were Hispanic and Asian. Cases that underwent splenectomy were younger, more often female, included a higher percentage of Hispanics, and had fewer comorbidities. One-thousand seven-hundred sixty-two (17.7%) of the ITP cases underwent splenectomy (17 had partial splenectomy). The median time from index hospitalization to splenectomy was 2.9 months (range, 1-207). The rates of splenectomy over the 20-year period are shown in Figure 2.
Characteristics of the cohort
| . | Total . | No splenectomy . | Splenectomy . | Chi-Square . | ||
|---|---|---|---|---|---|---|
| Parameter . | N . | n . | (%) . | n . | (%) . | P value . |
| All | 9976 | 8214 | 82.3% | 1762 | 17.7% | |
| Age | ||||||
| Age <60 | 5616 | 4442 | 79.1% | 1174 | 20.9% | <.0001 |
| Age ≥60 | 4360 | 3772 | 86.5% | 588 | 13.5% | |
| Gender | ||||||
| Males | 4168 | 3469 | 83.2% | 699 | 16.8% | .0479 |
| Females | 5808 | 4745 | 81.7% | 1063 | 18.3% | |
| Race/Ethnicity | ||||||
| NH white | 5821 | 4815 | 82.7% | 1006 | 17.3% | .0004 |
| NH Black | 723 | 621 | 85.9% | 102 | 14.1% | |
| Hispanic | 2271 | 1827 | 80.4% | 444 | 19.6% | |
| NH Asian/PI | 863 | 723 | 83.8% | 140 | 16.2% | |
| Other/Unknown | 298 | 228 | 76.5% | 70 | 23.5% | |
| Comorbities at ITP dx | ||||||
| 0-1 | 5814 | 4547 | 78.2% | 1267 | 21.8% | <.0001 |
| 2+ | 4162 | 3667 | 88.1% | 495 | 11.9% | |
| . | Total . | No splenectomy . | Splenectomy . | Chi-Square . | ||
|---|---|---|---|---|---|---|
| Parameter . | N . | n . | (%) . | n . | (%) . | P value . |
| All | 9976 | 8214 | 82.3% | 1762 | 17.7% | |
| Age | ||||||
| Age <60 | 5616 | 4442 | 79.1% | 1174 | 20.9% | <.0001 |
| Age ≥60 | 4360 | 3772 | 86.5% | 588 | 13.5% | |
| Gender | ||||||
| Males | 4168 | 3469 | 83.2% | 699 | 16.8% | .0479 |
| Females | 5808 | 4745 | 81.7% | 1063 | 18.3% | |
| Race/Ethnicity | ||||||
| NH white | 5821 | 4815 | 82.7% | 1006 | 17.3% | .0004 |
| NH Black | 723 | 621 | 85.9% | 102 | 14.1% | |
| Hispanic | 2271 | 1827 | 80.4% | 444 | 19.6% | |
| NH Asian/PI | 863 | 723 | 83.8% | 140 | 16.2% | |
| Other/Unknown | 298 | 228 | 76.5% | 70 | 23.5% | |
| Comorbities at ITP dx | ||||||
| 0-1 | 5814 | 4547 | 78.2% | 1267 | 21.8% | <.0001 |
| 2+ | 4162 | 3667 | 88.1% | 495 | 11.9% | |
P values are for comparison between splenectomy and non-splenectomy groups.
NH, non-Hispanic; dx, diagnosis; PI, Pacific Islander.
AbVTE and peripheral VTE
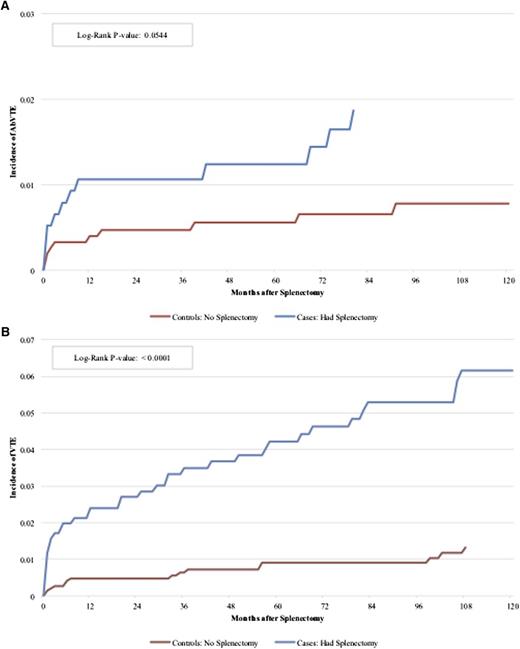
AbVTE developed in 109 cases: 27 splenectomized cases (cumulative incidence 1.6%) and 82 nonsplenectomized cases (cumulative incidence 1%), VTE developed in 215 cases: 75 splenectomized cases (cumulative incidence 4.3%) and 140 nonsplenectomy cases (cumulative incidence 1.7%), with a median follow-up of 60 months. The corresponding Kaplan-Meier plots are shown in Figure 3. Fifty-one cases in the entire cohort were coded as having a hypercoaguable state, and 33 cases were coded as having the antiphospholipid antibody. VTE developed in only 1 case with either of these conditions.
Incidence of AbVTE and VTE by splenectomy status. (A) The incidence of AbVTE comparing cases with splenectomy to controls with no splenectomy. P = .0544, log-rank. (B) The incidence of VTE comparing cases with splenectomy to controls with no splenectomy. P < .0001., log-rank. Median follow-up time 120 months.
Incidence of AbVTE and VTE by splenectomy status. (A) The incidence of AbVTE comparing cases with splenectomy to controls with no splenectomy. P = .0544, log-rank. (B) The incidence of VTE comparing cases with splenectomy to controls with no splenectomy. P < .0001., log-rank. Median follow-up time 120 months.
Because undergoing an operation may have provoked VTE, the hazards for “early” AbVTE and VTE (<90 days of splenectomy) and “late” (≥90 days) were determined separately (Table 2). Splenectomy was associated with increased hazard of early AbVTE (hazard ratio [HR] 5.4 [confidence interval (CI), 2.3-12.5]), but not late AbVTE (HR 1.5 [CI 0.9-2.6]). Splenectomy was associated with increased early (HR 5.2 [CI, 3.2-8.5]) and late VTE (HR 2.7 [CI, 1.9-3.8]). The median time from splenectomy to AbVTE was 9.3 months (range, 0-158) and to VTE was 20.9 months (range, 0-179). In the multivariable model, comorbid conditions predicted an increased risk of AbVTE, and older age predicted an increased risk of VTE. Asian and Pacific Islander cases had a lower risk of developing VTE, consistent with previous epidemiologic data.28,29
Cox proportional hazard model for AbVTE and VTE
| . | AbVTE . | VTE . | ||||
|---|---|---|---|---|---|---|
| Parameter . | Hazard ratio . | 95% Confidence limit . | P value . | Hazard ratio . | 95% Confidence limit . | P value . |
| Female sex | 1.1 | (0.7, 1.6) | .6082 | 1.0 | (0.8, 1.3) | .9518 |
| Age ≥60 years | 1.3 | (0.9, 2.0) | .1701 | 2.0* | (1.5, 2.6) | <.0001 |
| Race/Ethnicity (vs NH white) | ||||||
| NH black | 0.8 | (0.4, 1.7) | .5049 | 1.0 | (0.6, 1.7) | .9936 |
| Hispanic | 0.6 | (0.3, 1.0) | .0410 | 0.7 | (0.5, 1.0) | .0423 |
| NH Asian/PI | 0.9 | (0.5, 1.8) | .8321 | 0.2* | (0.1, 0.6) | .0011 |
| 2+ Comorbidities (vs 0-1 comorbidities) | 2.4* | (1.6, 3.6) | <.0001 | 1.3 | (1.0, 1.7) | 0.1058 |
| Splenectomy vs no splenectomy | ||||||
| VTE within 90 days of splenectomy | 5.4* | (2.3, 12.5) | <.0001 | 5.2* | (3.2, 8.5) | <.0001 |
| VTE 90+ days after splenectomy | 1.5 | (0.9, 2.6) | .1252 | 2.7* | (1.9, 3.8) | <.0001 |
| . | AbVTE . | VTE . | ||||
|---|---|---|---|---|---|---|
| Parameter . | Hazard ratio . | 95% Confidence limit . | P value . | Hazard ratio . | 95% Confidence limit . | P value . |
| Female sex | 1.1 | (0.7, 1.6) | .6082 | 1.0 | (0.8, 1.3) | .9518 |
| Age ≥60 years | 1.3 | (0.9, 2.0) | .1701 | 2.0* | (1.5, 2.6) | <.0001 |
| Race/Ethnicity (vs NH white) | ||||||
| NH black | 0.8 | (0.4, 1.7) | .5049 | 1.0 | (0.6, 1.7) | .9936 |
| Hispanic | 0.6 | (0.3, 1.0) | .0410 | 0.7 | (0.5, 1.0) | .0423 |
| NH Asian/PI | 0.9 | (0.5, 1.8) | .8321 | 0.2* | (0.1, 0.6) | .0011 |
| 2+ Comorbidities (vs 0-1 comorbidities) | 2.4* | (1.6, 3.6) | <.0001 | 1.3 | (1.0, 1.7) | 0.1058 |
| Splenectomy vs no splenectomy | ||||||
| VTE within 90 days of splenectomy | 5.4* | (2.3, 12.5) | <.0001 | 5.2* | (3.2, 8.5) | <.0001 |
| VTE 90+ days after splenectomy | 1.5 | (0.9, 2.6) | .1252 | 2.7* | (1.9, 3.8) | <.0001 |
NH, non-Hispanic; PI, Pacific Islander.
Statistically significant at P < .01.
Sepsis
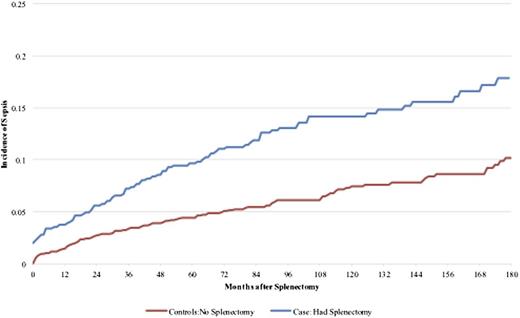
Sepsis developed in 1016 cases: 191 splenectomized cases (cumulative incidence 11.1%) and 825 nonsplenectomized cases (cumulative incidence 10.1%), with a median follow-up of 56 months. The corresponding Kaplan-Meier plots are shown in Figure 4. The cumulative incidence of early sepsis after splenectomy (<90 days) was 2.6% and of late sepsis (≥90 days) was 8.8%. Among the splenectomy cases, the median time from splenectomy to hospitalization with sepsis was 35.5 months (range, 0-219).
In the mulitvariable model for sepsis, splenectomy was a significant predictor of both early and late sepsis, with a more than threefold higher hazard for early sepsis (HR 3.3 [CI, 2.4-4.6]). For late sepsis, there was an interaction between splenectomy and number of comorbidities. Cases with none or one comorbidity had a HR of 1.6 (CI, 1.3-2.0), and for cases with 2 or more comorbidities, the HR was 3.1 (CI, 2.2-4.4). There was also an interaction between age and number of comorbidities. In addition to splenectomy, age ≥60 years, presence of comorbidities, non-Hispanic Blacks, and males were also significant predictors of sepsis (Table 3).
Cox proportional hazards for postsplenectomy sepsis
| . | Sepsis . | ||
|---|---|---|---|
| Parameter . | Hazard ratio . | 95% Confidence limit . | P value . |
| Female sex | 0.8* | (0.7, 0.9) | <.0001 |
| Age ≥60 years | 3.2* | (2.7, 3.9) | <.0001 |
| Race/Ethnicity (vs NH white) | |||
| NH black | 1.4* | (1.1, 1.8) | .0037 |
| Hispanic | 1.1 | (1.0, 1.3) | .1687 |
| NH Asian/PI | 1.2 | (1.0, 1.5) | .0964 |
| 2+ Comorbidities | |||
| Age <60 years | 3.6* | (2.9, 4.4) | <.0001 |
| Age ≥60 years | 6.1* | (5.1, 7.3) | <.0001 |
| Splenectomy vs no splenectomy | |||
| Sepsis within 90 days of splenectomy | 3.3* | (2.4, 4.6) | <.0001 |
| Sepsis 90+ days after splenenctomy | |||
| 0-1 Comorbidities | 1.6* | (1.3, 2.0) | <.0001 |
| 2+ Comorbidities | 3.1* | (2.2, 4.4) | .0016 |
| . | Sepsis . | ||
|---|---|---|---|
| Parameter . | Hazard ratio . | 95% Confidence limit . | P value . |
| Female sex | 0.8* | (0.7, 0.9) | <.0001 |
| Age ≥60 years | 3.2* | (2.7, 3.9) | <.0001 |
| Race/Ethnicity (vs NH white) | |||
| NH black | 1.4* | (1.1, 1.8) | .0037 |
| Hispanic | 1.1 | (1.0, 1.3) | .1687 |
| NH Asian/PI | 1.2 | (1.0, 1.5) | .0964 |
| 2+ Comorbidities | |||
| Age <60 years | 3.6* | (2.9, 4.4) | <.0001 |
| Age ≥60 years | 6.1* | (5.1, 7.3) | <.0001 |
| Splenectomy vs no splenectomy | |||
| Sepsis within 90 days of splenectomy | 3.3* | (2.4, 4.6) | <.0001 |
| Sepsis 90+ days after splenenctomy | |||
| 0-1 Comorbidities | 1.6* | (1.3, 2.0) | <.0001 |
| 2+ Comorbidities | 3.1* | (2.2, 4.4) | .0016 |
NH, non-Hispanic; PI, Pacific Islander.
Statistically significant at P < .01.
Mortality
The Kaplan-Meier 5- and 10-year survivals for the entire ITP cohort were 73% (CI, 72%-74%) and 64% (CI, 63%-65%), respectively. For the patients in whom acute VTE developed, the corresponding Kaplan-Meier 5- and 10-year survivals rates were 62% and 46%, respectively (CI could not be calculated). The AbVTE cases had a 5-year survival rate of 38% (there were an insufficient number of cases to calculate 10-year survival). For the patients in whom sepsis developed, the 5- and 10-year survival rates were 35% (CI, 32%-38%) and 27% (CI, 24%-30%).
In a logistic regression model adjusted for age, race/ethnicity, and number of comorbidities, AbVTE was associated with increased odds ratio for death (OR 3.3; CI, 2.1-5.1), whereas VTE was not (OR 1.4; CI 1.0-1.9). Development of sepsis was associated with increased odds of death (OR 4.7; CI, 4-5.5), as were age ≥60 years and a higher number of comorbidities (data not shown). In the adjusted model, splenectomy was associated with a significant reduction in the odds of death (OR 0.8; CI, 0.7-0.9).
Discussion
To our knowledge, this represents the largest population-based study that has examined the effect of splenectomy on the incidence of AbVTE, VTE, sepsis, and mortality in patients with ITP. Using a retrospective observational cohort design, we found that among patients who required at least 1 hospitalization for ITP, subsequent splenectomy was associated with an approximately fivefold higher risk for early AbVTE and VTE, and a 3-fold higher risk for late VTE when compared with patients with ITP who did not undergo splenectomy, after adjusting for potential cofounders. In contrast with other patient populations,28,30 the presence of medical comorbidities did not increase the risk for VTE, although comorbid conditions were shown to increase the risk for AbVTE.
Splenectomy was also associated with a higher risk of sepsis, both early and late, after the operation. In addition to splenectomy, the risk of sepsis was associated with expected risk factors, including older age and the number of comorbidities. We also found that male gender and black ethnicity predicted for a high risk of sepsis. Despite a demonstrated increased risk for AbVTE, VTE, and sepsis, splenectomy was associated with a reduction in the odds of death (OR 0.8; CI, 0.7-0.9).
The major strengths of this report include the large number of cases (N = 9976) and the long duration of follow-up (up to 228 months). Limitations include reliance on hospital discharge coding and lack of laboratory data. Reliance on hospitalization to identify ITP cases means that our findings may not be generalizable to patients who can be treated exclusively in the outpatient setting or who require hospitalization only for splenectomy. The patients we analyzed likely represented the more severe cases of ITP. Because we did not analyze patients who underwent splenectomy during the index hospitalization, it is likely that all patients included in our cohort did have some initial response to treatment and then subsequently treatment failed.
Although the accuracy of the ICD-9-CM coding in the Patient Discharge Database for ITP has not been validated, we only included patients hospitalized with a principal diagnosis of ITP. Our conservative definition might have resulted in undercounting the number of ITP cases, but our goal was not to estimate the incidence or prevalence of ITP, but rather to evaluate the effect of splenectomy. Because splenectomy is almost exclusively an inpatient procedure, it is unlikely that a significant number of splenectomized patients were missed.
The outcome measures AbVTE, VTE, and sepsis based on the presence or absence of specific ICD-9-CM codes. Although VTE codes have high positive predictive value when compared with abstracted chart documentation,26 codes for sepsis or AbVTE have not been similarly validated. We based the definition of “sepsis” on accepted coding guidelines and included cases where a code corresponding to sepsis, septicemia, and systemic inflammatory response syndrome were present in one of the first 3 diagnostic positions.
The cumulative incidence of AbVTE and VTE were higher in patients who underwent splenectomy; Cox proportional hazard modeling showed splenectomy to be independently associated with increased hazard of AbVTE and VTE. Patients undergoing splenectomy were younger and had fewer medical comorbidities (Table 1); traits that have been shown in most studies to lessen the risk of thromboembolism. It is possible that the increased risk associated with splenectomy was a postoperative phenomenon that might be seen with any major abdominal operation. Because the risk for postoperative thromboembolism has been shown to vary by type of operation,31 the site of postoperative thrombosis (ie, abdominal vs peripheral) might also vary in the early vs later postoperative period. Because AbVTE is typically thought of as a surgical complication, it is not surprising that there was a fivefold higher risk of AbVTE in the early postoperative period that did not persist. The cumulative incidence of 1.6% reported here is similar to that reported in other studies examining the rate of postsplenectomy portal vein thrombosis,32,33 although none of the patients who developed portal vein thrombosis in those series had underlying ITP. A small series of patients with β-thalassemia who underwent splenectomy reported an 8.3% incidence of postsplenectomy VTE,34 a difference likely caused by the higher burden of hypercoagulability in patients with β-thalassemia compared with ITP.
Reasons for the increased risk for VTE in splenectomized patients might include factors attributable to: (1) ITP, (2) splenectomy, and/or (3) the more aggressive medical therapies to which patients with relapsed or refractory disease might be exposed. Because smaller studies have suggested ITP itself to be a risk factor for thromboembolic events,23,35-37 it is possible that patients with more severe ITP, rather than the splenectomy, are at higher risk for VTE. Data from the Danish National Patient Registry38 indicated that the adjusted odds ratio for VTE in splenectomized patients was 32.6 vs the general population in the first 90 days post surgery, although the absolute incidence was low. Patients who developed thromboembolism post splenectomy were more likely to have an underlying hematologic malignancy, massive splenomegaly, thrombocytosis, or hemolytic anemia, features not commonly associated with ITP.
Within the ITP population, potential mechanisms by which splenectomy could increase the risk of VTE were recently reviewed39 and include loss of the spleen’s filtering activity, allowing for increased circulation and exposure of damaged red cells, cholesterol, and C-reactive protein. Animal models have suggested that splenic macrophages regulate inflammation40 and mobilization of splenic macrophages could promote thrombosis. Candidates for splenectomy are likely to be patients with relapsed or refractory ITP, a cohort also likely to be receiving aggressive medical therapy in the form of higher levels of immunosuppressive drugs and repeated hospitalization. It is possible that these risk factors increased the incidence of thromboembolism.
In a prospective analysis of 205 patients with ITP, Aledort et al reported the cumulative incidence of VTE to be 5% in the adult patients. Fifty percent of the VTE cases were in patients who had undergone splenectomy. Bennett et al, using an insurance claims dataset, reported an overall rate of thromboembolism of 6.9% in a cohort of 2783 patients with a median follow-up of 15 months. However, the events included stroke and myocardial infarction, there was no increased risk ratio for VTE, and there was no information on splenectomy.41 Another prospective analysis of 114 patients with ITP refractory to splenectomy reported an increased rate of VTE compared with the expected rate of VTE in the general population; however, this increase was not significant when patients with other VTE risk factors were removed from the analysis.23 In contrast, a more recent retrospective analysis of 233 patients with ITP who underwent splenectomy revealed an 8% incidence of VTE in the 10-year period of follow-up,42 a higher incidence than previously reported and herein.
In a study to evaluate the safety of romiplostim in patients with ITP, 4.9% of treated patients had a thrombotic event. Most patients who had thrombotic events had preexisting risk factors for thrombosis before initiation of therapy.36 The same group reported a cumulative incidence of 2.4% for the entire study cohort but did not specifically analyze the relationship of VTE to splenectomy.
The variable rates of VTE between these other studies and the present one are likely a result of differences in case ascertainment, referral bias, cohort size and duration of follow-up, treatment, and other unaccounted for demographic factors. Given the large size and diversity of the hospitals included in the present study, it may more accurately reflect the incidence of VTE across a range of practice settings.
It has been difficult to quantify the risk of infection for ITP patients who undergo splenectomy compared with those who do not. It should be noted that some studies have determined all serious infections, whereas others have looked only at sepsis, as in the present report.
Routine therapy for ITP is immunosuppressive. Because splenectomy is highly effective therapy for ITP, and response may obviate the need for further immunosuppressive therapy,10 the procedure might actually decrease the overall risk of infection in patients with ITP despite resultant loss of splenic function.
In an attempt to determine the attributable risk associated with splenectomy, a recent retrospective review analyzed the infectious outcomes of 3812 patients who underwent splenectomy for a variety of indications. The study showed that those who underwent splenectomy were at a 4.6-fold (CI, 3.8-5.5) higher risk for infection when compared with the general population from days 91 to 365 days post splenectomy. When compared with disease-matched controls, patients with ITP who underwent splenectomy had a trend toward increased rates of infection at >365 days post splenectomy that was not statistically significant (HR 1.4 [CI, 1.0-2.0]).17
Other studies have demonstrated an increased risk of postsplenectomy bacteremia and infection,43 with reported rates of 2.3% to 3.2%.15,16 The present study also demonstrates that patients with ITP who underwent splenectomy were at higher risk for both early and late sepsis compared with nonsplenectomized ITP patients.
It is worth noting that the cumulative incidence of sepsis in the nonsplenectomized ITP patients (10.1%), although lower than for those who had undergone splenectomy (11.1%), was not trivial. As would be expected, older age and presence of comorbidities were also associated with increased risk for sepsis. Interestingly, female gender was associated with a lower risk of sepsis. Being of black ethnicity was associated with a slightly increased risk of sepsis.
Sepsis and AbVTE were associated with an increased odds ratio for death, but VTE was not. Interestingly, splenectomy was associated with decreased odds of death in the same model. Splenectomy patients were younger and had fewer comorbidities. However, the odds of death were reduced even when adjusted for age, comorbidities, gender, ethnicity, and incident AbVTE/VTE/sepsis. We believe that patients selected for splenectomy are overall healthier, but we cannot exclude the possibility that splenectomy was somehow protective of death in this cohort. When compared with a population-based comparator group, Yong et al demonstrated a higher relative risk for death in the 90-day period after splenectomy (RR 2.3) that decreased to 0.5 in days 91 to 365, and decreased further to 0.4 at >365 post splenectomy. Taken together, this data imply that, although patients are at higher risk for AbVTE, VTE, and sepsis after splenectomy, they might have a reduced long-term risk of death.44
In the present report, 5- and 10-year Kaplan-Meier survival rates for the entire cohort (73% and 64%, respectively) were lower than previously reported. In a retrospective review of 152 patients with ITP, only 24 patients died during a 20-year follow-up period (84% survival). Of the entire cohort, the HR for death was 1.5 (CI, 1.1-2.2) compared with the general population. When patients with secondary ITP were excluded, the HR was 1.3 (CI, 0.89-2.0). However, patients with severe ITP (defined as persistent thrombocytopenia of <30.0 × 109/L 2 years after diagnosis) had a HR for death of 4.2 (CI, 1.7-10).45 Differences from our study may be a result of the present cohort being older (median age 55 vs 39 years) with more comorbidities and more likely to have severe disease.
In the 2011 updated treatment guidelines for ITP, the American Society of Hematology recommended splenectomy be considered in patients in whom front-line therapy with corticosteroids (Grade IB evidence) fails. The authors noted that a paucity of evidence prevents them from making specific recommendations regarding second-line therapies, although recommended thrombopoeitin receptor agonists for patients at risk of bleeding who relapse after splenectomy, have a contraindication to splenectomy, or in whom at least one other line of therapy (grade 1A recommendation) has failed. The authors suggest the use of thrombopoeitin receptor agonists for patients at risk for bleeding in whom one prior line of therapy before splenectomy (grade 2C recommendation) has failed. They note that only splenectomy is likely to provide sustained remission off treatment beyond 1 year.4
The International Working Group recognized that many patients and physicians prefer to delay or avoid splenectomy when patients relapse after front-line therapy. The panel stated that splenectomy should not be performed in patients who are too ill or frail, but the curative potential of splenectomy is superior to other available treatments.2 The International Working Group panel did not provide specific recommendations for sequencing of second-line therapies.
Despite the efficacy of splenectomy, the rate of splenectomy decreased during the period of this analysis. Possible reasons for this decline include physician and patient preference for medical over surgical therapy, as well as an increased awareness of the possible risks associated with splenectomy.
Patients with ITP refractory to primary therapy and those contemplating splenectomy should be made aware of both short- and longer-term complications. The risks of AbVTE, VTE, and sepsis were increased in patients who underwent splenectomy, with cumulative incidences of 1.6%, 4.3%, and 11.1%, respectively. The increased risk of AbVTE, VTE, and sepsis must be weighed against the efficacy of splenectomy for long-term disease control.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.B. and T.W. had full access to all data in the study and take responsibility for the integrity of the data and accuracy of the data analysis; T.W. and R.H.W. contributed to study concept and design; A.B. did statistical analysis; S.B drafted the manuscript and final revision; and T.W. and R.H.W. provided critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no completing financial interests.
Correspondence: Ted Wun, Division of Hematology Oncology, UC Davis Cancer Center, 4501 X St, Sacramento, CA 95817; e-mail: ted.wun@ucdmc.ucdavis.edu.