In this issue of Blood, Seano et al describe a new method to screen drugs that starve tumors by using umbilical cords, which allow nourishment and waste product exchange between mother and embryo.1
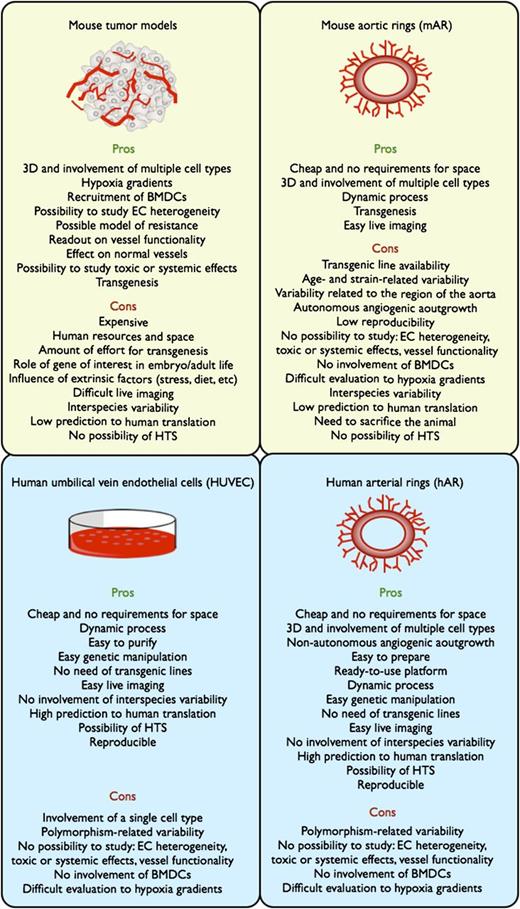
Scheme showing advantages and limitations of angiogenic assays. The yellow boxes refer to assays developed in mouse, and the sky-blue boxes refer to assays with human biomaterials. Pros and cons reflect the potential of these systems to study the angiogenic process and to validate pharmacologically or genetically the relevance of a certain pathway during angiogenesis. 3D, three-dimensional; BMDCs, bone marrow–derived cells; HTS, high-throughput screening; EC, endothelial cell.
Scheme showing advantages and limitations of angiogenic assays. The yellow boxes refer to assays developed in mouse, and the sky-blue boxes refer to assays with human biomaterials. Pros and cons reflect the potential of these systems to study the angiogenic process and to validate pharmacologically or genetically the relevance of a certain pathway during angiogenesis. 3D, three-dimensional; BMDCs, bone marrow–derived cells; HTS, high-throughput screening; EC, endothelial cell.
From the original concept proposed by Judah Folkman in 1971, several antiangiogenic drugs were shown to be capable of pruning the tumor vasculature and blocking cancer growth as a result of diminished nutrients’ delivery to the tumor.2,3 The translation of these drugs from mouse to human has revealed their tremendous potential, yet underscores their limitations.2,3 More than 80 antiangiogenic drugs are being tested worldwide in clinical trials, and each year patients with cancer or ocular diseases will be treated with these agents.2-4 Antiangiogenic treatments have shown efficacy in preventing progressive loss of vision and even improving eyesight in ophthalmologic patients. In cancer patients, these drugs have not fulfilled the expectations promised from preclinical studies. Most studies have demonstrated a modest increase in the overall survival of treated patients without offering an enduring cure.2-4 The reasons underlying the disappointing results are diverse, but one of the major causes is that many of the molecular pathways regulating angiogenesis are different in mice and humans, so that the overall results can differ significantly among species.5 In this issue, Seano et al report that rings of tissue prepared from human umbilical arteries when embedded into a tridimensional matrix are able to sprout in response to recombinant or tumor-derived proangiogenic factors, thus offering a robust and reproducible system for pharmacologic or genetic validation of targeted therapies.1
This new system advances the field in several ways (see figure). It offers the possibility for dynamic monitoring of the angiogenic process. The system can potentially be used for the development of a human model for the screening of antiangiogenic drugs, preventing flops caused by a lack of interspecies cross-reactivity.5 The assay is inexpensive, using biological material that would otherwise be discarded, and it requires fewer resources per experiment than in vivo assays, in mice or any other small animal models. But, as with in vivo systems, and differently from in vitro cell systems, the method proposed by Seano et al takes into account that blood vessels are a three-dimensional network of both endothelial cells and associated stromal cells (eg, mural cells).1 Of particular interest for cancer biologists is that the so-called human arterial rings (hARs) proposed by Seano et al when stimulated by tumor cell–released factors give rise to massively branched structures, which mimic tortuous, abnormal tumor vessels and respond less to antiangiogenic drugs, indicating that, as in a tumor, these vascular branches rely on a panoply of biological stimuli, and the inhibition of one of these pathways unleashes alternative signals that escape the antiangiogenic treatment.1 Finally, the ease of genetic modification of hARs in vitro bypasses the difficulties that scientists have encountered when knocking out genes that are required during the embryonic or adult life of the animal.5
Although hARs have advantages, there are still some limitations (see figure). The method raises some questions that need to be addressed for further improvement of this assay. First, endothelial cell heterogeneity has been long recognized so that embryonic endothelial cells (that are part of hARs) will be different from adult endothelial cells. In addition, arterial and venous endothelium from different tissue compartments present specific gene profiles.2,3 The animal models used thus far take into account this endothelial diversity, whereas using hARs represents a simplified approach. Furthermore, genome polymorphisms within the population can predict the response to antiangiogenic agents.6 As a consequence, the same drug tested on cords harvested from different individuals might present distinct sensitivity patterns.
Similar to all the in vitro systems, the proposed assay is not useful for determining systemic effects of the drugs on tumor-associated cells, organ functions or peripheral vessels, important to study mechanisms of refractoriness, toxic side effects or distant cancer cell extravasation.7,8 For example, inflammatory cells recruited to the tumor from the bone marrow participate in the angiogenic process and often confer resistance to antiangiogenic therapies.9 The same is true for low oxygen tension, namely hypoxia, which promotes vessel formation through the induction of proangiogenic factors in the neighboring tissue cells, and of maturation factors in the endothelial cells themselves.8,10 It will be interesting to assess how hARs respond to the addition of inflammatory cells and/or hypoxia to the system.
Finally, vessel function has been found to correlate more to tumor outcome than to vessel density.8,10 Given the presence of vascular lumens in branched hARs, the possibility to assess dynamic flow or other functional parameters will narrow the gap between this in vitro model and tumor blood vessels.
If these hARs are shown to respond to angiogenic stimuli after cryopreservation and thawing, the assay described by Seano et al will overall represent a powerful and ready-to-use tool for the design of high-throughput screening platforms of hundreds of compounds and their chemical derivatives, paving the way toward new opportunities for antiangiogenic therapy and cancer treatment.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal