Key Points
Hematopoietic progenitor kinase 1 (HPK1) regulates LFA-1 affinity and thereby controls adhesion and postadhesion functions of neutrophils.
Hematopoietic progenitor kinase 1 (HPK1) is critically involved in neutrophil trafficking during acute inflammation.
Recruitment of polymorphonuclear neutrophils (PMNs) to sites of acute inflammation critically depends on β2 integrins (CD11/CD18). Recently, the mammalian actin-binding protein 1 (mAbp1) was identified as an important adaptor protein regulating PMN trafficking downstream of β2 integrins. Here, we show that mAbp1 constitutively co-immunoprecipitated with hematopoietic progenitor kinase 1 (HPK1) in neutrophil-like differentiated HL-60 (dHL-60) cells. HPK1 was enriched at the lamellipodium of polarized dHL-60 cells, where it colocalized with mAbp1 and actin. Functional analysis of PMNs from HPK1-deficient mice showed that HPK1 was critical for CXCL1-induced lymphocyte function-associated antigen 1 (LFA-1)–mediated PMN adhesion to ICAM-1 under flow conditions. Accordingly, CXCL1-mediated induction of high-affinity LFA-1 required HPK1, but macrophage antigen 1 (Mac-1) affinity regulation was independent of HPK1. Intravital microscopy of the mouse cremaster muscle confirmed the defect of CXCL1-induced leukocyte adhesion in HPK1-deficient mice. Furthermore, β2 integrin–mediated post-adhesion processes—adhesion strengthening, spreading, and directed mechanotactic crawling of PMNs under flow conditions—involved HPK1 in vitro and in vivo. Upon intrascrotal administration of tumor necrosis factor α (TNF-α), PMN adhesion and extravasation were severely compromised in HPK1-deficient mice. In summary, our results indicate that HPK1 is critically involved in LFA-1–mediated PMN trafficking during acute inflammation.
Introduction
Polymorphonuclear neutrophils (PMNs) mediate host defense and inflammation. During the acute inflammatory response, PMNs are rapidly (within minutes) recruited from the blood to lesion sites.1 Although under tight temporal and spatial control, this response, called the multistep cascade of leukocyte recruitment, is extremely robust. The recruitment cascade of PMNs in postcapillary venules involves capture and rolling, activation, firm adhesion, adhesion strengthening, spreading, and intraluminal (mechanotactic) crawling of PMNs on the activated endothelium to extravasation sites where transendothelial diapedesis occurs. Upon extravasation, PMNs perform abluminal crawling on the pericytes of the vessel wall followed by interstitial migration.2 Although this process of PMN recruitment during acute inflammation is well defined on the cellular level, the molecular mechanisms controlling this cascade are still incompletely understood.
Inflammatory mediators released from the surrounding tissue prompt endothelial cells of postcapillary venules to upregulate expression of various surface molecules, including P-selectin, E-selectin, and intercellular adhesion molecule 1 (ICAM-1).1 Selectins mediate initial capture and rolling of PMNs, facilitating PMN activation via G protein–coupled receptors by chemokines such as CXCL1 presented at the luminal side of inflamed endothelial cells. The following recruitment steps, including slow rolling, firm adhesion, adhesion strengthening, spreading, intraluminal crawling, and PMN extravasation are mainly mediated by β2 integrins (CD11/CD18).1,3,4 This family of heterodimeric leukocyte adhesion molecules consists of four members among which lymphocyte function-associated antigen 1 (LFA-1; CD11a/CD18, αLβ2) and macrophage antigen 1 (Mac-1; CD11b/CD18, αMβ2) are most important for PMN recruitment. β2 integrins are known to exist in 3 conformational states with different ligand affinities.5 Bent inactive β2 integrins with low ligand affinity are predominant on nonactivated circulating PMNs. During PMN rolling on endothelial selectins via P-selectin glycoprotein ligand 1 (PSGL-1), ligand binding of PSGL-1 triggers extension of LFA-1 via inside-out signaling, which thereby gains intermediate affinity to its ligand ICAM-1 and can, in turn, mediate slow leukocyte rolling through transient interactions.6,-8 Finally, binding of CXCL1, for example, to its G protein–coupled receptor (i.e., CXCR2) induces further inside-out signaling that promotes partial dissociation of the alpha and beta subunits of extended LFA-1.6,8 This second conformational shift leads to an opening of the β2 integrin I domain promoted by a complex sequence of molecular events that regulate the high ligand affinity conformation of LFA-1 and thereby allow firm PMN arrest.8,9
However, β2 integrins not only mediate adhesive interactions, they also induce outside-in signaling processes that support postadhesion events, including adhesion strengthening, induction of shape change, and spreading.4,10,-12 Upon ligand binding of β2 integrins, immunoreceptor tyrosine-based activating motif (ITAM)–bearing side chains of transmembrane adaptors such as DAP12 or FcγRIIA are phosphorylated by Src-family kinases, which results in recruitment and activation of spleen tyrosine kinase (Syk).13,14 Syk is also known to be involved in PSGL-1–mediated inside-out signaling.15 However, during β2 integrin–mediated outside-in signaling, activated Syk has several downstream partners, including PLCγ, Vav, or the mammalian actin-binding protein 1 (mAbp1).14,16 mAbp1 is an adaptor protein that has recently been found to play an important role in PMN recruitment.17 Accordingly, mAbp1 was critically involved in PMN adhesion, spreading, and migration under flow conditions in vitro and in vivo.18,19 In addition, PMN extravasation during inflammation was markedly reduced in the absence of mAbp1.19 Fluorescence microscopy revealed that mAbp1 was recruited to the leading edge of polarized PMNs in a Syk-dependent manner, where it colocalized with F-actin.18 Upon ligand binding of β2 integrins, outside-in signaling induced the interaction of mAbp1 with actin and promoted the reinforcement of the high-affinity conformation of β2 integrins under flow conditions.18,19 Originally, mAbp1 was cloned as an interacting protein of hematopoietic progenitor kinase 1 (HPK1) in fibroblasts and was named HPK1-interacting protein of 55 kDa (HIP-55).20 By using cotransfected HEK293 T cells, Ensenat et al demonstrated that this interaction was mediated by the Src homology 3 (SH3) domain of mAbp1 and the second proline-rich domain of HPK1.20 Subsequently, evidence for the interaction between mAbp1 and HPK1 was also obtained in T Jurkat cells.21
HPK1 is a 97-kDa Ste20-like serine/threonine kinase predominantly expressed in hematopoietic cells of the adult organism.22 First described as an activator of the SAPK/JNK pathway,22 HPK1 was found to be involved in nuclear factor κB activation23 and apoptosis of T cells24 and is known to negatively affect lymphocyte adhesion.25,26 HPK1 can exert its function by interacting with several adaptor proteins in a variety of different signaling complexes thereby fine-tuning regulation of different cellular processes, including differentiation, proliferation, adhesion, and apoptosis of lymphocytes.24,,,-28 Thus, there is a growing body of evidence that HPK1 plays an important role in the regulation of the adaptive immune system, but the functional impact of HPK1 for innate immunity and especially PMN recruitment remains elusive. Therefore, this study was designed to delineate the functional impact of HPK1 for PMN trafficking during the acute inflammatory response.
We found that HPK1 constitutively co-immunoprecipitated with the adaptor protein mAbp1 in neutrophil-like differentiated HL-60 (dHL-60) cells. HPK1 was enriched at the lamellipodium of polarized dHL-60 cells upon β2 integrin–mediated adhesion where it colocalized with mAbp1 and actin. Functional analysis revealed that HPK1 was important for CXCL1-induced adhesion under flow conditions by regulating the induction of the high-affinity conformation of LFA-1. Moreover, HPK1 was involved in adhesion strengthening, spreading, and mechanotactic crawling of PMNs under flow conditions. In cremaster muscle venules treated with tumor necrosis factor α (TNF-α), leukocyte extravasation was severely compromised in the absence of HPK1. Thus, we identified HPK1 as an important regulator of LFA-1–mediated PMN recruitment during acute inflammation.
Materials and methods
Plasmids, antibodies, and reagents
Please see supplemental Methods.
Mice
HPK1−/− mice that carried a PGK1-neo selection cassette in antisense orientation in the second half of exon 1, disrupting protein expression, were maintained on the C57BL/6 background.29 All animal experiments were institutionally approved by Regierung von Oberbayern, Munich, Germany.
Isolation of murine PMNs and cell culture
PMNs were isolated from murine bone marrow by using a discontinuous Percoll gradient as described.16 Isolated PMNs were cultivated for 24 hours in RPMI 1640 medium supplemented with 20% WEHI-3B–conditioned medium. Murine WEHI-3B cells (ACC 26) and human HL-60 cells (ACC 3) were obtained from the German Resource Centre for Biological Material (Braunschweig, Germany). dHL-60 cells were differentiated with 1.3% dimethylsulfoxide (DMSO) for 6 days. Stable lentiviral transduction of HL-60 cells was performed as described.18
Co-immunoprecipitation and confocal microscopy
Co-immunoprecipitation experiments were performed as previously described.18 Briefly, dHL-60 cells stably expressing mAbp1-GFP or dHL-60 control cells were seeded onto fibrinogen-coated dishes or were left in suspension and were incubated at 37°C for 10 minutes. Upon induction of adhesion by the addition of 1 mM Mn2+ for 15 minutes, cells were lysed and co-immunoprecipitation was performed by using GFP-Trap beads (Chromotek; Martinsried, Germany). Fluorescence staining and confocal microscopy of dHL-60 cells are described in supplemental Methods.
Intravital microscopy
Intravital microscopy of the mouse cremaster muscle was performed by using an upright microscope (Leica; Wetzlar, Germany) with an SW40×/0.75NA saline immersion objective, as previously reported.12 Microvascular parameters were measured by using a digital image processing system.30 Postcapillary venules were recorded to calculate rolling flux fraction (percentage of rolling leukocytes relative to the number of leukocytes entering the vessel) and leukocyte adhesion efficiency (percentage of adherent leukocytes per square millimeter relative to white blood cell count in µL−1). Leukocytes were considered adherent when attached at the same position for >30 seconds.31 To analyze CXCL1-induced adhesion, rolling flux fraction and adhesion efficiency were measured at different time points following systemic application of CXCL1 (600 ng) via the carotid artery and were compared with control values obtained from the same vessels prior to CXCL1 injection. Leukocyte recruitment during TNF-α–induced acute inflammation was studied with intravital microscopy 2.5 hours after intrascrotal injection of TNF-α (500 ng). Leukocyte rolling and adhesion were assessed as mentioned above. Time-lapse recordings were conducted for 10 minutes to investigate leukocyte crawling on the inflamed endothelium as described.32 Leukocytes with a displacement of >15 µm were tracked by using ImageJ (National Institutes of Health, Bethesda, MD). Calculation of crawling parameters and plotting of rose diagrams were carried out as described.33 Leukocytes ending up in a 60° angle symmetrically divided by the vector of blood flow were defined as leukocytes crawling in the direction of blood flow.
Flow chamber assays and fluorescence-activated cell sorting analysis of isolated PMNs
Please see supplemental Methods.
Histologic analysis of cremaster whole mounts
Please see supplemental Methods.
Statistical analysis
Data shown represent mean ± standard deviation or standard error of the mean, as indicated. Statistical significance (P < .05) was calculated by using the Student t test or Mann-Whitney rank sum test as implemented in Sigma Stat V3.5 (Systat Software, Chicago, IL).
Results
Adaptor protein mAbp1 and HPK1 constitutively co-immunoprecipitated and colocalized at the lamellipodium upon β2 integrin–mediated adhesion
To study the interaction between mAbp1 and HPK1 in myeloid cells, we performed co-immunoprecipitation experiments with neutrophil-like dHL-60 cells. We used wild-type dHL-60 cells for control and dHL-60 cells expressing mAbp1-GFP (dHL-60-mAbp1-GFP) for specific mAbp1-GFP pulldown. Cells were exposed to immobilized fibrinogen, a known β2 integrin ligand, and were treated with Mn2+ to induce adhesion by stabilizing the high-affinity integrin conformation,34 or cells were left untreated for control. After cell lysis, co-immunoprecipitation with GFP nanotraps was performed, and HPK1, mAbp1, and actin were detected by western blotting (Figure 1A). As expected, adhesion induced the interaction between mAbp1 and actin.18 Moreover, HPK1 co-immunoprecipitated with mAbp1 in unstimulated nonadherent dHL-60-mAbp1-GFP cells as well as in adherent cells. Using wild-type dHL-60 cells as negative control, no (co)-immunoprecipitation was observed, demonstrating the specificity of the technique that was used. Next, confocal microscopy was used to study the localization of mAbp1 and HPK1 in adherent dHL-60 cells (Figure 1B). When exposed to immobilized fibrinogen in the presence of Mn2+ (upper panel), the cells underwent a shape change and formed lamellipodium-like structures enriched with HPK1 colocalizing with mAbp1. Upon exposure to immobilized ICAM-1 and interleukin-8 (IL-8), the cells polarized and formed lamellipodia displaying an enrichment of HPK1, which colocalized with mAbp1 (lower panel) and actin (Figure 1C). Thus, mAbp1 and HPK1 seemed to interact constitutively, and the induction of β2 integrin–mediated adhesion led to an enrichment of the HPK1-mAbp1 module at the lamellipodium, where it colocalized with actin.
The mAbp1 interacting protein HPK1 was involved in induction of adhesion of murine PMNs under flow conditions in vitro. (A) Western blot of whole-cell lysates and co-immunoprecipitates of wild-type dHL-60 cells and dHL-60 cells expressing an mAbp1-GFP construct (dHL-60-mAbp1-GFP) upon specific mAbp1-GFP pulldown with GFP-nanotraps. Cells were either exposed to immobilized fibrinogen (250 µg/mL) and treated with Mn2+ (1 mM) for 15 minutes at 37°C or left in suspension without stimulation. mAbp1, HPK1, and actin were detected by immunoblotting by using specific antibodies. Western blot is representative for 3 independent experiments. (B,C) Confocal microscopy of dHL-60-mAbp1-GFP cells stained for HPK1 and actin. Cells were treated with Mn2+ (1 mM) and exposed to immobilized fibrinogen (250 µg/mL) (B upper panel), or were seeded on coverslips coated with ICAM-1 (12.5 µg/mL) and IL-8 (1 µg/mL) (B lower panel, and C) for 15 minutes at 37°C. After fixation and staining, confocal laser scanning microscopy was conducted. (B) Colocalization (yellow) of mAbp1-GFP (green) and HPK1 (red) at the lamellipodium (arrowheads) of polarized dHL-60 cells. (C) Colocalization (white) of HPK1 (red) and actin (cyan) at the lamellipodium (arrowheads) of polarized dHL-60 cells. Images are representative of at least 3 independent experiments; bar = 10 µm. (D) Induction of adhesion of isolated murine PMNs under flow conditions (1 dyne/cm2). Flow chambers were coated with P-selectin (10 µg/mL), ICAM-1 (12.5 µg/mL), and CXCL1 (5 µg/mL). PMNs were treated with a nonbinding control antibody (crtl immunoglobulin G [IgG]), or function blocking anti–Mac-1, or anti–LFA-1 antibodies (30 µg/mL) as indicated. Numbers indicate relative PMN adhesion after 10 minutes in percent of all interacting PMNs (100%). n = 4; mean ± standard deviation (SD); *P < .05; **P < .001; n.s. = not significant.
The mAbp1 interacting protein HPK1 was involved in induction of adhesion of murine PMNs under flow conditions in vitro. (A) Western blot of whole-cell lysates and co-immunoprecipitates of wild-type dHL-60 cells and dHL-60 cells expressing an mAbp1-GFP construct (dHL-60-mAbp1-GFP) upon specific mAbp1-GFP pulldown with GFP-nanotraps. Cells were either exposed to immobilized fibrinogen (250 µg/mL) and treated with Mn2+ (1 mM) for 15 minutes at 37°C or left in suspension without stimulation. mAbp1, HPK1, and actin were detected by immunoblotting by using specific antibodies. Western blot is representative for 3 independent experiments. (B,C) Confocal microscopy of dHL-60-mAbp1-GFP cells stained for HPK1 and actin. Cells were treated with Mn2+ (1 mM) and exposed to immobilized fibrinogen (250 µg/mL) (B upper panel), or were seeded on coverslips coated with ICAM-1 (12.5 µg/mL) and IL-8 (1 µg/mL) (B lower panel, and C) for 15 minutes at 37°C. After fixation and staining, confocal laser scanning microscopy was conducted. (B) Colocalization (yellow) of mAbp1-GFP (green) and HPK1 (red) at the lamellipodium (arrowheads) of polarized dHL-60 cells. (C) Colocalization (white) of HPK1 (red) and actin (cyan) at the lamellipodium (arrowheads) of polarized dHL-60 cells. Images are representative of at least 3 independent experiments; bar = 10 µm. (D) Induction of adhesion of isolated murine PMNs under flow conditions (1 dyne/cm2). Flow chambers were coated with P-selectin (10 µg/mL), ICAM-1 (12.5 µg/mL), and CXCL1 (5 µg/mL). PMNs were treated with a nonbinding control antibody (crtl immunoglobulin G [IgG]), or function blocking anti–Mac-1, or anti–LFA-1 antibodies (30 µg/mL) as indicated. Numbers indicate relative PMN adhesion after 10 minutes in percent of all interacting PMNs (100%). n = 4; mean ± standard deviation (SD); *P < .05; **P < .001; n.s. = not significant.
LFA-1–mediated induction of adhesion was defective in HPK1−/− PMNs under flow conditions
To elucidate the functional impact of HPK1 on PMN trafficking, we studied induction of adhesion under flow conditions in vitro by using PMNs isolated from HPK1−/− and HPK1+/+ control mice. First of all, the expression levels of mAbp1 in HPK1+/+ and HPK1−/− PMNs were studied and found to be similar in both cell types (supplemental Figure S1). For functional analysis, PMNs were perfused through flow chambers coated with P-selectin, ICAM-1, and CXCL1 at physiological flow conditions in the presence of anti–LFA-1 or anti–Mac-1 function blocking antibodies or a nonbinding control antibody. Relative adhesion of PMNs was significantly reduced from 90% ± 3% of HPK1+/+ PMNs to 52% ± 10% of HPK1−/− PMNs in the presence of a nonbinding control antibody (Figure 1D). Blocking Mac-1 had only minor effects, suggesting that Mac-1 was dispensable for induction of adhesion in this experimental setting. In contrast, blocking LFA-1 markedly decreased adhesion of HPK1+/+ PMNs to adhesion levels seen in the absence of HPK1, whereas adhesion of HPK1−/− PMNs was almost unaffected. Thus, HPK1 specifically regulated CXCL1-induced adhesion to ICAM-1 via LFA-1 under flow conditions.
HPK1 regulated the affinity of LFA-1, but not Mac-1 in PMNs
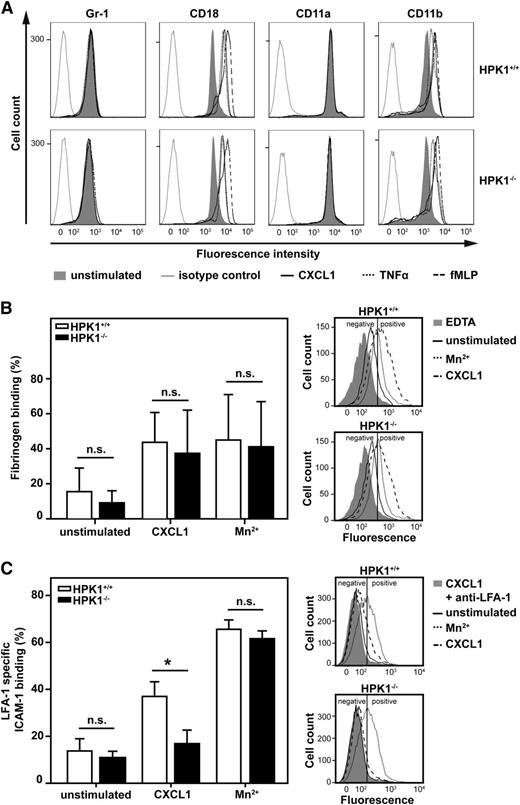
To exclude the possibility that compromised PMN maturation or diminished β2 integrin expression in the absence of HPK1 caused the observed defect, fluorescence-activated cell sorting analysis for surface expression of Gr-1, CD18, CD11a, and CD11b was performed. The expression levels of all epitopes of HPK1−/− PMNs were similar when compared with HPK1+/+ PMNs (Figure 2A). Upregulation of CD11b/CD18 in HPK1−/− PMNs was also unaffected upon stimulation with CXCL1, TNF-α, or N-formyl-methyl-leucyl-phenylalanine compared with HPK1+/+ PMNs. Thus, compromised induction of adhesion in the absence of HPK1−/− was not due to impaired PMN maturation or decreased β2 integrin expression. Next, we studied the role of HPK1 for the control of ligand-binding affinity of the β2 integrins LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18). Binding of soluble fibrinogen to isolated murine PMNs was quantified by using flow cytometry as a measurement of Mac-1 affinity. When stimulated with CXCL1, fibrinogen binding of HPK1+/+ PMNs was increased compared with unstimulated PMNs, as expected (Figure 2B). Addition of Mn2+, which stabilizes the high-affinity conformation of Mac-1 while bypassing inside-out signaling,34 also increased fibrinogen binding compared with unstimulated control. However, there was no difference in fibrinogen binding between HPK1+/+ and HPK1−/− PMNs, suggesting that HPK1 was dispensable for the affinity regulation of Mac-1. LFA-1 affinity regulation of murine PMNs was measured by LFA-1–specific binding of soluble ICAM-1 in the presence of function-blocking anti–Mac-1 antibody. In unstimulated PMNs, LFA-1–specific ICAM-1 binding was comparable between HPK1+/+ and HPK1−/− PMNs (Figure 2C). Upon CXCL1 application, LFA-1–specific ICAM-1 binding of HPK1+/+ PMNs was increased compared with unstimulated cells, but this response was significantly compromised in the absence of HPK1. However, induction of the high-affinity conformation by Mn2+ increased LFA-1–specific ICAM-1 binding in both HPK1+/+ and HPK1−/− PMNs. This finding suggests that HPK1 specifically regulated the induction of the high-affinity conformation of LFA-1, but not Mac-1 during inside-out signaling upon CXCL1 stimulation.
HPK1-deficient PMNs showed normal affinity regulation of Mac-1, but defective CXCL1-mediated upregulation of LFA-1 affinity. (A) Flow cytometric analysis of cell-surface expression of Gr-1, CD18, CD11a, and CD11b. Isolated murine PMNs were stimulated with CXCL1 (100 ng/mL), TNF-α (100 ng/mL), N-formyl-methyl-leucyl-phenylalanine (10 µM), or left unstimulated for 20 minutes at 37°C. Histograms are representative of 3 independent experiments. (B) Binding of soluble fibrinogen to isolated murine PMNs measured by flow cytometry. PMNs were stimulated with CXCL1 (100 ng/mL), Mn2+ (3 mM), or left unstimulated. Bar chart (left) shows percentage of cells with positive fibrinogen binding, calculated by using a threshold that defined 95% of EDTA-treated (2 mM) PMNs as negative. Representative fluorescence-activated cell sorting (FACS) histograms are shown on the right. n = 6. (C) LFA-1–specific binding of soluble ICAM-1/Fc to isolated murine PMNs measured by flow cytometry. PMNs were preincubated with anti–Mac-1 blocking antibody to prevent Mac-1 binding of ICAM-1/Fc and were stimulated with CXCL1 (100 ng/mL), Mn2+ (5 mM), or left unstimulated. Bar chart (left) indicates percentage of cells with positive LFA-1–specific ICAM-1/Fc binding, calculated by using a threshold that defined 95% of PMNs treated with CXCL1 and anti–LFA-1 antibody as negative. Similar results were obtained with 3 mM Mn2+. Representative FACS histograms are shown on the right. n = 4 (unstimulated, CXCL1); n = 3 (Mn2+). Diagrams show mean ± SD; *P < .05; n.s. = not significant.
HPK1-deficient PMNs showed normal affinity regulation of Mac-1, but defective CXCL1-mediated upregulation of LFA-1 affinity. (A) Flow cytometric analysis of cell-surface expression of Gr-1, CD18, CD11a, and CD11b. Isolated murine PMNs were stimulated with CXCL1 (100 ng/mL), TNF-α (100 ng/mL), N-formyl-methyl-leucyl-phenylalanine (10 µM), or left unstimulated for 20 minutes at 37°C. Histograms are representative of 3 independent experiments. (B) Binding of soluble fibrinogen to isolated murine PMNs measured by flow cytometry. PMNs were stimulated with CXCL1 (100 ng/mL), Mn2+ (3 mM), or left unstimulated. Bar chart (left) shows percentage of cells with positive fibrinogen binding, calculated by using a threshold that defined 95% of EDTA-treated (2 mM) PMNs as negative. Representative fluorescence-activated cell sorting (FACS) histograms are shown on the right. n = 6. (C) LFA-1–specific binding of soluble ICAM-1/Fc to isolated murine PMNs measured by flow cytometry. PMNs were preincubated with anti–Mac-1 blocking antibody to prevent Mac-1 binding of ICAM-1/Fc and were stimulated with CXCL1 (100 ng/mL), Mn2+ (5 mM), or left unstimulated. Bar chart (left) indicates percentage of cells with positive LFA-1–specific ICAM-1/Fc binding, calculated by using a threshold that defined 95% of PMNs treated with CXCL1 and anti–LFA-1 antibody as negative. Similar results were obtained with 3 mM Mn2+. Representative FACS histograms are shown on the right. n = 4 (unstimulated, CXCL1); n = 3 (Mn2+). Diagrams show mean ± SD; *P < .05; n.s. = not significant.
CXCL1-mediated induction of leukocyte adhesion in vivo was almost absent in HPK1−/− mice
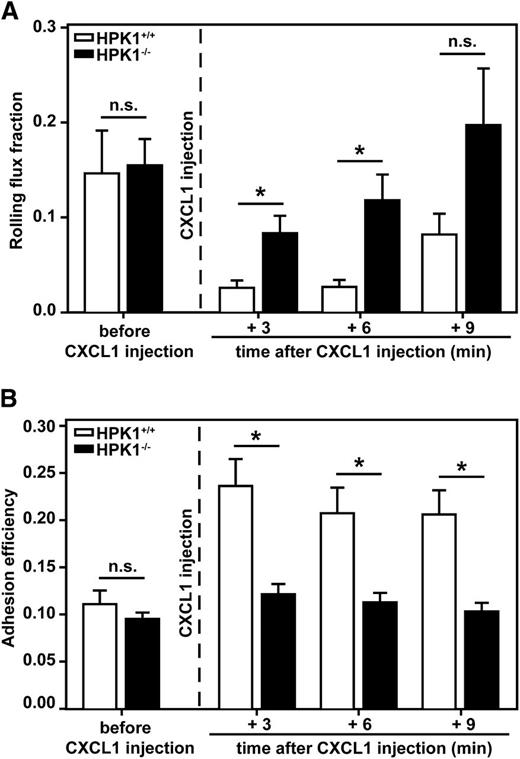
To analyze the in vivo relevance of our findings, leukocyte rolling and adhesion were studied in postcapillary venules of the inflamed mouse cremaster muscle. White blood cell count and hemodynamic parameters of analyzed vessels of HPK1+/+ and HPK1−/− mice were comparable (Table 1). Prior to CXCL1 injection, similar rolling flux fractions were observed in postcapillary venules of HPK1+/+ and HPK1−/− mice (Figure 3A). As expected, systemic administration of CXCL1 caused a striking drop in the rolling flux fraction after 3, 6, and 9 minutes in HPK1+/+ mice when compared with control values obtained before CXCL1 injection. In contrast, CXCL1 application in HPK1−/− mice only mildly decreased the rolling flux fraction when compared with the data obtained prior to CXCL1 injection, and it led to significantly elevated rolling flux fractions when compared with that in HPK1+/+ mice. Whereas the leukocyte adhesion efficiency (Figure 3B) was similar in both groups prior to CXCL1 injection, application of CXCL1 led to an increased adhesion efficiency in HPK1+/+ mice after 3 minutes, which slightly declined after 6 and 9 minutes, as expected.35 In contrast, CXCL1-induced leukocyte adhesion was almost absent in HPK1−/− mice, demonstrating a profound leukocyte adhesion defect in HPK1−/− mice in response to CXCL1.
Microvascular parameters of cremaster muscle venules before CXCL1 application
| . | Mice . | Venules . | Diameter (µm) . | Length (µm) . | Centerline velocity (µm/s) . | Shear rate (s−1) . | WBC (µL−1) . |
|---|---|---|---|---|---|---|---|
| HPK1+/+ | 5 | 10 | 27 ± 1 | 274 ± 23 | 1780 ± 127 | 1667 ± 164 | 6073 ± 615 |
| HPK1−/− | 6 | 15 | 26 ± 1 | 221 ± 16 | 1953 ± 215 | 1868 ± 203 | 6246 ± 621 |
| n.s. | n.s. | n.s. | n.s. | n.s. |
| . | Mice . | Venules . | Diameter (µm) . | Length (µm) . | Centerline velocity (µm/s) . | Shear rate (s−1) . | WBC (µL−1) . |
|---|---|---|---|---|---|---|---|
| HPK1+/+ | 5 | 10 | 27 ± 1 | 274 ± 23 | 1780 ± 127 | 1667 ± 164 | 6073 ± 615 |
| HPK1−/− | 6 | 15 | 26 ± 1 | 221 ± 16 | 1953 ± 215 | 1868 ± 203 | 6246 ± 621 |
| n.s. | n.s. | n.s. | n.s. | n.s. |
Table shows mean ± SEM. WBC, white blood cell count; n.s., not significant.
CXCL1-mediated leukocyte adhesion was almost absent in HPK1-deficient mice. (A) Leukocyte rolling flux fraction and (B) leukocyte adhesion efficiency in postcapillary venules of mouse cremaster muscle as assessed by intravital microscopy. Leukocyte rolling and adhesion were measured at different time points in each vessel: before CXCL1 injection as well as 3, 6, and 9 minutes after systemic administration of CXCL1 (600 ng) via the carotid artery. n = 10 venules from 5 HPK1+/+ mice and 15 venules from 6 HPK1−/− mice. Diagrams show mean ± standard error of the mean (SEM); *P < .05; n.s. = not significant.
CXCL1-mediated leukocyte adhesion was almost absent in HPK1-deficient mice. (A) Leukocyte rolling flux fraction and (B) leukocyte adhesion efficiency in postcapillary venules of mouse cremaster muscle as assessed by intravital microscopy. Leukocyte rolling and adhesion were measured at different time points in each vessel: before CXCL1 injection as well as 3, 6, and 9 minutes after systemic administration of CXCL1 (600 ng) via the carotid artery. n = 10 venules from 5 HPK1+/+ mice and 15 venules from 6 HPK1−/− mice. Diagrams show mean ± standard error of the mean (SEM); *P < .05; n.s. = not significant.
LFA-1–mediated adhesion strengthening and spreading of HPK1−/− PMNs were reduced under flow conditions in vitro
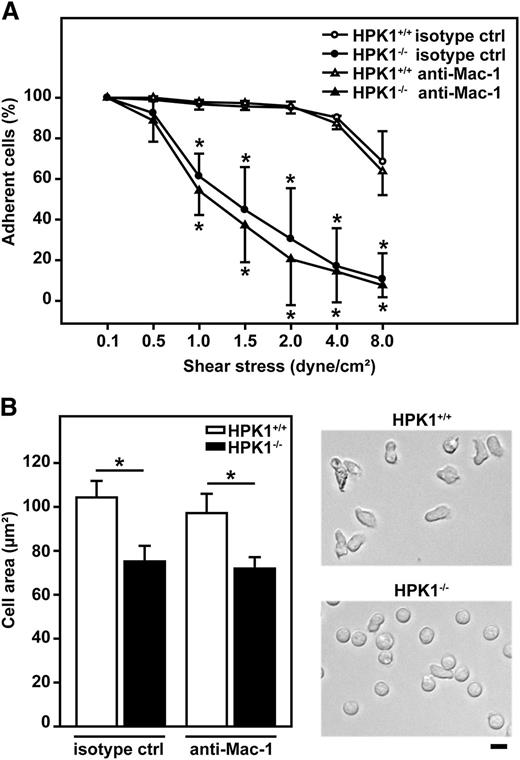
Postadhesion functions of PMNs were studied in vitro by using flow chamber assays. For analysis of adhesion strengthening, gradually increasing shear stress was applied to isolated murine PMNs adherent in flow chambers coated with P-selectin, ICAM-1, and CXCL1. At 1 dyne/cm2, 97% ± 2% of initially adherent HPK1+/+ PMNs (100%) remained adherent in the presence of an isotype control antibody (Figure 4A). This percentage was significantly lower in HPK1−/− PMNs (61% ± 9%). The observed detachment of HPK1−/− PMNs was even more pronounced at higher shear. Under shear stress of 8 dyne/cm2, 69% ± 12% of HPK1+/+ PMNs remained adherent, whereas this percentage was significantly reduced to only 11% ± 10% in HPK1−/− PMNs (Figure 4 and supplemental Video V1). The detachment process of HPK1+/+ and HPK1−/− PMNs was almost unaffected in the presence of a function blocking anti–Mac-1 antibody when compared with the data obtained in the presence of an isotype control antibody, suggesting that adhesion strengthening critically involved LFA-1 but was rather independent of Mac-1. To analyze spreading, PMNs were exposed to immobilized P-selectin, ICAM-1, and CXCL1 for 10 minutes, and flow of 1 dyne/cm2 was applied for 5 minutes before the cell area was quantified (Figure 4B). When compared with HPK1+/+ PMNs with a mean cell area of 104 ± 6 µm2, the cell area of HPK1−/− PMNs was significantly diminished to 75 ± 6 µm2 in the presence of an isotype control antibody. This was also apparent in the microscopic images (Figure 4B). Spreading was unaffected in the presence of an anti–Mac-1 function-blocking antibody when compared with isotype controls, indicating a predominant role of LFA-1 and its regulation by HPK1 for PMN spreading on ICAM-1 under flow conditions.
HPK1 was important for LFA-1–mediated adhesion strengthening and spreading of PMN under flow conditions in vitro. For flow chamber assays, isolated murine PMNs were treated with an isotype control antibody (30 µg/mL) or an anti–Mac-1 blocking antibody (30 µg/mL) and seeded into flow chambers coated with P-selectin (10 µg/mL), ICAM-1 (12.5 µg/mL), and CXCL1 (5 µg/mL). After 10 minutes of static conditions, flow was applied as indicated. (A) Detachment assay of adherent murine PMNs in response to gradually increasing shear stress (0.1 to 8.0 dyne/cm2) at intervals of 90 seconds. Adhesion strengthening is represented by the percentage of nondetaching cells compared with initially adherent cells at 0.1 dyne/cm2 (100%). n = 3 independent experiments with a total of 315 (HPK1+/+ isotype control), 281 (HPK1−/− isotype control), 266 (HPK1+/+ anti–Mac-1), and 235 (HPK1−/− anti–Mac-1) analyzed cells. (B) Spreading of adherent PMNs after 5 minutes of constant flow (1 dyne/cm2), quantified by the measurement of cell area (left panel). Representative microscopic images (right panel) are shown. Bar = 10 µm. n = 3 (isotype control); n = 4 (anti–Mac-1) independent experiments with a total of 221 (HPK1+/+ isotype control), 318 (HPK1−/− isotype control), 344 (HPK1+/+ anti–Mac-1), and 460 (HPK1−/− anti–Mac-1) analyzed cells. Diagrams (A,B) show mean ± SD; *P < .05 for HPK1+/+ values versus corresponding HPK1−/− values.
HPK1 was important for LFA-1–mediated adhesion strengthening and spreading of PMN under flow conditions in vitro. For flow chamber assays, isolated murine PMNs were treated with an isotype control antibody (30 µg/mL) or an anti–Mac-1 blocking antibody (30 µg/mL) and seeded into flow chambers coated with P-selectin (10 µg/mL), ICAM-1 (12.5 µg/mL), and CXCL1 (5 µg/mL). After 10 minutes of static conditions, flow was applied as indicated. (A) Detachment assay of adherent murine PMNs in response to gradually increasing shear stress (0.1 to 8.0 dyne/cm2) at intervals of 90 seconds. Adhesion strengthening is represented by the percentage of nondetaching cells compared with initially adherent cells at 0.1 dyne/cm2 (100%). n = 3 independent experiments with a total of 315 (HPK1+/+ isotype control), 281 (HPK1−/− isotype control), 266 (HPK1+/+ anti–Mac-1), and 235 (HPK1−/− anti–Mac-1) analyzed cells. (B) Spreading of adherent PMNs after 5 minutes of constant flow (1 dyne/cm2), quantified by the measurement of cell area (left panel). Representative microscopic images (right panel) are shown. Bar = 10 µm. n = 3 (isotype control); n = 4 (anti–Mac-1) independent experiments with a total of 221 (HPK1+/+ isotype control), 318 (HPK1−/− isotype control), 344 (HPK1+/+ anti–Mac-1), and 460 (HPK1−/− anti–Mac-1) analyzed cells. Diagrams (A,B) show mean ± SD; *P < .05 for HPK1+/+ values versus corresponding HPK1−/− values.
Direction of mechanotactic crawling was altered in HPK1−/− PMNs
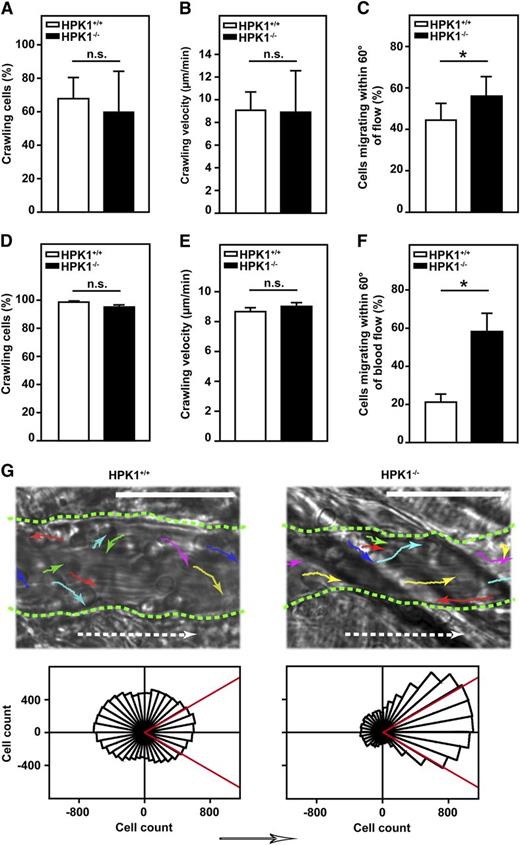
To further investigate the importance of HPK1 for trafficking of PMNs, mechanotactic crawling was analyzed. Shear of 1 dyne/cm2 was applied to isolated 60°murine PMNs adherent in flow chambers coated with P-selectin, ICAM-1, and CXCL1. Although significantly more HPK1−/− than HPK1+/+ PMNs detached under flow conditions as described above, analysis of remaining nondetached HPK1−/− PMNs showed that the percentage of adherent cells that performed crawling was not altered when compared with HPK1+/+ PMNs (Figure 5A). This was also true for the crawling velocity (Figure 5B). However, detailed analysis of the crawling direction revealed a slight but significant difference between HPK1+/+ and HPK1−/− PMNs (Figure 5C). Here, the percentage of PMNs crawling within a 60° angle, which is symmetrically divided by the vector of flow, was increased in HPK1−/− PMNs, suggesting that the ability to crawl against or perpendicular to the direction of flow was diminished in the absence of HPK1. To study the effect of HPK1 on intraluminal crawling under physiologic conditions, we performed intravital microscopy of the mouse cremaster muscle. Because leukocyte-endothelial interactions upon systemic administration of CXCL1 are too transient to allow analysis of leukocyte crawling, the model of intrascrotal injection of TNF-α 2.5 hours prior to microscopy was chosen to study leukocyte crawling in vivo. Here, almost every adherent cell crawled (Figure 5D). Similar to the in vitro findings, leukocyte crawling velocity was approximately 9 µm/min and was not affected by the absence of HPK1 (Figure 5E). However, crawling direction was severely affected in HPK1−/− mice. Although in HPK1+/+ mice, most leukocytes crawled against or perpendicular to the direction of blood flow and only 21% ± 4% of PMNs crawled in the direction of flow, the majority of leukocytes in HPK1−/− mice (58% ± 9%) crawled within 60° of the direction of blood flow (Figure 5F). This finding, also illustrated by the rose diagrams (Figure 5G), suggests that HPK1 was important for controlling the direction of PMN crawling under flow conditions.
Directed mechanotactic crawling was compromised in HPK1-deficient PMNs. Mechanotactic crawling of isolated murine PMNs evaluated in vitro. (A-C) Flow chambers coated with P-selectin (10 µg/mL), ICAM-1 (12.5 µg/mL), and CXCL1 (5 µg/mL) were used. Flow of 1 dyne/cm2 was applied and crawling was recorded for 10 minutes by using live-cell imaging, followed by offline analysis with ImageJ. At least 635 cells were analyzed for each strain. n = 9 flow chambers with cells from 5 mice each. Diagrams show mean ± SD; *P < .05; n.s. = not significant. (D-G) Intraluminal leukocyte crawling in inflamed postcapillary venules, assessed by intravital microscopy of the mouse cremaster muscle 2.5 hours after intrascrotal injection of TNF-α (500 ng). At least 189 cells were analyzed for each strain; n = 6 venules from 4 mice each. Diagrams show mean ± SEM; *P < .05; n.s. = not significant. (A,D) Percentage of crawling cells compared with number of totally adherent cells. Cells that exceeded a displacement of more than one cell diameter (Euclidean distance) were considered as crawling. (B,E) Mean crawling velocities were analyzed offline in time-lapse videos with temporal resolution of 3 seconds (in vivo) or 5 seconds (in vitro). (C,F) Percentage of cells ending up in a 60° angle that is symmetrically divided by the vector of flow. (G) Representative microscopic images (upper panel) with single-cell migration tracks (colored arrows) of leukocytes crawling on the inflamed vessel wall of postcapillary venules in TNF-α–treated cremaster muscles of HPK1+/+ and HPK1−/− mice. Dotted white arrows indicate direction of blood flow and dotted green lines depict the vessel walls; bar = 50 µm. Rose diagrams (lower panel) were generated by overlaying the single-cell tracks of all tracked cells, rotating an angular sector with an interior angle of 90° by 1° steps, and counting the cells that ended up in that angle. The counts were grouped in 10° intervals with the radius of each wedge representing the accumulated cell number. The arrow indicates the direction of blood flow.
Directed mechanotactic crawling was compromised in HPK1-deficient PMNs. Mechanotactic crawling of isolated murine PMNs evaluated in vitro. (A-C) Flow chambers coated with P-selectin (10 µg/mL), ICAM-1 (12.5 µg/mL), and CXCL1 (5 µg/mL) were used. Flow of 1 dyne/cm2 was applied and crawling was recorded for 10 minutes by using live-cell imaging, followed by offline analysis with ImageJ. At least 635 cells were analyzed for each strain. n = 9 flow chambers with cells from 5 mice each. Diagrams show mean ± SD; *P < .05; n.s. = not significant. (D-G) Intraluminal leukocyte crawling in inflamed postcapillary venules, assessed by intravital microscopy of the mouse cremaster muscle 2.5 hours after intrascrotal injection of TNF-α (500 ng). At least 189 cells were analyzed for each strain; n = 6 venules from 4 mice each. Diagrams show mean ± SEM; *P < .05; n.s. = not significant. (A,D) Percentage of crawling cells compared with number of totally adherent cells. Cells that exceeded a displacement of more than one cell diameter (Euclidean distance) were considered as crawling. (B,E) Mean crawling velocities were analyzed offline in time-lapse videos with temporal resolution of 3 seconds (in vivo) or 5 seconds (in vitro). (C,F) Percentage of cells ending up in a 60° angle that is symmetrically divided by the vector of flow. (G) Representative microscopic images (upper panel) with single-cell migration tracks (colored arrows) of leukocytes crawling on the inflamed vessel wall of postcapillary venules in TNF-α–treated cremaster muscles of HPK1+/+ and HPK1−/− mice. Dotted white arrows indicate direction of blood flow and dotted green lines depict the vessel walls; bar = 50 µm. Rose diagrams (lower panel) were generated by overlaying the single-cell tracks of all tracked cells, rotating an angular sector with an interior angle of 90° by 1° steps, and counting the cells that ended up in that angle. The counts were grouped in 10° intervals with the radius of each wedge representing the accumulated cell number. The arrow indicates the direction of blood flow.
PMN adhesion and extravasation were defective in TNF-α–treated cremaster muscle of HPK1−/− mice
To study the impact of HPK1 for leukocyte infiltration into the tissue during a robust proinflammatory response, we used the model of intrascrotal injection of TNF-α. Rolling flux fraction and rolling velocity were comparable between HPK1+/+ and HPK1−/− mice 2.5 hours after intrascrotal injection of TNF-α, as assessed by intravital microscopy (Figure 6A-B). Hemodynamic parameters (Table 2) of analyzed vessels showed no difference between the two mouse strains. However, leukocyte adhesion efficiency was significantly reduced in HPK1−/− mice when compared with HPK1+/+ mice (Figure 6C). Histologic examination of Giemsa-stained whole mounts of murine cremaster muscles treated with TNF-α allowed the quantification of leukocytes within the vessels and in the tissue (supplemental Figure S2). Compared with HPK1+/+ mice, HPK1−/− mice revealed a significantly reduced number of intravascular leukocytes confirming the adhesion defect observed by intravital microscopy (Figure 6D). Moreover, the number of perivascular leukocytes in the tissue was significantly reduced (Figure 6E), demonstrating the biological importance of HPK1 for leukocyte infiltration of the tissue during an acute inflammatory response.
Leukocyte adhesion and extravasation were defective in TNF-α–treated cremaster muscle of HPK1-deficient mice. Intravital microscopy (A-C) of postcapillary venules in mouse cremaster muscle 2.5 hours after intrascrotal injection of TNF-α (500 ng). (A) Leukocyte rolling flux fraction, (B) rolling velocity, and (C) leukocyte adhesion efficiency were analyzed offline. n = 32 venules from 5 HPK1+/+ mice and 32 venules from 4 HPK1−/− mice; accumulated frequency of rolling velocity includes 259 cells from 5 HPK1+/+ mice and 275 cells from 4 HPK1−/− mice. Number of (D) intravascular and (E) perivascular leukocytes as quantified in Giemsa-stained cremaster muscle whole mounts of HPK1+/+ and HPK1−/− mice. Cremaster muscles were fixed and stained 2.5 hours after intrascrotal injection of TNF-α (500 ng), and the number of leukocytes was assessed histologically. n = 50 venules from 3 HPK1+/+ mice and 48 venules from 3 HPK1−/− mice. (A-E) Diagrams show mean ± SEM; *P < .05; **P < .001; n.s. = not significant.
Leukocyte adhesion and extravasation were defective in TNF-α–treated cremaster muscle of HPK1-deficient mice. Intravital microscopy (A-C) of postcapillary venules in mouse cremaster muscle 2.5 hours after intrascrotal injection of TNF-α (500 ng). (A) Leukocyte rolling flux fraction, (B) rolling velocity, and (C) leukocyte adhesion efficiency were analyzed offline. n = 32 venules from 5 HPK1+/+ mice and 32 venules from 4 HPK1−/− mice; accumulated frequency of rolling velocity includes 259 cells from 5 HPK1+/+ mice and 275 cells from 4 HPK1−/− mice. Number of (D) intravascular and (E) perivascular leukocytes as quantified in Giemsa-stained cremaster muscle whole mounts of HPK1+/+ and HPK1−/− mice. Cremaster muscles were fixed and stained 2.5 hours after intrascrotal injection of TNF-α (500 ng), and the number of leukocytes was assessed histologically. n = 50 venules from 3 HPK1+/+ mice and 48 venules from 3 HPK1−/− mice. (A-E) Diagrams show mean ± SEM; *P < .05; **P < .001; n.s. = not significant.
Microvascular parameters of cremaster muscle venules 2.5 hours after intrascrotal TNF-α injection
| . | Mice . | Venules . | Diameter (µm) . | Length (µm) . | Centerline velocity (µm/s) . | Shear rate (s−1) . | WBC (µL−1) . |
|---|---|---|---|---|---|---|---|
| HPK1+/+ | 5 | 32 | 35 ± 2 | 260 ± 3 | 2103 ± 264 | 1430 ± 143 | 3357 ± 357 |
| HPK1−/− | 4 | 32 | 36 ± 1 | 265 ± 4 | 2175 ± 243 | 1593 ± 203 | 4072 ± 555 |
| n.s. | n.s. | n.s. | n.s. | n.s. |
| . | Mice . | Venules . | Diameter (µm) . | Length (µm) . | Centerline velocity (µm/s) . | Shear rate (s−1) . | WBC (µL−1) . |
|---|---|---|---|---|---|---|---|
| HPK1+/+ | 5 | 32 | 35 ± 2 | 260 ± 3 | 2103 ± 264 | 1430 ± 143 | 3357 ± 357 |
| HPK1−/− | 4 | 32 | 36 ± 1 | 265 ± 4 | 2175 ± 243 | 1593 ± 203 | 4072 ± 555 |
| n.s. | n.s. | n.s. | n.s. | n.s. |
Table shows mean ± SEM. Abbreviations are explained in Table 1.
Discussion
In this study, we showed that HPK1 was critically involved in LFA-1–mediated PMN trafficking during acute inflammation. Analysis of isolated murine PMNs revealed that CXCL1-mediated induction of adhesion to immobilized ICAM-1 under flow conditions was diminished in HPK1−/− PMNs. Although blocking Mac-1 did not substantially affect adhesion, blocking LFA-1 markedly decreased adhesion of HPK1+/+ PMNs to adhesion levels seen in the absence of HPK1. These results suggest that efficient CXCL1-induced adhesion to ICAM-1 under flow was dependent on LFA-1 and HPK1. Whereas residual adhesion upon blocking LFA-1 was similar in HPK1+/+ and HPK1−/− PMNs, the effect of HPK1 on PMN adhesion was still present when Mac-1 was blocked. This suggests that HPK1 specifically regulated the function of LFA-1, but not Mac-1. However, LFA-1 and Mac-1 surface expression of PMNs as well as PMN maturation measured as Gr-1 expression were unaffected by the absence of HPK1. An ICAM-1 binding assay, specifically reporting the high-affinity state of LFA-1,8,36 revealed that HPK1 was important for CXCL1-mediated inside-out signaling and induction of high-affinity LFA-1, but Mn2+-mediated induction of high-affinity LFA-1, bypassing inside-out signaling,34 was independent of HPK1. Interestingly, HPK1 was dispensable for binding of soluble fibrinogen by PMNs. Although Mac-1, as well as CD11c/CD18 binds fibrinogen, Mac-1 represents the major fibrinogen receptor on PMNs because expression of CD11c/CD18 is particularly low on this cell type compared with Mac-1.37 Thus, HPK1 seems to specifically regulate the affinity of LFA-1, whereas Mac-1 function is likely to be independent of HPK1. Intravital microscopy confirmed the pivotal role of HPK1 for CXCL1-induced leukocyte adhesion. Upon systemic CXCL1 injection, promoting high-affinity LFA-1 and thus mediating leukocyte arrest,1,8,38 induction of leukocyte adhesion was almost completely absent in HPK1−/− mice. Intrascrotal injection of TNF-α provides a more robust model of local inflammation and induces upregulation of endothelial CXCL1 and E-selectin expression.15,39 Intravital microscopy of TNF-α–treated cremaster muscles of HPK1−/− mice showed normal slow leukocyte rolling, which is dependent on intermediate-affinity LFA-1. However, high-affinity LFA-1–mediated leukocyte adhesion was also significantly compromised in HPK1−/− mice upon TNF-α injection.
Several postadhesion functions, including adhesion strengthening and spreading, were compromised in HPK1−/− PMNs under flow conditions when compared with HPK1+/+ PMNs. These functions were almost unaffected upon Mac-1 blocking, suggesting a predominant role of LFA-1 in the experimental setting used. Analysis of subsequent mechanotactic crawling revealed that the percentage of crawling cells and the crawling velocity were similar in HPK1+/+ and HPK1−/− PMNs. However, directed mechanotactic crawling was affected by the absence of HPK1, especially in vivo. Here, the majority of HPK1−/− PMNs were able to crawl only in the direction of blood flow, whereas HPK1+/+ PMNs crawled in all directions. Phillipson et al suggested that mechanotactic crawling perpendicular to blood flow is important for PMNs to find optimal transmigration sites.40 The overall ability of PMNs to crawl under flow conditions is known to primarily depend on Mac-1 because the percentage of crawling PMNs was reduced after blocking Mac-1 but not LFA-1 in vivo.40,,-43 This is in line with the finding that the percentage of PMNs crawling on truncated ICAM-1, which lacked the binding site for Mac-1, was reduced compared with full-length ICAM-1 in vitro.18 Recently, Dixit et al showed that high-affinity LFA-1, but not Mac-1, promoted mechanotactic crawling perpendicular to the direction of flow.44 Our findings clearly suggest a role of HPK1 in mechanotactic crawling perpendicular to and against the direction of flow. Thus, HPK1 seemed to be required not only for CXCL1-induced adhesion by regulating the affinity state of LFA-1, but also for the control of subsequent postadhesion events of PMN, including adhesion strengthening, spreading, and mechanotactic crawling perpendicular to or against the direction of flow. The biological significance of HPK1 for LFA-1–mediated PMN recruitment during acute inflammation was unraveled by using the model of TNF-α–induced inflammation in the mouse cremaster muscle, where leukocyte accumulation in the tissue was severely compromised in the absence of HPK1.
In previous studies, the adaptor protein mAbp1 was found to be critical for PMN trafficking.17,-19 mAbp1 is involved in β2 integrin–mediated outside-in signaling downstream of Syk, it translocates to the plasma membrane in a Syk-dependent manner, and it colocalizes with actin upon ligand binding of β2 integrins.18 Here, we found that HPK1 constitutively co-immunoprecipitated with mAbp1 in dHL-60 cells independent of β2 integrin–mediated cell activation whereas β2 integrin–mediated adhesion promoted the interaction of mAbp1 with actin,18 suggesting that the HPK1-mAbp1 module was translocated to the cytoskeleton upon adhesion. Confocal microscopy of adherent cells showed that both molecules were enriched at the lamellipodium where HPK1 also colocalized with actin. Similar to HPK1−/− PMNs, mAbp1−/− PMNs show compromised adhesion, adhesion strengthening, and spreading under flow conditions as well as disturbed mechanotactic crawling and diminished extravasation in response to TNF-α.18,19 However, mAbp1 was not downregulated in HPK1−/− PMNs compared with HPK1+/+ PMNs. Thus, the striking similarities between mAbp1−/− and HPK1−/− mice further support the hypothesis that HPK1 and mAbp1 may at least partially control the same signaling events regulating PMN trafficking. Surprisingly, HPK1 seems to have a fundamentally different function in lymphocytes compared with PMNs. HPK1 contributes to a negative feedback loop downregulating T-cell receptor (TCR) signaling45,-47 and negatively regulates T-cell adhesion and spreading on immobilized ICAM-1/Fc under static conditions.25 Similar effects were reported when HPK1 was knocked down in WEHI 231 cells.26 In T cells, HPK1 is involved in various signaling pathways, including TCR and E-prostanoid receptor signaling,48,49 and regulates NFAT–, AP-1–, and nuclear factor κB–mediated26,27,48 gene transcription. Accordingly, HPK1 is thought to participate in lymphocyte maturation, differentiation, activation, proliferation, and apoptosis and thereby implies a complex pattern of regulation.24,-26,50 Possible downstream targets of HPK1 in PMNs include the SH2 domain–containing leukocyte protein of 76 kDa (SLP-76), which interacts with HPK1 via its SH2 domain.51 In T cells, SLP-76 is phosphorylated by HPK1, leading to binding of 14-3-3, ubiquitination, and degradation of SLP-76 with subsequent attenuation of TCR signaling.45,-47,52 Moreover, HPK1 competes with ADAP for SLP-76 binding in T cells and thereby decreases the amount of active Rap1, an activator of high-affinity LFA-1.25 In PMNs, SLP-76 is involved in integrin activation, as well as in outside-in signaling and subsequent cell spreading.53,54 Interestingly, Tan et al suggested positive and negative regulatory functions of HPK1 in T cells, depending on the associated adaptor proteins,28 which may also account for the diverging effects of HPK1 in myeloid and lymphoid cells. However, further studies are required to provide a better understanding of HPK1 signaling in PMNs.
Taken together, our results showed that HPK1 was important for CXCL1-mediated induction of PMN adhesion in vitro and in vivo by regulating the affinity of LFA-1. In addition, HPK1 was critically involved in postadhesion functions of PMNs, namely adhesion strengthening, spreading, and directed mechanotactic crawling under flow conditions. In vivo analysis unraveled the pivotal impact of HPK1 for PMN extravasation in response to TNF-α. We conclude that HPK1 is critically involved in LFA-1–mediated recruitment of PMN during the acute inflammatory response. Further studies have to show whether the absence of HPK1 ameliorates the outcome of PMN-driven acute inflammatory diseases and may define the inhibition of HPK1 as a potential therapeutic concept in the treatment of inflammatory diseases.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jennifer Truong, Katy Niedung, and Susanne Bierschenk for excellent technical assistance and Angelika Höhe for analyzing data.
This study was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 914 project A2 [B.W.] and project B1 [M.S.]) and the FöFoLe program of Ludwig-Maximilians-Universität München.
Authorship
Contribution: S.M.J. designed and carried out research, analyzed data, and wrote the manuscript. R.P. and D.B. performed research and analyzed data. C.N. and M.S. designed research. F.K. provided the HPK1−/− mice. B.W. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Barbara Walzog, Department of Cardiovascular Physiology and Pathophysiology, Walter Brendel Centre of Experimental Medicine, Ludwig-Maximilians-Universität München, Schillerstrasse 44, D-80336 München, Germany; e-mail: walzog@lrz.uni-muenchen.de.

![Figure 1. The mAbp1 interacting protein HPK1 was involved in induction of adhesion of murine PMNs under flow conditions in vitro. (A) Western blot of whole-cell lysates and co-immunoprecipitates of wild-type dHL-60 cells and dHL-60 cells expressing an mAbp1-GFP construct (dHL-60-mAbp1-GFP) upon specific mAbp1-GFP pulldown with GFP-nanotraps. Cells were either exposed to immobilized fibrinogen (250 µg/mL) and treated with Mn2+ (1 mM) for 15 minutes at 37°C or left in suspension without stimulation. mAbp1, HPK1, and actin were detected by immunoblotting by using specific antibodies. Western blot is representative for 3 independent experiments. (B,C) Confocal microscopy of dHL-60-mAbp1-GFP cells stained for HPK1 and actin. Cells were treated with Mn2+ (1 mM) and exposed to immobilized fibrinogen (250 µg/mL) (B upper panel), or were seeded on coverslips coated with ICAM-1 (12.5 µg/mL) and IL-8 (1 µg/mL) (B lower panel, and C) for 15 minutes at 37°C. After fixation and staining, confocal laser scanning microscopy was conducted. (B) Colocalization (yellow) of mAbp1-GFP (green) and HPK1 (red) at the lamellipodium (arrowheads) of polarized dHL-60 cells. (C) Colocalization (white) of HPK1 (red) and actin (cyan) at the lamellipodium (arrowheads) of polarized dHL-60 cells. Images are representative of at least 3 independent experiments; bar = 10 µm. (D) Induction of adhesion of isolated murine PMNs under flow conditions (1 dyne/cm2). Flow chambers were coated with P-selectin (10 µg/mL), ICAM-1 (12.5 µg/mL), and CXCL1 (5 µg/mL). PMNs were treated with a nonbinding control antibody (crtl immunoglobulin G [IgG]), or function blocking anti–Mac-1, or anti–LFA-1 antibodies (30 µg/mL) as indicated. Numbers indicate relative PMN adhesion after 10 minutes in percent of all interacting PMNs (100%). n = 4; mean ± standard deviation (SD); *P < .05; **P < .001; n.s. = not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/20/10.1182_blood-2012-08-451385/2/m_4184f1.jpeg?Expires=1767733389&Signature=HuR2SBG-MhTb9UFLkJ7x8KL-dS12tLM~f6TBnFLY9bzdViLRZBQkgWOF~hQrIHfz8XCTiWCdlmRby4LWBlcG4O~ttNPhKz3bY01UXgfm~iR97Ybi1zRi4sENmc6xhYB~-jdSSJ1a9EvxjGZHKDlEfHkvkQh8Y5q~wZnieJEsfhkyJNBi9f6AmT1BORcFnv9Ki3bOTr6nHzU1ARF3GwyAd~RRuhnjWKMpaMyjO2UZRFOVUtJPZgzbvvlVDo7ovmiIljppSDa7XX~y5HvCH~IL24WHGxNrGaLvDnxZojAODHQf~fi9iZvec5NW0QckkUrtS10JSe9-kFcJzQFX9MW-LA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)