Key Points
IgA and IgM human plasma cells express a functional BCR on their cell surface and can therefore respond to antigenic stimulation.
Plasma cells (PCs) are terminally differentiated cells of the B-cell lineage that secrete antibodies at a high rate and are thought to lack the expression of the B-cell receptor (BCR). Here, we report that human IgA and IgM, unlike IgG, PCs express a membrane functional BCR associated with the Igα/Igβ heterodimer. BCR cross-linking on IgA and IgM PCs led to Ca2+ mobilization and extracellular signal-regulated kinase 1/2 and AKT phosphorylation and impacted survival of IgA PCs. These findings demonstrate a significant difference between human IgG, IgM, and IgA PCs and suggest that the IgA PC repertoire may be modulated by specific antigens with implications for the regulation of the mucosal immune system.
Introduction
B cells express clonotype-specific surface immunoglobulins (Ig’s), which associate to the Igα/Igβ heterodimer to constitute the B-cell receptor (BCR).1,2 Binding of the specific antigen to the BCR triggers a signaling cascade that leads, in concert with other signals, to cell activation, proliferation, and generation of memory B cells and plasma cells (PCs).3,4 BCR expression is required for the survival of memory B cells5 but is thought to be lost on mature PCs due to a secretory switch in the Ig messenger RNA (mRNA).6
Long-lived PCs persist in bone marrow (BM) and, in the case of IgA PCs, in mucosal tissues, homing to specific niches and continually secreting antibodies, thus maintaining serological memory.7,,-10 Serum IgG antibodies and consequently IgG PCs can persist for a lifetime, although displacement by newly generated PCs9 and susceptibility to Fcγ Receptor IIB-mediated apoptosis11 increase their turnover. In contrast, the IgA PCs present in the lamina propria (LP) show a high turnover rate.12 These findings suggest different mechanisms of regulation of IgG and IgA PCs.
Study design
Cells cultures
Human tissues were obtained according to the rules of the Cantonal Ethic Committee (Comitato Etico Cantonale, CH-6501 Bellinzona). This study was conducted in accordance with the Declaration of Helsinki. Bone marrow plasma cells (BM-PCs) were isolated from patients undergoing hip surgery using CD138-Phycoerythrin (PE) and anti-PE magnetic beads and further purified by cell sorting when cultured for enzyme-linked immunospot (ELISPOT) assay. Lamina propria plasma cells (LP-PCs) were isolated from colon tissue obtained from healthy areas of surgically resected specimens and were enriched by magnetic selection of CD38 positive cells. Circulating memory B cells were isolated by cell sorting as CD19+/CD27+. PCs were cultured in complete RPMI supplemented with 10% fetal calf serum and 10 ng/mL of recombinant interleukin 6. For in vitro differentiation, total memory B cells were pulsed for 16 hours with cytosine guanine dinucleotide 2006 and plated for 5 days on a monolayer of immortalized mesenchymal stromal cells.13 Survival of BM-PCs was evaluated by ELISPOT on 96-well plates coated with anti-Fc antibodies. Detection of spots was performed with biotinylated anti-Fc or light chains followed by peroxydase-labeled streptavidin and 3-amino-9-ethylcarbazole.
Antibodies
Ig’s were detected by antigen-binding fragments [F(abʹ)2] of goat anti-human heavy or light chains (all from Southern Biotech). Purified anti-phospho–extracellular signal-regulated kinase (ERK), anti–phospho-Spleen tyrosine kinase (Syk)Y525-526, anti-phospho-AKT (from Cell Signaling), as well as anti-B lymphocyte-induced maturation protein-1 (Blimp-1) and anti–Paired box protein-5 (Pax-5) (from Santa Cruz), were revealed by goat anti-rabbit Alexa Fluor 488 (Invitrogen). BCR cross-linking was performed with purified F(abʹ)2 of goat anti-κ and anti-λ chain antibodies (from Southern Biotech), allowing the homogeneous and concomitant cross-linking of all BCRs.
Cytometry and microscopy
BM and in vitro differentiated PCs were detected as CD138+/intracellular Ighigh. Colon LP-PCs were detected as CD3–/CD20–/CD38high/intracellular IgAhigh. Staining for transcription factors and phosphorylated molecules was performed using 4% formaldehyde fixation followed by permeabilization with 90% cold methanol. Internalization of the Ig molecules was evaluated by the disappearance of light chain staining after cross-linking with anti-Fc fragment antibodies at 4°C (for control) and 37°C. Staining intensities were expressed as the ratio of mean fluorescence intensity (rMFI) to control staining to correct for differences in background. BCR internalization was evaluated by confocal microscopy 20 minutes following cross-linking with anti-IgA-Cy5 at 37°C or 4°C as control. Surface IgA+, surface IgM+, surface IgA-, IgM-, and BM-PCs were isolated by cell sorting; Igα, Igβ, and surface and secreted Ig complementary DNA were quantified by reverse transcription–polymerase chain reaction.
Results and discussion
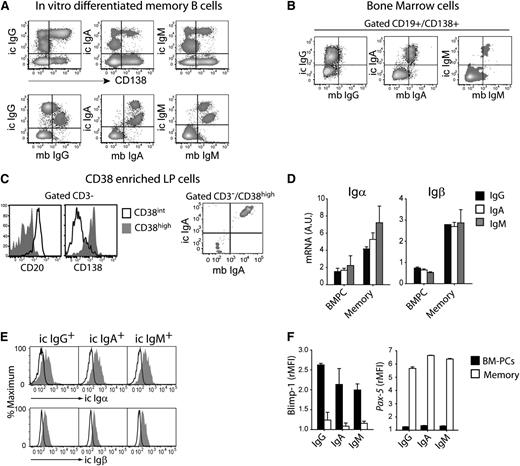
To develop an in vitro culture method that would support PC survival, we plated cytosine guanine dinucleotide–activated memory B cells on monolayers of BM-derived mesenchymal cells.13 In these cultures, a fraction of activated B cells acquired CD138 and expressed high levels of intracellular Ig, consistent with PC differentiation (Figure 1A, upper panel). Surprisingly, although IgG PCs downregulated surface IgG expression, IgA and IgM PCs expressed their respective isotype both intracellularly and on the plasma membrane (Figure 1A, lower panel). Importantly, concordant Ig’s were also detected on the plasma membrane of IgA and IgM, but not IgG, PCs isolated from BM or colon LP indicating that this property is a characteristic of PCs present in their physiological niches (Figure 1B-C). These results were confirmed by quantitative polymerase chain reaction (supplementary Figure 1) and suggest that the complete switch from surface to secreted Ig is limited to IgG PCs.
Surface BCR expression on human IgM and IgA PCs. (A) In vitro differentiated memory cells were stained for CD138 and membrane (mb) and intracellular (ic) Ig; 1 representative experiment of 4. (B) Expression of ic and mb IgG, IgA, and IgM in CD19+CD138+-gated BM-PCs; 1 representative experiment of 3. (C) Expression of CD20 and CD138 on the CD38-enriched fraction of colon LP (left panel). Expression of mb and ic IgA in CD3–/CD38high LP-PCs (right panel); 1 experiment of 2. (D) Igα and Igβ mRNA in BM-PCs and memory B cells normalized to 18S RNA. Mean + standard error of the mean (SEM) of 3 donors. (E) Expression of intracellular epitopes (gray histograms) of Igα and Igβ on IgG, IgA, and IgM BM-PCs. Control staining, open histograms; 1 experiment of 3. (F) Expression of Blimp-1 and Pax-5 on BM-PCs and memory B cells. Mean of rMFI + SEM of duplicates; 1 experiment of 3.
Surface BCR expression on human IgM and IgA PCs. (A) In vitro differentiated memory cells were stained for CD138 and membrane (mb) and intracellular (ic) Ig; 1 representative experiment of 4. (B) Expression of ic and mb IgG, IgA, and IgM in CD19+CD138+-gated BM-PCs; 1 representative experiment of 3. (C) Expression of CD20 and CD138 on the CD38-enriched fraction of colon LP (left panel). Expression of mb and ic IgA in CD3–/CD38high LP-PCs (right panel); 1 experiment of 2. (D) Igα and Igβ mRNA in BM-PCs and memory B cells normalized to 18S RNA. Mean + standard error of the mean (SEM) of 3 donors. (E) Expression of intracellular epitopes (gray histograms) of Igα and Igβ on IgG, IgA, and IgM BM-PCs. Control staining, open histograms; 1 experiment of 3. (F) Expression of Blimp-1 and Pax-5 on BM-PCs and memory B cells. Mean of rMFI + SEM of duplicates; 1 experiment of 3.
To dissect a possible heterogeneity among PCs subsets, we analyzed the expression of the BCR-associated Igα and Igβ and of master transcription factors Blimp-1 and Pax-5 that control the PC and B-cell differentiation state.14,15 Igα and Igβ mRNA were detected by quantitative reverse-transcription polymerase chain reaction in IgA, IgM, and IgG BM-PCs, albeit at a reduced level when compared with memory B cells (Figure 1D). Furthermore, antibodies directed against the intracellular portion of Igα and Igβ stained all BM-PCs irrespective of the Ig isotype (Figure 1E). In addition, all BM-PCs expressed Blimp-1 but not Pax-5, HLA-DR, CD62L, or Ki-67 (Figure 1F and not shown). These results are consistent with the phenotypic characterization of the cells as terminally differentiated PCs and suggest that all PCs express Igα and Igβ, although at reduced levels.
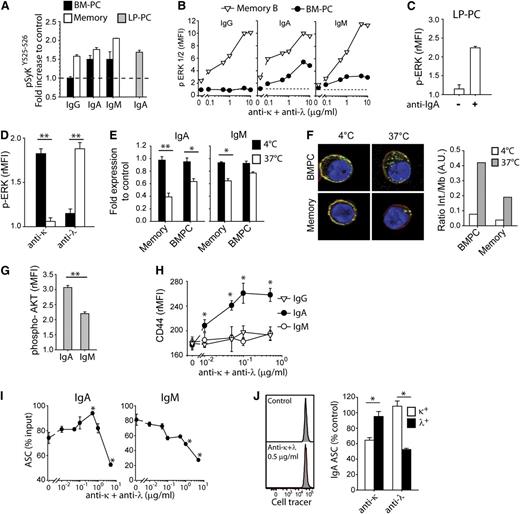
To assess the functionality of the BCR present on IgA and IgM PCs, we measured, at the single cell level, signaling events triggered by BCR cross-linking. In IgA and IgM, but not IgG, PCs from BM or LP, BCR cross-linking led to the phosphorylation of Syk at tyrosines 525 and 526 present in the catalytic site,16,17 the level of phosphorylation being comparable to that observed in memory B cells (Figure 2A). Furthermore, IgA and IgM BM-PCs, as well as IgA LP-PCs, showed BCR-induced ERK1/2 phosphorylation with dose response and kinetics comparable to those observed in activated memory B cells (Figure 2B-C and data not shown). Only κ- or λ-expressing cells responded to anti-κ or anti-λ antibodies, respectively, showing that ERK phosphorylation is highly specific and occurs directly through BCR and not through Fc receptors in IgA and IgM BM-PCs (Figure 2D and not shown). Finally, an increase in intracellular [Ca2+] was detected by flow cytometry in IgA and IgM BM-PCs by concomitant staining and cross-linking of the BCR (supplemental Figure 2A-B). Taken together, these results indicate that human IgA and IgM, but not IgG, PCs express a functional BCR.
Human IgM and IgA PCs express a functional BCR. (A) Increase in phospho-SykY525-526 staining in BM-PCs (black bars), memory B cells (white bars), and LP-PCs (gray bar) following cross-linking with 2.5 μg/mL anti–light chain F(abʹ)2 antibody fragments. Mean of rMFI + SEM of duplicates. PC gates as in Figure 1. One experiment of 3. (B) ERK1/2 phosphorylation of BM-PCs (black circles) and memory B cells (white triangles) 15 minutes after stimulation with increasing doses of anti–light chain antibodies; 1 experiment of 3. (C) Levels of ERK1/2 phosphorylation in LP-PCs cultured for 15 minutes in the presence or absence of anti-IgA F(abʹ)2 antibodies. Mean of rMFI + SEM of duplicates; 1 representative experiment of 2. (D) Levels of ERK1/2 phosphorylation of κ (black bars) or λ (white bars) IgA BM-PCs after 15 minutes in the presence of 2.5 μg/mL anti-κ or anti-λ light chain F(abʹ)2 antibodies. Mean + SEM of duplicate; 1 experiment of 3. (E) Internalization of IgM and IgA on BM-PCs and memory B cells cultured with 2.5 μg/mL anti-IgM or IgA heavy chain F(abʹ)2 fragments. Shown is the surface staining of light chains after 20 minutes at 4°C (black bars) or 37°C (white bars). Mean of fold expression to control (concomitant staining of heavy and light chains at 4°C) + SEM of duplicates; 1 experiment of 3. (F) Confocal images of IgA BM-PCs and memory B cells incubated for 20 minutes with anti-IgA-Cy5 (green) and counterstained with CD45 Alexa Fluor 700 (red) as a membrane marker and 4,6 diamidino-2-phenylindole (blue). Images are representative of 2 independent experiments. Quantification of the ratio of internal vs membrane IgA (right panel). (G) Expression of phospho-AKT (Ser 473) on IgA and IgM BM-PCs cultured for 20 minutes with 2.5 μg/mL anti–light chain F(abʹ)2 antibodies. Mean of rMFI + SEM of duplicates; 1 representative experiment of 3. (H) Expression of CD44 on IgG, IgA, and IgM BM-PCs cultured for 12 hours with increasing doses of F(abʹ)2 anti–light chain antibodies. Mean of rMFI + SEM of duplicates; 1 experiment of 3. (I) Survival of IgA and IgM BM-PCs cultured for 4 days in the presence of increasing doses of anti–light chain F(abʹ)2 antibodies. Mean of % input cells + SEM of duplicates; 1 representative experiment of 3. (J) Cell tracer dilution of IgA BM-PCs after 4 days in culture with (lower histogram) or without (upper histogram) 0.5 μg/mL of anti–light chain F(abʹ)2 antibodies (left panel). One experiment out of 2. Survival of κ (black bars) or λ (white bars) IgA BM-PCs upon being cultured for 4 days in the presence of 10 μg/mL anti-κ or anti-λ light chain F(abʹ)2 antibodies. Mean + SEM of duplicate; 1 experiment of 3 (right panel). *P < .05; **P = .01 in Student t test.
Human IgM and IgA PCs express a functional BCR. (A) Increase in phospho-SykY525-526 staining in BM-PCs (black bars), memory B cells (white bars), and LP-PCs (gray bar) following cross-linking with 2.5 μg/mL anti–light chain F(abʹ)2 antibody fragments. Mean of rMFI + SEM of duplicates. PC gates as in Figure 1. One experiment of 3. (B) ERK1/2 phosphorylation of BM-PCs (black circles) and memory B cells (white triangles) 15 minutes after stimulation with increasing doses of anti–light chain antibodies; 1 experiment of 3. (C) Levels of ERK1/2 phosphorylation in LP-PCs cultured for 15 minutes in the presence or absence of anti-IgA F(abʹ)2 antibodies. Mean of rMFI + SEM of duplicates; 1 representative experiment of 2. (D) Levels of ERK1/2 phosphorylation of κ (black bars) or λ (white bars) IgA BM-PCs after 15 minutes in the presence of 2.5 μg/mL anti-κ or anti-λ light chain F(abʹ)2 antibodies. Mean + SEM of duplicate; 1 experiment of 3. (E) Internalization of IgM and IgA on BM-PCs and memory B cells cultured with 2.5 μg/mL anti-IgM or IgA heavy chain F(abʹ)2 fragments. Shown is the surface staining of light chains after 20 minutes at 4°C (black bars) or 37°C (white bars). Mean of fold expression to control (concomitant staining of heavy and light chains at 4°C) + SEM of duplicates; 1 experiment of 3. (F) Confocal images of IgA BM-PCs and memory B cells incubated for 20 minutes with anti-IgA-Cy5 (green) and counterstained with CD45 Alexa Fluor 700 (red) as a membrane marker and 4,6 diamidino-2-phenylindole (blue). Images are representative of 2 independent experiments. Quantification of the ratio of internal vs membrane IgA (right panel). (G) Expression of phospho-AKT (Ser 473) on IgA and IgM BM-PCs cultured for 20 minutes with 2.5 μg/mL anti–light chain F(abʹ)2 antibodies. Mean of rMFI + SEM of duplicates; 1 representative experiment of 3. (H) Expression of CD44 on IgG, IgA, and IgM BM-PCs cultured for 12 hours with increasing doses of F(abʹ)2 anti–light chain antibodies. Mean of rMFI + SEM of duplicates; 1 experiment of 3. (I) Survival of IgA and IgM BM-PCs cultured for 4 days in the presence of increasing doses of anti–light chain F(abʹ)2 antibodies. Mean of % input cells + SEM of duplicates; 1 representative experiment of 3. (J) Cell tracer dilution of IgA BM-PCs after 4 days in culture with (lower histogram) or without (upper histogram) 0.5 μg/mL of anti–light chain F(abʹ)2 antibodies (left panel). One experiment out of 2. Survival of κ (black bars) or λ (white bars) IgA BM-PCs upon being cultured for 4 days in the presence of 10 μg/mL anti-κ or anti-λ light chain F(abʹ)2 antibodies. Mean + SEM of duplicate; 1 experiment of 3 (right panel). *P < .05; **P = .01 in Student t test.
The endocytosis of the BCR, which allows B cells to take up and present antigens on major histocompatibility complex class II molecules, has also been shown to regulate AKT phosphorylation,18 a pathway involved in control of cell survival. When compared with memory B cells, IgA PCs showed a slighty reduced capacity to internalize the BCR (more pronounced in IgM PCs; Figure 2E); nonetheless, internalized IgA could be detected as multiple spots distributed all over the cytoplasm (Figure 2F). When cross-linked with anti–light chain antibodies, IgM and in particular IgA PCs from BM or colon LP showed increased AKT phosphorylation (Figure 2G and not shown). Furthermore, upon BCR cross-linking, only IgA BM-PCs upregulated CD44, an adhesion molecule involved in PC survival19 (Figure 2H).
To test whether BCR cross-linking might affect survival of IgA PCs, we cultured IgA and IgM BM-PCs in the presence of cross-linking antibodies and measured their survival after 4 days by ELISPOT. An increased survival of IgA PCs (but not of IgM PCs) was evident at low doses of cross-linking antibodies (Figure 2I) with no sign of cell division (Figure 2J, left panel). At high doses, both IgA and IgM recovery was significantly impaired (Figure 2I) in an apparently BCR-dependent manner (Figure 2J, right panel).
This study highlights an unexpected property of IgM and IgA PCs: the surface expression of a functional BCR. Importantly, this was shown for in vitro differentiated PCs as well as for PCs isolated ex vivo from human BM and gut LP. To date, surface IgA expression has been reported in mice gut LP-PCs.20 These findings imply that IgM and IgA, unlike IgG, PCs can directly respond to their antigen. Moreover, BCR cross-linking can impact positively on the survival of IgA PCs. Our observation may be partially relevant to understanding the dynamics of IgA PCs in the gut and their capacity to adapt to commensals.12 We propose that the selection of IgA PCs (and most likely IgA plasma blasts) in the LP could be partially due to the capacity of these cells to respond positively to their antigen at low doses, a mechanism that would favor PCs whose antigen is present at the mucosal surface.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the L.E.M. laboratory of the San Giovanni Hospital for collecting BM samples and Davide Corti for critical reading of the paper.
This work was supported by funding from the Swiss National Science Foundation (N. 126027) (A.L.), the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement (250348), the Helmut Horten Foundation (A.L.), and the San Raffaele University Milan (D.P.).
D.P. is a PhD student of the International PhD School in Molecular Medicine, San Raffaele University, Milan.
Authorship
Contribution: D.P. designed and performed experiments and analyzed data; E.M. performed experiments and analyzed data; M.B. and G.G. analyzed data; A.L. and F.S. analyzed data and wrote the manuscript; and D.J. designed and coordinated the study, performed and analyzed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Jarrossay, Institute for Research in Biomedicine, via Vela 6, 6500 Bellinzona, Switzerland; e-mail: david.jarrossay@irb.usi.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal