Abstract
Natural killer (NK) cells have important functions in cancer immunosurveillance, BM allograft rejection, fighting infections, tissue homeostasis, and reproduction. NK cell–based therapies are promising treatments for blood cancers. Overcoming their currently limited efficacy requires a better understanding of the molecular mechanisms controlling NK cell development and dampening their effector functions. NK cells recognize the loss of self-antigens or up-regulation of stress-induced ligands on pathogen-infected or tumor cells through invariant NK cell receptors (NKRs), and then kill such stressed cells. Two second-messenger pathways downstream of NKRs are required for NK cell maturation and effector responses: PIP3 generation by PI3K and generation of diacylglycerol and IP3 by phospholipase-Cγ (PLCγ). In the present study, we identify a novel role for the phosphorylated IP3 metabolite inositol (1,3,4,5)tetrakisphosphate (IP4) in NK cells. IP4 promotes NK cell terminal differentiation and acquisition of a mature NKR repertoire. However, in mature NK cells, IP4 limits NKR-induced IFNγ secretion, granule exocytosis, and target-cell killing, in part by inhibiting the PIP3 effector-kinase Akt. This identifies IP4 as an important novel regulator of NK cell development and function and expands our understanding of the therapeutically important mechanisms dampening NK cell responses. Our results further suggest that PI3K regulation by soluble IP4 is a broadly important signaling paradigm.
Key Points
By producing soluble IP4, inositol-trisphosphate 3-kinase B promotes NK-cell terminal maturation but limits NK-cell effector functions.
IP4 acts in part by limiting phosphoinositide 3-kinase signaling via the effector kinase Akt downstream of NK-cell receptors.
Introduction
Natural killer (NK) cells are innate lymphocytes that respond rapidly to certain viruses, tumors, and cellular stress.1-4 Their importance is evidenced by NK cell–deficient patients, who succumb early in life to herpesvirus infections. Other studies have implicated NK cells in fighting influenza virus infections, in mediating cancer immunosurveillance and BM allograft rejection, and in tissue homeostasis and reproduction.1-5 NK cell–based therapies are promising cancer treatments. Their currently limited efficacy will be improved by a better understanding of the molecular mechanisms controlling NK cell development and dampening effector functions.2,3,5
Conventional NK cell development proceeds through a series of developmental stages with characteristic cell surface markers, sequential acquisition of various NK cell receptors (NKRs) and a progressive gain of NK cell function.6-16 Mature NK cells have 2 main effector functions: direct target cell destruction via release of cytolytic granules and secretion of proinflammatory cytokines and chemokines. Recently, 2 mature NK cell populations with distinct functional characteristics were defined.16,17 CD11b+CD27+ NK cells traffic within the lymphoid compartment and respond readily to stimulation by secretion of cytolytic granules and IFNγ. They can further mature into CD11b+CD27− NK cells. These circulate in blood and tissue and are longer lived, but have a higher activation threshold and produce less IFNγ.17
A balance of signals from both activating and inhibitory NKRs controls NK cell responsiveness.6-15 In particular, the acquisition of inhibitory NKRs recognizing MHC class I (MHCI) and other self-antigens is required to render developing NK cells functionally competent. Conversely, inhibitory NKR engagement on mature NK cells prevents the inappropriate attack of normal body cells, thus establishing self-tolerance.14,15 Reduced inhibitory NKR ligand expression on virus-infected or cancer cells can trigger lysis by NK cells.6-13 Moreover, activating NKRs including NK1.1, NKG2D, FcγRIIIa/b, and certain Ly49 proteins in mice recognize de novo expressed viral or stress-induced ligands6-13 and signal directly via cytoplasmic domains or indirectly through transmembrane adapters.18 Many effectors may differ between different activating NKRs, but all ultimately activate one or both of the lipid metabolizing enzymes PI3K and phospholipase-Cγ (PLCγ). Both enzymes convert the membrane phospholipid phosphatidylinositol(4,5)-bisphosphate (PIP2) into distinct second messengers. Both enzymes are important for NK cell effector functions, but only PI3K is required for NK cell maturation.4
PI3K phosphorylates PIP2 into phosphatidylinositol(3,4,5)-trisphosphate (PIP3), a membrane ligand for signaling proteins containing pleckstrin homology (PH) or other PIP3-binding domains.4,19 PIP3 recruitment facilitates their activation and function by colocalizing them with upstream activators or downstream effectors. PI3K deficiency or inhibition impairs NK cell maturation, IFNγ production, and cytotoxicity.4,19-21
PLCγ hydrolyzes PIP2 into the membrane lipid diacylglycerol and soluble inositol(1,4,5)-trisphosphate (IP3). Diacylglycerol recruits and facilitates activation of protein kinase C, RasGRP, and other effectors.22 In NK cells, protein kinase Cθ activation promotes IFNγ production but is dispensable for cytolytic granule release.4,18 Ca2+ mobilization by IP3 is necessary for cytolytic granule exocytosis.4,18 PLCγ deficiency increases susceptibility to MCMV infection and cancer by impairing NK cell effector functions.4,23-25
We and others recently identified IP3 3-kinase B (IP3 3-KB, ItpkB) as a key regulator of leukocyte development and function.26-32 ItpkB−/− mice are immunodeficient, with severe T-lymphopenia, anergic B cells, and complex neutrophil phenotypes.26-32 By catalyzing a similar chemical reaction to that of PI3K, ItpkB phosphorylates IP3 into inositol(1,3,4,5)–tetrakisphosphate (IP4).33 As a soluble PIP3 analog, IP4 can bind to PH domains and either promote or inhibit PIP3 binding.26-29,33 Many PH domains bind PIP3 and IP4 with similar affinities.19 A major open question is how many PI3K functions are regulated by IP4.33
Given the importance of PI3K in NK cell development and function, we assessed how ItpkB and thus IP4 deficiency affect these processes. We found that ItpkB promotes NK cell terminal maturation and acquisition of a mature NKR repertoire. However, IP4 limits mature NK cell IFNγ secretion, granule exocytosis, and clearance of MHCI−/− target cells, in part by dampening the activity of the PIP3 effector kinase Akt. This identifies ItpkB as a key regulator of NK cell biology, broadens the physiologic importance of PI3K regulation by IP4, and expands our currently limited understanding2,3 of the therapeutically important mechanisms dampening NK cell function.
Methods
Mice and cells
Our ItpkB−/− mice were described previously.26,30,32 All mice were housed in specific pathogen–free facilities monitored by the Division of Comparative Medicine at Washington University School of Medicine or The Scripps Research Institute Department of Animal Resources. All animal studies were approved by The Scripps Research Institute institutional animal care and use committee or Washington University Animal Studies Committee and conform to all relevant regulatory standards. Primary splenic NK cells were purified by negative selection with the mouse NK cell enrichment kit (StemCell Technologies). Splenocytes and purified NK cells were assayed directly or cultured as specified in RPMI-1640/10% FCS. YAC-1 (H-2a), RMA/S (H-2b), and RMA/S-Rae1 (H-2b) cells were grown in RPMI 1640-Glutamax/10% FCS/penicillin/streptomycin. To generate IL-2– or IL-15–expanded NK cells (LAK), splenic NK cells were expanded for 2 days in RPMI 1640-Glutamax/10% FCS/penicillin/streptomycin, nonessential amino acids, sodium pyruvate, 10−5M β-mercaptoethanol, and either 1000 IU/mL of recombinant IL-2 or 100 ng/mL of IL-15.
Cell staining
BM cells and splenocytes (106 per stain) were incubated with 2.4G2 anti-Fc receptor (CD16/32) Abs for 5 minutes at room temperature, followed by staining with combinations of anti-CD3 APCCy7, NK1.1 PE or Pacific Blue, Ly49D FITC or APC, Ly49H APC, NKG2ACE FITC, CD27 PE, Dx5 APC, Ly49A Pacific Blue and KLRG1 PerCPCy5.5 Abs. All Abs were from BioLegend or eBiosciences unless otherwise indicated. Intracellular pAkt was analyzed after formaldehyde/methanol fixation, permeabilization, and incubation with 1:100 diluted anti–AKT-pS473 Abs (Cell Signaling Technology) and anti–rabbit IgG–Alexa Fluor 488. Intracellular granzyme B and total Akt were analyzed via fixation with Cytofix/Cytoperm (BD Biosciences) and staining with anti-GrzB–APC or isotype control-APC (1:4) or anti-Akt (1:30) Abs and anti–rabbit IgG F(ab)2-FITC (1:100) in Perm/Wash buffer (BD Biosciences). Apoptosis of NK cells or target cells was assayed by staining with Annexin V APC and 7-amino-actinomycin D viability dye in annexin V staining buffer (BioLegend). All flow cytometry samples were analyzed on an LSR II flow cytometer (BD Biosciences) and processed using FlowJo 9.5 software (TreeStar).
BM chimeras
For mixed chimeras, BM from CD45.2+ItpkB+/+ or CD45.2+ItpkB−/− and congenic CD45.1+ItpkB+/+ mice was flushed out of femurs and tibias and filtered through a 40-μM cell strainer. After erythrocyte lysis, T and B cells were depleted using streptavidin microbeads and biotinylated Abs against CD3, CD4, CD8, B220, and CD19 (Miltenyi Biotec). After mixing CD45.2+ItpkB+/+ or CD45.2+ItpkB−/− and CD45.1+ItpkB+/+ competitor BM at the indicated ratios, 1 × 106 cells were injected intravenously into the tail veins of lethally irradiated (2 × 550 rads, at least 3 hours apart one day before reconstitution) CD45.1+CD45.2+ recipient mice. These mice were fed trimethoprim/sulfamethoxazole in water for 2 weeks and analyzed after 6.5 weeks. For retroviral chimeras, ItpkB−/− donor mice were treated with 5-fluorouracil 5 days before BM harvest as above. The BM was cultured with TPO/SCF for 2 days before spinoculation with retroviral supernatants harvested from packaging cells 48 hours after transfection with empty or wild-type (WT) murine ItpkB encoding pCMViresGFP. Two days later, the transduced BM was injected intravenously into lethally irradiated recipients for analysis 5-10 weeks later.
To measure the in vivo clearance of MHCI−/− target cells, B6 and MHCI−/− splenocytes were labeled with 0.25 and 1.67μM CFSE, respectively. A total of 107 cells mixed at a 1:1 ratio were injected intravenously into WT or ItpkB−/− mice. Residual targets in recipient spleens were assessed 24 hours later.
IFNγ production
Splenocytes (2 × 106) were stimulated with plate-bound anti-NK1.1 Abs at the indicated concentrations for 5 hours in the presence of 2μM monensin (eBiosciences). Cells were harvested and stained for surface CD3, NKp46, and intracellular IFNγ after fixation and permeabilization (BD Biosciences).
Degranulation assay
106 splenocytes were cocultured with 2 × 105 target cells or stimulated with plate-bound anti-NK1.1, anti-NKp46, or anti-2B4 Abs for 2 hours in the presence of 0.5 μg of anti-CD107a–Alexa Fluor 488 (BioLegend). Conjugates were formed by centrifugation at 300g for 1 minute. Cells were harvested and stained for surface CD3, NK1.1, and CD69. In some cases, 107 splenocytes/mL were preincubated with 0.5% DMSO, 10μM Akt inhibitor VIII (Calbiochem), 10μM 2,6-di-O-Butyryl-Ins (1,3,4,5)P4/AM-0.02% Pluronic (Echelon Biosciences), or 50μM LY294002 for 1 hour before coculture. Coculture diluted the agents by 50%.
Cytotoxicity assays
Target cells (5 × 103) were loaded with 51Cr and incubated with NK cells for the indicated times at 37°C at the indicated effector/target cell ratios in duplicate. Using mean lysis values, the percent specific lysis was calculated as 100 × (experimental release − spontaneous release)/(total release − spontaneous release).
Results
NK cell maturation requires ItpkB
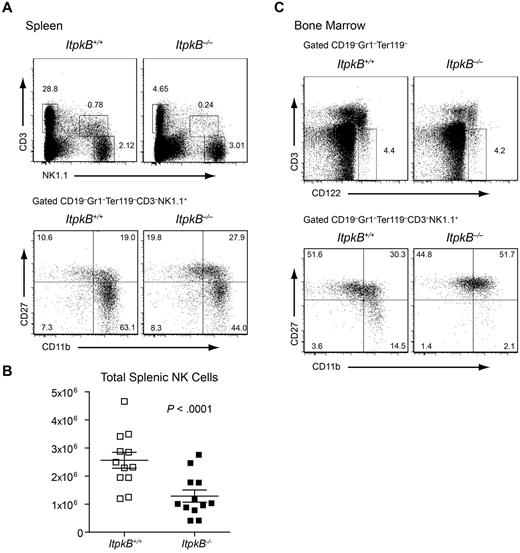
NKR ligation induces IP4 in NK cells.34 Among all mammalian IP3 3-kinases, NK cells only express ItpkB highly (supplemental Figures 1 and 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Therefore, we hypothesized that ItpkB controls NK cell development or function by producing IP4. To investigate whether ItpkB controls NK cell development, we first compared the proportions and total numbers of splenic NK cells in ItpkB−/− and WT littermate mice on the C57BL/6 genetic background. Because of their profound T-cell deficiency,22,31,32 ItpkB−/− mice had WT-level splenic NK cell proportions (Figure 1A), but the total splenic NK cell numbers were significantly reduced (Figure 1B). In the BM, we observed similar proportions of CD3−CD122+ NK-lineage cells16 in ItpkB−/− and WT mice (Figure 1C). Immature and mature NK cells can be distinguished by differential expression of CD11b and CD27.17 ItpkB−/− mice had mildly increased splenic and near normal BM CD11b−CD27+ immature NK cell proportions. More mature CD11b+CD27+ “rapid responder” NK cell proportions were increased. In contrast, ItpkB−/− spleens and, more profoundly, BM had reduced (> 6-fold in the BM) proportions of CD11b+CD27− more mature, longer-lived NK cells (Figure 1A,C), suggesting that ItpkB promotes mature NK cell differentiation.
A maturational defect causes reduced NK cell numbers in ItpkB−/− mice. Splenocytes (A) and BM cells (C) from WT and ItpkB−/− mice were stained for CD3 and NK1.1 or the indicated lineage markers, CD122, CD27, and CD11b to distinguish terminal maturational stages17 and analyzed by flow cytometry. WT level CD3−NK1.1+ NK cell (spleen; A) and CD3−CD122+ NK cell precursor (BM; C) percentages reflect the profound CD3+NK1.1− T-cell deficiency in ItpkB−/− mice.26,31,32 However, CD11b+CD27− mature NK cells were considerably reduced in ItpkB−/− spleens and BM. (B) Paired Student t test analysis (n = 12) revealed a significant reduction (P < .0001) of total splenic NK cell numbers in ItpkB−/− mice.
A maturational defect causes reduced NK cell numbers in ItpkB−/− mice. Splenocytes (A) and BM cells (C) from WT and ItpkB−/− mice were stained for CD3 and NK1.1 or the indicated lineage markers, CD122, CD27, and CD11b to distinguish terminal maturational stages17 and analyzed by flow cytometry. WT level CD3−NK1.1+ NK cell (spleen; A) and CD3−CD122+ NK cell precursor (BM; C) percentages reflect the profound CD3+NK1.1− T-cell deficiency in ItpkB−/− mice.26,31,32 However, CD11b+CD27− mature NK cells were considerably reduced in ItpkB−/− spleens and BM. (B) Paired Student t test analysis (n = 12) revealed a significant reduction (P < .0001) of total splenic NK cell numbers in ItpkB−/− mice.
ItpkB−/− mice have a NK cell–intrinsic developmental defect
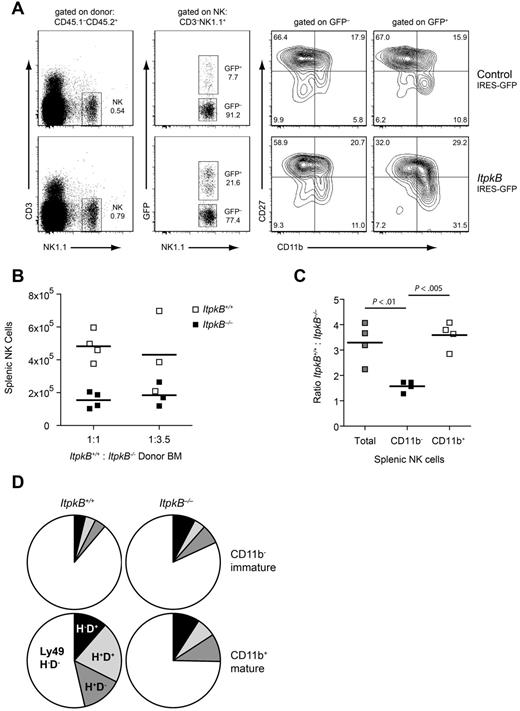
To determine whether ItpkB is required within developing NK cells, we generated BM chimeras. CD45.1−CD45.2+ItpkB−/− donor hematopoietic stem cells (HSCs) were infected with bicistronic retroviruses expressing green fluorescent protein (GFP) alone (control) or together with WT ItpkB and injected into lethally irradiated CD45.1+CD45.2+ recipients. Development of donor-derived NK cells was evaluated 5-10 weeks later (CD45.1−CD45.2+CD3−NK1.1+; supplemental Figure 3). GFP+ NK cells expressing WT ItpkB efficiently developed into CD11b+CD27− mature splenic NK cells (31.5%; Figure 2A). In contrast, uninfected HSCs (GFP−) or empty retrovirus infected GFP+ HSCs failed to efficiently generate CD11b+CD27− mature NK cells (11.0% and 10.8%, respectively; Figure 2A). Therefore, the NK cell maturational defects in ItpkB−/− mice result from a cell-intrinsic ItpkB deficiency.
A cell-intrinsic defect impairs maturation of ItpkB−/− NK cells. (A) Purified ItpkB−/− donor HSCs (CD45.1−CD45.2+) were infected with bicistronic retroviruses expressing GFP alone (control) or together with WT ItpkB and injected intravenously into lethally irradiated CD45.1+CD45.2+ recipients. Five to 10 weeks later, ItpkB−/− donor HSC-derived cells were identified by CD45.1−CD45.2+ expression (supplemental Figure 3A). Retrovirally transduced cells were identified by GFP expression. NK cells reconstituted with WT ItpkB (GFP+; lower right), but not empty vector (top panel) or nontransduced cells (GFP−) efficiently developed into CD11b+CD27− NK cells. (B-C) Lethally irradiated CD45.1+CD45.2+ recipients were injected with the indicated ratios of lymphocyte-depleted WT CD45.1+:ItpkB−/− CD45.2+ BM cells and analyzed 6.5 weeks later. (B) Total WT (□) or ItpkB−/− (■) CD3−NK1.1+ NK cell numbers in individual recipients. Horizontal lines denote the means of the respective populations. (C) WT:ItpkB−/− total number ratios of splenic CD3−NK1.1+ NK cells (Total), CD3−NK1.1+NKG2D+CD11b− immature, and CD3−NK1.1+NKG2D+CD11b+ mature NK cells. Statistical significance of the indicated comparisons was assessed via paired Student t test (n = 4). (D) Pie charts showing the percentage of immature (CD11b−; top) or mature (CD11b+; bottom) splenic NK cells expressing the indicated Ly49D and H combinations in the ItpkB+/+ (left) or ItpkB−/− (right) compartment. Qualitatively similar results were found for ItpkB+/+:ItpkB−/− HSC injection ratios of 5:1 and 1:3.5 or when CD3−NK1.1+NKG2D+ cells were analyzed (not shown). Results shown are representative of 2 independent experiments.
A cell-intrinsic defect impairs maturation of ItpkB−/− NK cells. (A) Purified ItpkB−/− donor HSCs (CD45.1−CD45.2+) were infected with bicistronic retroviruses expressing GFP alone (control) or together with WT ItpkB and injected intravenously into lethally irradiated CD45.1+CD45.2+ recipients. Five to 10 weeks later, ItpkB−/− donor HSC-derived cells were identified by CD45.1−CD45.2+ expression (supplemental Figure 3A). Retrovirally transduced cells were identified by GFP expression. NK cells reconstituted with WT ItpkB (GFP+; lower right), but not empty vector (top panel) or nontransduced cells (GFP−) efficiently developed into CD11b+CD27− NK cells. (B-C) Lethally irradiated CD45.1+CD45.2+ recipients were injected with the indicated ratios of lymphocyte-depleted WT CD45.1+:ItpkB−/− CD45.2+ BM cells and analyzed 6.5 weeks later. (B) Total WT (□) or ItpkB−/− (■) CD3−NK1.1+ NK cell numbers in individual recipients. Horizontal lines denote the means of the respective populations. (C) WT:ItpkB−/− total number ratios of splenic CD3−NK1.1+ NK cells (Total), CD3−NK1.1+NKG2D+CD11b− immature, and CD3−NK1.1+NKG2D+CD11b+ mature NK cells. Statistical significance of the indicated comparisons was assessed via paired Student t test (n = 4). (D) Pie charts showing the percentage of immature (CD11b−; top) or mature (CD11b+; bottom) splenic NK cells expressing the indicated Ly49D and H combinations in the ItpkB+/+ (left) or ItpkB−/− (right) compartment. Qualitatively similar results were found for ItpkB+/+:ItpkB−/− HSC injection ratios of 5:1 and 1:3.5 or when CD3−NK1.1+NKG2D+ cells were analyzed (not shown). Results shown are representative of 2 independent experiments.
The efficiency of NK cell maturation increased proportionally with ItpkB expression levels. NK cells derived from HSCs with lower ItpkB expression (GFPlow) contributed less to the CD11b+CD27− subset than GFPhigh NK cells expressing more ItpkB (supplemental Figure 3B). Together with an intermediate defect in ItpkB+/− mice (data not shown), this indicates that ItpkB expression levels limit the efficiency of NK cell maturation.
The lymphopenic environment in ItpkB−/− mice might mask the severity of maturational defects because there is little competition for growth factors compared with a WT environment. To examine the effect of ItpkB deficiency in a lympho-replete environment, we analyzed WT:ItpkB−/− mixed BM chimeras. Congenically marked WT and ItpkB−/− HSCs were mixed in different ratios and injected into lethally irradiated recipients. A 1:1 WT:ItpkB−/− donor HSC ratio led to a > 3-fold enrichment of host-derived WT over ItpkB−/− NK cells (Figure 2B-C). CD11b− immature NK cells were equivalently represented among genotypes; however, ItpkB−/− CD11b+ mature NK cells were strongly underrepresented (Figure 2C). Even when ItpkB−/− HSCs were in excess (1:3.5), ItpkB−/− splenic NK cells remained underrepresented compared with WT NK cells (Figure 2B), suggesting that ItpkB is required within developing NK cells and does not control NK cell development through paracrine effects or by acting in other hematopoietic cells.
Mature ItpkB−/− NK cells express an immature NKR repertoire
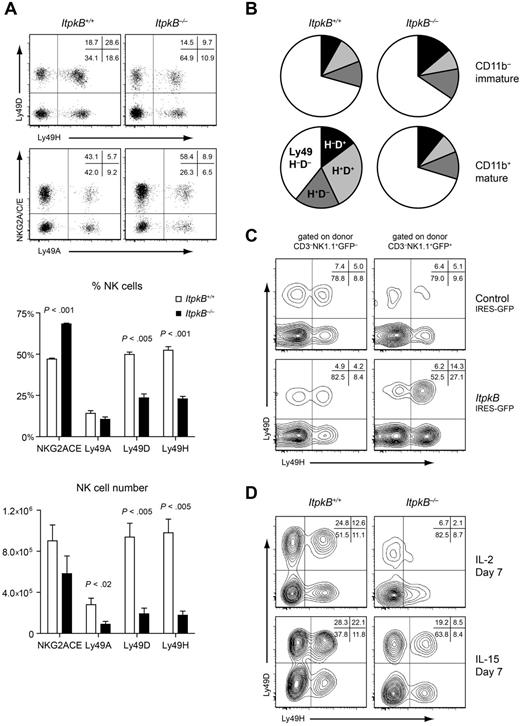
NK cell diversity is generated by the stochastic but sequential acquisition of multiple NKRs.6-15 NKG2A/CD94 expression on the most immature CD11b− NK cells is followed by the acquisition of Ly49 NKRs. Among these, activating Ly49D and Ly49H are typically expressed later at the mature CD11b+ stage.10 NKG2A/C/E+ NK cell proportions were enriched in ItpkB−/− mice (Figure 3A). In contrast, Ly49D and Ly49H representation was greatly reduced (Figure 3A) even in the absence of the Ly49 ligand MHCI (supplemental Figure 4). Therefore, the Ly49 repertoire change is not a consequence of altered ligands. Comparison of Ly49D and Ly49H expression on CD11b− immature and CD11b+ mature NK cells showed that ItpkB−/− mature NK cells maintain an immature Ly49 repertoire (Figure 3B), even in competitive BM chimeras (Figure 2D). This was rescued by retroviral ItpkB reexpression in BM chimeras (Figure 3C and supplemental Figure 3B). In contrast, culturing with cytokines that can activate and expand NK cells (IL-2) or promote their maturation (IL-15) did not compensate for ItpkB loss (Figure 3D). Therefore, ItpkB deficiency skews mature NK cells toward an immature NKR repertoire independently of ligand or cytokine availability. Interestingly, similar representation of NK cells expressing NKG2D, 2B4 or NKp46 in WT and ItpkB−/− mice (supplemental Figure 5) indicates that ItpkB does not control the representation of all NKRs.
ItpkB−/− NK cells express an immature NKR repertoire that persists in IL-2 or IL-15 culture but is normalized upon ectopic ItpkB expression. (A-D) CD94-coupled (NKG2A/C/E) and Ly49 NKRs were assessed on splenic NK cells by flow cytometry. (A) Top, representative dot plots indicating the percentage of cells in the respective quadrants. Center and bottom, bar graphs depicting mean ± SEM, percentages or total numbers of splenic NK cells expressing the indicated NKRs in WT (open bars) or ItpkB−/− (black bars) mice (n = 5). (B) Pie charts showing the percentage of immature (CD11b−; top) or mature (CD11b+; bottom) splenic NK cells expressing the indicated Ly49D/H combinations in ItpkB+/+ or ItpkB−/− mice, respectively. Results shown are representative of at least 4 independent experiments. (C) Retrovirally transduced BM chimeras were generated as in Figure 2A. Shown is Ly49D/H expression on GFP− versus GFP+ItpkB−/− donor-derived splenic NK cells transduced with empty (top) or WT ItpkB (bottom) expressing retroviruses. (D) Ly49D/H expression on purified WT and ItpkB−/− splenic NK cells cultured for 7 days in IL-2 (1000 U/mL; top) or IL-15 (100 ng/mL; bottom).
ItpkB−/− NK cells express an immature NKR repertoire that persists in IL-2 or IL-15 culture but is normalized upon ectopic ItpkB expression. (A-D) CD94-coupled (NKG2A/C/E) and Ly49 NKRs were assessed on splenic NK cells by flow cytometry. (A) Top, representative dot plots indicating the percentage of cells in the respective quadrants. Center and bottom, bar graphs depicting mean ± SEM, percentages or total numbers of splenic NK cells expressing the indicated NKRs in WT (open bars) or ItpkB−/− (black bars) mice (n = 5). (B) Pie charts showing the percentage of immature (CD11b−; top) or mature (CD11b+; bottom) splenic NK cells expressing the indicated Ly49D/H combinations in ItpkB+/+ or ItpkB−/− mice, respectively. Results shown are representative of at least 4 independent experiments. (C) Retrovirally transduced BM chimeras were generated as in Figure 2A. Shown is Ly49D/H expression on GFP− versus GFP+ItpkB−/− donor-derived splenic NK cells transduced with empty (top) or WT ItpkB (bottom) expressing retroviruses. (D) Ly49D/H expression on purified WT and ItpkB−/− splenic NK cells cultured for 7 days in IL-2 (1000 U/mL; top) or IL-15 (100 ng/mL; bottom).
Enhanced clearance of MHCI-deficient target cells in ItpkB−/− mice
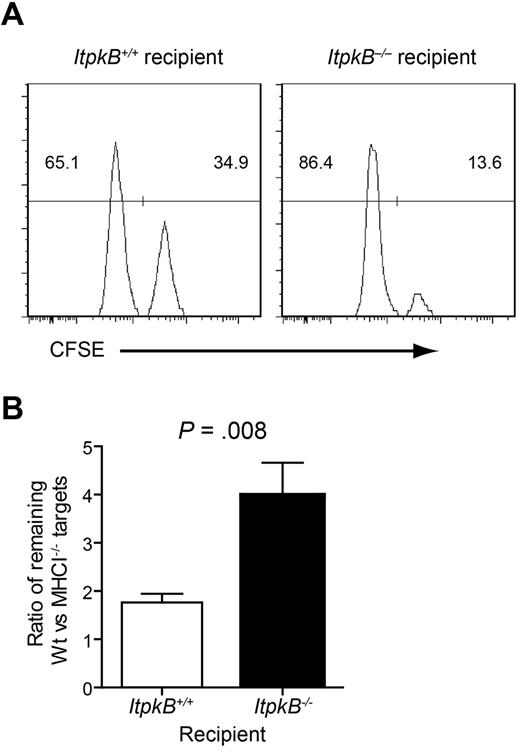
NK cell effector functions can be triggered by engagement of activating NKRs or by disrupting inhibitory NKR signaling. Inhibitory Ly49 and NKG2A NKRs monitor MHCI expression either directly by binding distinct MHCI proteins or indirectly by binding MHCI leader sequences presented by the MHCI-like molecule Qa-1.11 To determine whether ItpkB−/− NK cells respond normally to the loss of Ly49 inhibitory signals, we assessed their ability to clear differentially CFSE-labeled WT and MHCI-deficient (MHCI−/−) target cells in vivo. MHCI+ targets (WT, CFSElow) resist NK cell lysis and were used as an internal control. Clearance of MHCI−/− targets (CFSEhigh) 24 hours after injection was quantified as the ratio of remaining WT to MHCI−/− targets. Ratios > 1 quantify the clearance efficiency of MHCI−/− cells. Surprisingly, clearance of MHCI−/− targets in ItpkB−/− recipients (ratio = 3.7) was enhanced compared with WT recipients (ratio = 1.7; Figure 4). Therefore, ItpkB−/− NK cells cleared MHCI−/− targets more efficiently than WT NK cells.
Enhanced clearance of MHCI-deficient target cells by ItpkB−/− NK cells. (A) MHCI+/+ or MHCI−/− splenocytes were labeled with low or high CFSE amounts, respectively. A 1:1 mixture was injected IV into WT versus ItpkB−/− mice. CFSElow versus CFSEhigh target cell content in recipient spleens was assessed 24 hours later. (B) Statistical analysis of the ratio (± SEM) of WT control to MHCI−/− targets harvested from recipient spleens (n = 5) revealed significantly underrepresented MHCI−/− targets in ItpkB−/− recipients (P = .008, paired t test), indicating increased MHCI−/− target clearance.
Enhanced clearance of MHCI-deficient target cells by ItpkB−/− NK cells. (A) MHCI+/+ or MHCI−/− splenocytes were labeled with low or high CFSE amounts, respectively. A 1:1 mixture was injected IV into WT versus ItpkB−/− mice. CFSElow versus CFSEhigh target cell content in recipient spleens was assessed 24 hours later. (B) Statistical analysis of the ratio (± SEM) of WT control to MHCI−/− targets harvested from recipient spleens (n = 5) revealed significantly underrepresented MHCI−/− targets in ItpkB−/− recipients (P = .008, paired t test), indicating increased MHCI−/− target clearance.
Enhanced IFNγ production by ItpkB−/− NK cells
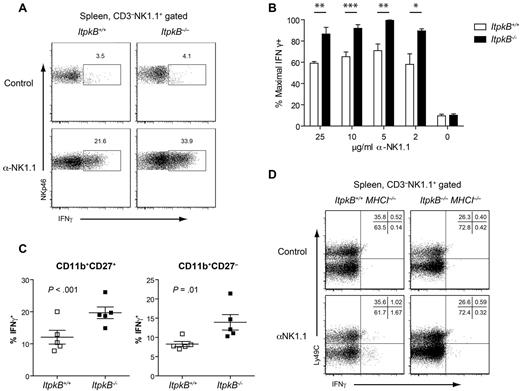
In addition to cell lysis, the secretion of inflammatory cytokines and chemokines, including IFNγ, is a major NK cell function that enhances inflammation and recruits APCs.1 Intriguingly, a higher proportion of ItpkB−/− NK cells (33.9%) than WT controls (21.6%; Figure 5A-B) produced IFNγ after NK1.1-activating NKR stimulation. This enhanced responsiveness does not reflect the altered mature NK cell subset representation because both CD11b+CD27+ and CD11b+CD27− NK cells produced more IFNγ (Figure 5C).
Enhanced IFNγ production by licensed but not unlicensed ItpkB−/− NK cells. (A) WT or ItpkB−/− splenic NK cells were stimulated with plate-bound anti-NK1.1 Abs (5 μg/mL) in the presence of 2μM monensin for 5 hours. IFNγ production was analyzed by intracellular FACS. (B) Splenic NK cells were stimulated with the indicated plate-bound anti-NK1.1 Ab concentrations. IFNγ secretion was normalized to the maximum observed (100%) and is shown as mean ± SEM; n = 5 different experiments. Asterisks denote statistical significance for the indicated comparisons as determined by Student t test: ***P < .001; **P < .01; and *P < .05. (C) IFNγ production by CD11b+CD27+ versus CD11b+CD27− splenic NK cell mature subpopulations stimulated as in panel A. Horizontal bars denote mean ± SEM (n = 5). P values for genotype comparisons were obtained via paired Student t test. (D) Splenic NK cells from WT and ItpkB−/− mice crossed to a TAP-deficient (MHCI−/−) background, which lacks licensing self-ligands, were stimulated as in panel A and evaluated for IFNγ production.
Enhanced IFNγ production by licensed but not unlicensed ItpkB−/− NK cells. (A) WT or ItpkB−/− splenic NK cells were stimulated with plate-bound anti-NK1.1 Abs (5 μg/mL) in the presence of 2μM monensin for 5 hours. IFNγ production was analyzed by intracellular FACS. (B) Splenic NK cells were stimulated with the indicated plate-bound anti-NK1.1 Ab concentrations. IFNγ secretion was normalized to the maximum observed (100%) and is shown as mean ± SEM; n = 5 different experiments. Asterisks denote statistical significance for the indicated comparisons as determined by Student t test: ***P < .001; **P < .01; and *P < .05. (C) IFNγ production by CD11b+CD27+ versus CD11b+CD27− splenic NK cell mature subpopulations stimulated as in panel A. Horizontal bars denote mean ± SEM (n = 5). P values for genotype comparisons were obtained via paired Student t test. (D) Splenic NK cells from WT and ItpkB−/− mice crossed to a TAP-deficient (MHCI−/−) background, which lacks licensing self-ligands, were stimulated as in panel A and evaluated for IFNγ production.
NK cells acquire functional responsiveness through licensing by engagement of inhibitory NKRs that recognize self-MHCI.6,13,15 Consequently, NK cells that develop in an MHCI−/− environment produce little IFNγ. To determine whether ItpkB−/− NK cells might have gained functional competency without licensing, ItpkB−/− mice were crossed onto an MHCI−/− background. MHCI−/− and ItpkB−/−MHCI−/− NK cells both hyporesponded to NK1.1 stimulation (Figure 5D). Therefore, despite their hyperresponsiveness, ItpkB−/− NK cells still require licensing.
Enhanced degranulation of ItpkB−/− NK cells
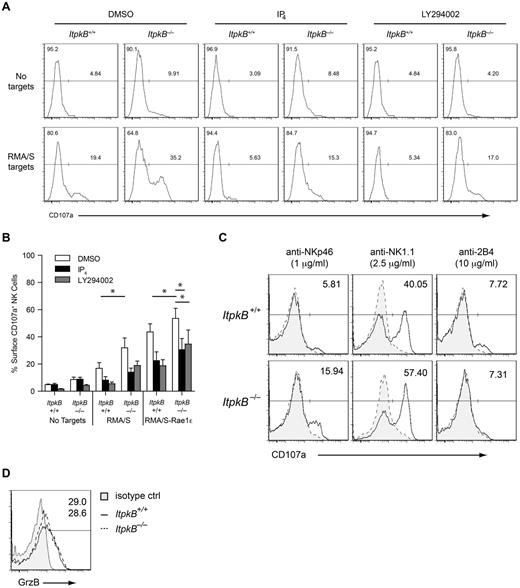
To determine whether the enhanced target cell clearance and IFNγ production by ItpkB−/− NK cells associate with increased degranulation, we assessed NK cell surface CD107a/LAMP1 levels upon target cell coculture. CD107a is normally localized to lysosomal compartments and cytolytic granules. Their fusion with the plasma membrane during degranulation and target cell lysis is correlated with increased CD107a surface levels.35 Surface CD107a was mildly increased on unstimulated ItpkB−/− (9.9%) versus WT NK cells (4.8%; Figure 6A-B). Exposure to MHCI−/− RMA/S tumor target cells induced considerably higher CD107a surface expression on ItpkB−/− (35.2%) than WT NK cells (19.4%). RMA/S cells ectopically expressing Rae1ϵ, the activating ligand for the NKR NKG2D, tended to induce higher CD107a levels on ItpkB−/− versus WT NK cells (ItpkB−/−: 53.6%; WT: 43.6%). In vitro cross-linking of NK1.1 or NKp46, but not 2B4, caused higher CD107a up-regulation on ItpkB−/− than WT splenic NK cells (Figure 6C). NK cells of both genotypes contained similar intracellular amounts of the lytic enzyme granzyme B (Figure 6D). These data indicate that ItpkB deficiency increases primary NK cell cytotoxic granule release in response to inhibitory NKR ligand loss (RMA/S cells) or to the sole engagement of NK1.1 or NKp46.
ItpkB and IP4 inhibit NK cell degranulation. (A) WT or ItpkB−/− splenic NK cells were pretreated with DMSO, cell-permeable IP4, or the PI3K inhibitor LY294002 for 1 hour and then cocultured with RMA/S targets for 2 hours. Degranulation was assessed by cell-surface staining of CD107a. (B) Degranulation (mean ± SEM, n = 3) by WT and ItpkB−/− splenic NK cells in response to media, RMA/S, or RMA/S-Rae1ϵ target cells after pretreatment with DMSO, cell-permeable IP4, or LY294002. Asterisks indicate statistical significance of P < .05 for the indicated comparisons (paired Student t test, n = 3). (C) WT and ItpkB−/− splenic NK cells were stimulated without (gray shaded, hatched histograms) or with (open solid histograms) the indicated concentrations of plate-bound Abs against NKp46, NK1.1, or 2B4 for 2 hours. Degranulation was assessed as in panel A. Numbers indicate the percentage of cells in the CD107a+ gate after stimulation. (D) WT and ItpkB−/− NK cells express similar levels of granzyme B. Splenocytes from WT (solid open histogram) and ItpkB−/− (hatched open histogram) mice were stained for surface CD3 and NK1.1, followed by intracellular staining with anti–granzyme B (open histograms) or isotype control (shaded gray histogram, isotype control, WT only) Abs and analyzed by flow cytometry. Numbers denote the percentage of cells in the indicated granzyme B (GrzB)+ gate for ItpkB+/+ and ItpkB−/− mice.
ItpkB and IP4 inhibit NK cell degranulation. (A) WT or ItpkB−/− splenic NK cells were pretreated with DMSO, cell-permeable IP4, or the PI3K inhibitor LY294002 for 1 hour and then cocultured with RMA/S targets for 2 hours. Degranulation was assessed by cell-surface staining of CD107a. (B) Degranulation (mean ± SEM, n = 3) by WT and ItpkB−/− splenic NK cells in response to media, RMA/S, or RMA/S-Rae1ϵ target cells after pretreatment with DMSO, cell-permeable IP4, or LY294002. Asterisks indicate statistical significance of P < .05 for the indicated comparisons (paired Student t test, n = 3). (C) WT and ItpkB−/− splenic NK cells were stimulated without (gray shaded, hatched histograms) or with (open solid histograms) the indicated concentrations of plate-bound Abs against NKp46, NK1.1, or 2B4 for 2 hours. Degranulation was assessed as in panel A. Numbers indicate the percentage of cells in the CD107a+ gate after stimulation. (D) WT and ItpkB−/− NK cells express similar levels of granzyme B. Splenocytes from WT (solid open histogram) and ItpkB−/− (hatched open histogram) mice were stained for surface CD3 and NK1.1, followed by intracellular staining with anti–granzyme B (open histograms) or isotype control (shaded gray histogram, isotype control, WT only) Abs and analyzed by flow cytometry. Numbers denote the percentage of cells in the indicated granzyme B (GrzB)+ gate for ItpkB+/+ and ItpkB−/− mice.
ItpkB limits NK cell degranulation by producing the PI3K antagonist IP4
To determine whether ItpkB regulates granule secretion directly, we evaluated the ability of a cell-permeable IP4 ester, 2,6-di-O-Butyryl-Ins (1,3,4,5)P4/AM, to reverse the enhanced degranulation of ItpkB−/− NK cells and inhibit WT NK cell degranulation. In contrast to the highly hydrophilic, membrane-impermeable IP4, 2,6-di-O-Butyryl-Ins (1,3,4,5)P4/AM can cross cell membranes and releases intracellular IP4 after its hydrolysis by ubiquitous cellular esterases. IP4/AM has been used by many investigators to experimentally increase endogenous IP4 concentrations.28,30 NK cells were pretreated with cell-permeable IP4 for 1 hour, followed by coculture with tumor cell targets for 2 additional hours. Pretreatment with cell-permeable IP4 reduced RMA/S target-induced CD107a surface up-regulation on ItpkB−/− NK cells to WT levels without IP4 treatment (15.3% vs 19.4%), but did not affect basal CD107a levels on unstimulated cells (Figure 6A-B) or NK cell viability (supplemental Figure 6). Cell-permeable IP4 also reduced CD107a expression on WT NK cells in response to all stimuli. Therefore, the ItpkB product IP4 limits NKR-induced degranulation. In contrast, NKR-induced CD69 up-regulation was unaffected by IP4 (supplemental Figure 7B). Therefore, IP4 does not limit all aspects of NK cell activation.
NK cell degranulation and target cell killing require PI3K.4,20,21 To investigate the mechanism by which IP4 limits degranulation, we hypothesized that IP4 competitively inhibits the activation of PIP3 effectors,26,28,32,33 leading to increased NKR-induced PI3K signaling in ItpkB−/− NK cells. To determine whether increased PI3K signaling in ItpkB−/− NK cells contributes to the enhanced degranulation, we first assessed the ability of PI3K inhibitors to normalize CD107a surface expression on ItpkB−/− NK cells. As described previously,20 pretreatment of WT NK cells with the PI3K inhibitor LY294002 at nontoxic doses reduced RMA/S or RMA/S-Rae1ϵ target cell–induced surface CD107a up-regulation (Figures 6A-B and 7B). Moreover, similar to IP4 pretreatment, PI3K inhibition reduced the RMA/S cell-induced hyperdegranulation of ItpkB−/− NK cells to the levels found in inhibitor-untreated, RMA/S cell–exposed WT NK cells (17.0% vs 19.4%).
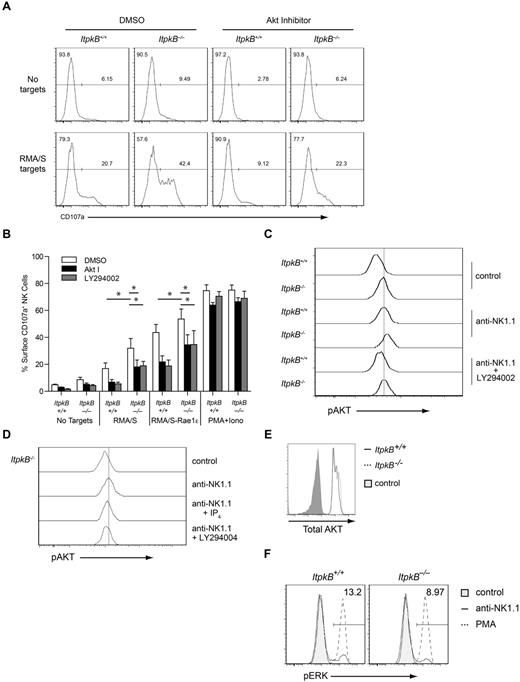
Akt promotes NK cell degranulation. (A) WT and ItpkB−/− splenic NK cells were pretreated with DMSO, Akt inhibitor VIII, or LY294002 for 1 hour before coculture with RMA/S targets for 2 hours. Degranulation was assessed by cell-surface staining of CD107a. Results shown are representative of 3 independent experiments. (B) Degranulation (mean ± SEM, n = 3) by WT and ItpkB−/− splenic NK cells stimulated with RMA/S or RMA/S-Rae1ϵ cells or PMA/ionomycin after pretreatment with DMSO, Akt inhibitor VIII, or LY294002. Asterisks indicate statistical significance of P < .05 for the indicated comparisons (paired Student t test, n = 3). (C-D) Akt activation was assessed in WT and ItpkB−/− splenic NK cells stimulated without (control) or with Abs against NK1.1 without (anti-NK1.1) or together with 25μM PI3K-inhibitor (anti-NK1.1 + LY294002) or 50μM cell-permeable IP4-ester (anti-NK1.1 + IP4) by intracellular staining with phospho-Akt-S473 (pAKT)–specific Abs. Results shown are representative of 3 independent experiments. (E) WT and ItpkB−/− NK cells contain similar total Akt protein amounts. Total Akt protein levels in WT (black open histogram) or ItpkB−/− (hatched open histogram) splenic CD3−NK1.1+ NK cells were determined via intracellular staining and flow cytometry. Shaded gray histogram indicates the negative control stain. Results shown are representative of 3 independent experiments. (F) ItpkB−/− and WT splenic NK cells were stimulated without (control, shaded gray histograms) or with anti-NK1.1 Abs (solid open histograms) or PMA (hatched open histograms) for 10 minutes, followed by flow cytometric analysis for phospho-Erk (pThr202/pTyr204) content (pERK). Numbers indicate the percentage of pERK+ cells after stimulation with plate-bound anti-NK1.1 Abs (5 μg/mL).
Akt promotes NK cell degranulation. (A) WT and ItpkB−/− splenic NK cells were pretreated with DMSO, Akt inhibitor VIII, or LY294002 for 1 hour before coculture with RMA/S targets for 2 hours. Degranulation was assessed by cell-surface staining of CD107a. Results shown are representative of 3 independent experiments. (B) Degranulation (mean ± SEM, n = 3) by WT and ItpkB−/− splenic NK cells stimulated with RMA/S or RMA/S-Rae1ϵ cells or PMA/ionomycin after pretreatment with DMSO, Akt inhibitor VIII, or LY294002. Asterisks indicate statistical significance of P < .05 for the indicated comparisons (paired Student t test, n = 3). (C-D) Akt activation was assessed in WT and ItpkB−/− splenic NK cells stimulated without (control) or with Abs against NK1.1 without (anti-NK1.1) or together with 25μM PI3K-inhibitor (anti-NK1.1 + LY294002) or 50μM cell-permeable IP4-ester (anti-NK1.1 + IP4) by intracellular staining with phospho-Akt-S473 (pAKT)–specific Abs. Results shown are representative of 3 independent experiments. (E) WT and ItpkB−/− NK cells contain similar total Akt protein amounts. Total Akt protein levels in WT (black open histogram) or ItpkB−/− (hatched open histogram) splenic CD3−NK1.1+ NK cells were determined via intracellular staining and flow cytometry. Shaded gray histogram indicates the negative control stain. Results shown are representative of 3 independent experiments. (F) ItpkB−/− and WT splenic NK cells were stimulated without (control, shaded gray histograms) or with anti-NK1.1 Abs (solid open histograms) or PMA (hatched open histograms) for 10 minutes, followed by flow cytometric analysis for phospho-Erk (pThr202/pTyr204) content (pERK). Numbers indicate the percentage of pERK+ cells after stimulation with plate-bound anti-NK1.1 Abs (5 μg/mL).
In neutrophils, IP4 competitively inhibits PIP3 recruitment of the PI3K effector kinase Akt.27,28 To investigate whether IP4 dampens NK cell degranulation by inhibiting Akt, we first evaluated whether Akt inhibition by the highly selective, allosteric Akt-inhibitor VIII36-38 could limit target cell-induced CD107a surface up-regulation. Indeed, Akt inhibition strongly reduced WT NK cell degranulation in response to RMA/S or RMA/S-Rae1ϵ targets (Figure 7A-B) without significantly affecting NK cell viability and RMA/S cell–induced CD69 up-regulation on NK cells (supplemental Figures 6 and 7A). This suggests that Akt is an important proximal PIP3 effector required for granule secretion but not CD69 up-regulation. Moreover, Akt inhibition reduced RMA/S-induced ItpkB−/− NK cell hyperdegranulation to inhibitor-untreated WT NK cell levels (22.3% vs 20.7%; Figure 7A-B). The similar abilities of cell-permeable IP4, PI3K, or Akt inhibitors to reverse the hyperdegranulation of ItpkB−/− NK cells are consistent with a model in which IP4 limits NK cell degranulation by antagonizing NKR-induced Akt recruitment by PI3K/PIP3. Supporting this view, ItpkB−/− NK cells contained more active phosphorylated Akt than WT NK cells both before and after NK1.1-stimulation, whereas the total Akt protein content was similar and PI3K inhibition reduced active Akt content (Figure 7C-E). Moreover, IP4 can prevent Akt membrane recruitment in hematopoietic and nonhematopoietic cells27-29 and partially reversed the NK1.1-induced Akt hyperphosphorylation in ItpkB−/− NK cells (Figure 7D). Neither PI3K inhibition nor IP4 treatment significantly affected basal phospho-Akt levels (data not shown). This was not unexpected, because basal PIP3-mediated Akt recruitment and activation have already occurred before the compounds are administered. In contrast to active Akt, basal and anti-NK1.1 or phorbol 12-myristate 13-acetate (PMA) induced amounts of active Erk were not increased in ItpkB−/− versus WT NK cells (Figure 7F). Therefore, ItpkB does not limit all aspects of NKR signaling.
Bypassing NKR activation via PMA/ionomycin stimulation induced comparable surface CD107a on ItpkB−/− and WT NK cells and was unaffected by PI3K or Akt inhibitors (Figure 7B). WT and ItpkB−/− NK cells contained similar amounts of the lytic granule component granzyme B (Figure 6D). Therefore, ItpkB−/− and WT NK cells contain similar overall numbers of cytolytic granules and ItpkB, PI3K, and Akt control degranulation by regulating proximal NKR signaling.
Discussion
NK cells provide an important first line of defense against viral infections and cellular transformation by secreting proinflammatory cytokines and directly killing target cells.1-4 Their effector functions are held in check by a delicate balance of signals from inhibitory and activating NKRs. Inhibitory NKR engagement and the “dampening” of NKR signaling through negative effectors have crucial but puzzlingly dichotomic functions in NK cell biology: They are essential for the acquisition of functional competence during NK cell “education” under certain conditions but also warrant mature NK cell tolerance to healthy self-tissue by preventing hyperresponsiveness.14,15 Elucidating the molecular mechanisms educating NK cells is an important problem.12,13 Incomplete understanding of the mechanisms governing NK cell education, tolerance, maturation, and distinguishing licensing from inhibiting effector functions is a barrier to the development of safe and efficacious NK cell therapies.2,3,5,12 In the present study, we identify production of the soluble messenger IP4 by ItpkB as a novel dampening mechanism of NKR signaling that promotes NK cell maturation but limits NK cell responsiveness. ItpkB deficiency in NK cells cell-autonomously alters representation and NKR repertoire of mature NK cell subsets, increases the proportion of NK cells responding to NKR stimulation, and augments the magnitude of their effector functions, including γIFN production, cytolytic granule release, and in vivo clearance of MHCI−/− target cells. This was at least in part caused by defective dampening of PI3K-mediated Akt activation by IP4, as evidenced by hyperactive Akt in ItpkB−/− NK cells and reversal of the hyperdegranulation and Akt hyperactivation by treatment with cell-permeable IP4 or with selective Akt or PI3K small-molecule inhibitors. Attempts to analyze in vitro cytotoxicity were limited by the known too low cytotoxicity of unprimed primary NK cells and a tendency of ItpkB−/− but not WT NK cells to overexpand and then die in the usual 1-week IL-2 or IL-15 in vitro culture (data not shown). Using 2-day cytokine-expanded NK cells, we surprisingly found no significant cytotoxicity differences between WT and ItpkB−/− NK cells (supplemental Figure 8). Therefore, ItpkB−/− NK cells may be hyperresponsive to γc-cytokines in vitro, but cytokine priming in vitro may also abrogate the cytotoxicity difference, possibly by increasing WT NK cell degranulation to the already higher levels in ItpkB−/− NK cells. This notwithstanding, our combined data show that ItpkB limits several aspects of primary NK cell function through IP4 antagonism with PI3K.
PI3Kγ/δ are key mediators of NKR signaling through production of the membrane-lipid PIP3, which recruits and activates multiple signaling proteins including Akt- and Tec-family (TFK) kinases by binding to PH and other domains.4,19-21 The critical PI3K downstream effectors in NK cells still require elucidation, but may include Akt.39,40 PI3K-deficient mice have less mature CD11b+CD27+ NK cells, abnormal Ly49 repertoires, and reduced NKR-mediated cytokine production, target cell lysis, and lymphoma/melanoma cell clearance because of impaired NKR signaling and migration defects.4,19-21 Often, PI3K signaling is limited through PIP3 dephosphorylation by phosphatase-and-tensin-homolog (PTEN) or SH2 domain-containing inositol-5′-phosphatase (SHIP1).4,22 In contrast, ItpkB does not directly affect PIP3 levels. Instead, IP4 binds to PH domains in PI3K effectors and primes or competitively inhibits PIP3 binding.22,26-29,33 Because PH domains can have different affinities for PIP3 versus IP4, IP4 can selectively regulate a subset of PI3K effectors.26,33 Interestingly, IP4 can serve as a substrate for PTEN and SHIP1 in vitro.33 Therefore, IP4 might also indirectly control PI3K signaling by competing with PIP3 as a SHIP1/PTEN substrate.
Among the mechanisms that limit PI3K signaling in NK cells, PTEN remains unexplored.4 SHIP1 acts downstream of certain NKRs.18,41 SHIP1−/− mice showed grossly normal NK cell development but increased Ly49A, C/I, and 2B4 and decreased Ly49D, G2, and NKG2A/C/E/CD94 expression, resulting in impaired allogeneic BM graft rejection.41 SHIP1−/− NK cells up-regulate 2B4 and the inhibitory phosphatase SHP-1, compromising cytolysis on a H2b MHCI-background.42,43 A background- and 2B4-independent defect of SHIP1−/− NK cells is impaired NKR induction of γIFN.4,44 Increased peripheral NK cell numbers may reflect reduced turnover because of increased 2B4/Ly49 homeostatic signaling and Akt hyperactivation in SHIP1−/− NK cells.4,41,44 A new study suggests that SHIP-1 may control NK cell development and function through combined NK cell–intrinsic and –extrinsic mechanisms.45
Despite similar Akt hyperactivity in NK cells (Figure 7C-D), ItpkB−/− mice show contrasting reduced mature NK cell numbers, increased NKG2A/C/E/CD94, seemingly unaltered Ly49A, NKG2D, NKp46, and 2B4 but reduced Ly49D/H representation, and the aforementioned hyperactivity and γIFN overproduction (Figures 1,Figure 2,Figure 3,Figure 4,Figure 5,Figure 6–7 and supplemental Figures 3-5). It remains to be determined whether these profound differences from SHIP1−/− mice reflect differing increases in cellular PIP3 activity or impaired specific IP4 functions unrelated to PIP3 inhibition33 in ItpkB−/− but not SHIP1−/− NK cells or perturbed positive functions of the SHIP-product PI(3,4)P24,33 in SHIP1−/− but not ItpkB−/− NK cells. By binding specific PH domains, PI(3,4)P2 can recruit certain PI3K effectors including Akt.4,33 Therefore, SHIP1 can limit via PIP3 turnover, augment via PI(3,4)P2 production, or modify via shifting the PIP3/PI(3,4)P2 balance PI3K and Akt function depending on cell type, expressed PI3K effectors, and context.4,22
Understanding the specific molecular mechanism of IP4 action has been challenging given the large number of potential IP4 effectors.22,33 How IP4 affects these in vivo remains mostly unexplored. In thymocytes, low-dose IP4 promotes PIP3 binding to the TFK Itk, possibly through a cooperative-allosteric mechanism involving PH domain oligomerization.26 This is essential for the generation of mature T cells.26 High-dose IP4 can inhibit Itk PH domain binding to PIP3 in vitro.26 In neutrophils and hematopoietic progenitors, IP4 inhibits Akt membrane recruitment and activation by competing with PIP3 binding to its PH domain.27,28 Therefore, IP4 can promote or inhibit PIP3 function. IP4 may have less well characterized additional functions.33 This can explain why the phenotypes of ItpkB−/− and PI3Kγ/δ-deficient mice are not exact opposites. ItpkB promotes NK cell maturation but limits effector functions. PI3K promotes both processes.4,20,21 Whether the dichotomy of IP4 function is cell-type, receptor, or context specific is an interesting area for future research. Identifying the specific IP4 effectors in NK cells and assessing the effects of varying the cellular concentrations of PIP3 versus its soluble counterpart IP4 will help discern overlapping versus distinct ItpkB, PI3K, and SHIP1 functions and address the major open question of how may PI3K functions are subject to IP4 control.33
Akt promotes cell survival, proliferation, and metabolism and is a paramount mediator of immunoreceptor signaling via PI3K.46 Akt is required for IL-2–mediated NK cell survival47 and is activated after human KIR NKR engagement.39 In human NK cells, binding of the endocytosed soluble nonclassic MHCI protein HLA-G to the endosomal NKR CD158d activates DNA-PK to phosphorylate Akt, which then activates NFκB to produce proinflammatory and angiogenic cytokines and chemokines.40 In the present study, we found that receptor-proximal Akt signaling in NK cells is required for “conventional” NKR-mediated degranulation (Figure 7). Akt hyperactivity in ItpkB−/− NK cells and reversal of Akt hyperphosphorylation and their hyperdegranulation by Akt inhibitor or cell-permeable IP4 treatment (Figures 6 and 7) are reminiscent of the Akt hyperactivity in ItpkB−/− neutrophils and hematopoietic progenitors27,28 and of the ability of exogenous IP4 to inhibit Akt membrane translocation and activation in many cell types.22,27-29,33 Therefore, we propose that ItpkB limits NK cell degranulation via IP4 inhibition of Akt. Intriguingly, IP4 does not control all aspects of NKR signaling, because NK1.1-induced Erk activation was not significantly impaired in ItpkB−/− NK cells (Figure 7F) and target cell–induced CD69 up-regulation on NK cells was unaffected by Akt inhibition and exogenous IP4 (supplemental Figure 7).
Determining whether the ItpkB−/− NK cell phenotype arises exclusively from Akt hyperactivation or involves the deregulation of other ItpkB effectors will require the codisruption of ItpkB and Akt. NK cells do express other candidate IP4 effectors, including the PIP3-binding TFKs Itk and Tec.48 They also express IP4/PIP3 nonbinding Rlk. Although TFKs activate PLCγ1-dependent signals important for IFNγ and granule secretion,4,18 no NK cell defects were reported for Itk−/− or Itk−/−Rlk−/− mice, possibly because of TFK redundancy. NKR stimulation induced Itk phosphorylation in human NK cell clones. Overexpressed WT Itk enhanced and Itk knock-down mildly impaired NKR-induced Ca2+ mobilization and FcR, but not NKG2D-induced granule release and cytotoxicity.49 Therefore, TFKs may control NKR signaling in complex and redundant manners. IP4 regulation in NK cells, which could be bimodal and may not affect Rlk,26,33 remains to be studied. Additional PIP3 effectors that might be controlled by IP4 in NK cells are Rho-family GTPase-activating-proteins and exchange factors including Vav1.18,20
Overall, IP4 regulates NK cell responsiveness through several different mechanisms. IP4 tempers effector responses indirectly by promoting the development of CD11b+CD27− NK cells with higher activation thresholds and decreased effector potential.17 In addition, IP4 directly inhibits NK cell effector functions by dampening Akt activity and possibly controlling other effectors. Whatever the precise molecular mechanism, our studies have unveiled production of the soluble messenger IP4 by ItpkB as a physiologically important novel dampening mechanism for NKR signaling and NK cell function. IP4 may set a stringent threshold for the release of tissue-damaging cytotoxic enzymes and proinflammatory cytokines. Recent reviews have pinpointed the importance of elucidating dampening mechanisms for improving NK cell therapeutic efficacy in infectious diseases and cancers.2,3 The ItpkB−/− NK cell hyperactivity is in striking contrast to the NK cell hyporesponsiveness in mice lacking other “dampening effectors,” including SHIP1 and SHP.4,41-44,50 In the future, selective and specific small-molecule ItpkB inhibition might thus provide avenues to improve NK cell function, either ex vivo before therapeutic engraftment or in patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Boreth Eam, Nancy Mathis, and Lyn'Al Nosaka for technical help and the Scripps Research Institute and Washington University vivariums for expert mouse husbandry.
This work was supported by the National Institutes of Health (grants AI070845 and GM088647 to K.S., grant AI089805 to Y.H.H., and predoctoral training grant AI007606 to L.S.), The Leukemia & Lymphoma Society (Scholar Award 1440-11 to K.S.), and the Deutsche Forschungsgemeinschaft (Fellowship SI 1547/1-1 to S.S.). This is Scripps Research Institute manuscript number 21752.
National Institutes of Health
Authorship
Contribution: K.S. and Y.H.H. conceived, directed, and supervised the studies, designed and performed experiments, analyzed and interpreted data, and wrote the manuscript; E.P., S.S., L.S., and S.R. conducted the experiments and analyzed and interpreted data; A.R.F. and W.M.Y. provided advice and critically read the manuscript; and J.A.W. and A.H.J. provided technical assistance.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karsten Sauer, Department of Immunology and Microbial Science, The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037; e-mail: ksauer@scripps.edu; or Yina Hsing Huang, Washington University School of Medicine, 660 S Euclid Ave, Box 8118, St Louis, MO 63110; e-mail: yhuang@pathology.wustl.edu.
References
Author notes
K.S. and E.P. contributed equally to this work.