Key Points
RA regulates donor T-cell trafficking during GVHD.
The RA receptor-α signaling pathway plays a critical role in the pathophysiology of GVHD.
Damage to the gastrointestinal tract during graft-versus-host disease (GVHD) is one of the major causes of morbidity and mortality in allogeneic hematopoietic stem cell transplant (HSCT) recipients. In the current study, we identified a critical role for the retinoic acid (RA) signaling pathway in the induction and propagation of gastrointestinal GVHD. The administration of exogenous RA significantly increased expression of the gut-homing molecules, CCR9 and α4β7, on donor T cells in mesenteric lymph nodes, and augmented the accumulation of proinflammatory CD4+ and CD8+ T cells within the gut mucosa, leading to a selective exacerbation of colonic GVHD and increased overall mortality. Conversely, depletion of RA in recipient mice by vitamin A deprivation resulted in a dramatic reduction of gut-homing molecule expression on donor T cells after HSCT. Significantly, absence of the RA receptor-α on donor T cells markedly attenuated the ability of these cells to cause lethal GVHD. This observation was attributable to a significant reduction in pathological damage within the colon. These findings identify an organ-specific role for RA in GVHD and provide evidence that blockade of the RA signaling pathway may represent a novel strategy for mitigating the severity of colonic GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially life-saving therapeutic modality for patients with hematological malignancies and nonmalignant disorders. Successful outcomes, however, are compromised by graft-versus-host disease (GVHD), which remains the primary complication of this treatment and the leading cause of morbidity and mortality.1,-3 GVHD is induced by donor T cells recognizing host alloantigens expressed by host antigen presenting cells (APCs).4,5 This results in the activation and expansion of donor T cells and leads to proinflammatory cytokine production and the induction of cytotoxic T-cell responses, both of which can cause tissue damage.2,3,6 Acute GVHD typically develops in a restricted set of organs including the skin, liver, and gastrointestinal tract. Of these target organs, the gastrointestinal tract is of particular importance.7 Compelling data in experimental animal models indicate that the gut is not only a major target organ of GVHD but also plays a crucial role in the amplification of systemic GVHD severity.3,8,9 Clinically, involvement of the gastrointestinal tract in patients with acute GVHD is a major cause of morbidity and mortality.
The gut-associated lymphoid tissue, which consists of Peyer’s patches, mesenteric lymph nodes (MLNs), and lymphoid cells in the lamina propria and epithelium, is not only responsible for eliciting, but also regulating, immune responses in the intestinal mucosa.10 The adaptive immune responses that occur in the gut are modulated by a complex interplay of regulatory mechanisms within these lymphoid tissue sites. Recently, all-trans retinoic acid (RA) has emerged as a critical regulator of gut immunity.11 RA is an active metabolite of vitamin A that is involved in many important biological processes in vivo.12,13 Within the immune system, RA influences many immune cell lineages and regulates an array of immune responses.11 RA is produced by a population of CD103+ dendritic cells in the gut and plays a pivotal role in the regulation of inflammation within the colon.14,15 RA is also able to enhance the stability of Foxp3 in natural Tregs (nTregs)16 and to facilitate the conversion of CD4+Foxp3─ T cells into induced Tregs (iTregs) by upregulating Foxp3.17,-19 Recent studies have demonstrated that RA can influence the lineage decisions of CD4+ T cells. Culture of naive CD4+ T cells under TH17 polarizing conditions in the presence of RA has been shown to reduce the number of interleukin (IL)-17–secreting cells while resulting in a commensurate increase in the number of iTregs.20,-22 Thus, RA appears able to alter the balance between effector and regulatory arms of the immune system similar to what has been described for blockade of IL-6 signaling.23 Additionally, RA has been shown to augment the expression of gut-homing receptors, such as CCR9 and α4β7, on T cells under steady-state conditions24 and to mediate the recruitment of Tregs into sites of inflammation.25 The ability to drive gut homing along with the capacity to stabilize nTreg function and facilitate the induction of iTregs, even in the presence of inflammation, suggests that administration of RA might be a strategy for reducing inflammatory responses during GVHD, particularly within the colon microenvironment. The purpose of this study was to define the role of RA in the pathophysiology of GVHD and to determine to what extent endogenous and exogenous RA was able to modulate the balance between inflammation and tolerance during GVH reactivity.
Materials and methods
Mice
C57BL/6 (B6; H-2b), Balb/cJ (H-2d), C.129S7 Rag-1 (Balb/c Rag), and B6 Foxp3EGFP mice26 were purchased from the Jackson Laboratory (Bar Harbor, ME) or bred in the Animal Resource Center (ARC) at the Medical College of Wisconsin (MCW). RAR-α–deficient (RAR-α─/─) mice (B6129 background) were kindly provided by Dr Pierre Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France).27 Vitamin A-deficient (VAD) and vitamin A-sufficient (VAS) mice were generated as previously described.28 All animals were housed in the Association for Assessment and Accreditation of Laboratory Animal Care–accredited ARC of the MCW. All experiments were carried out under protocols approved by the MCW Institutional Animal Care and Use Committee.
Reagents
All-trans RA was purchased from Sigma-Aldrich (St Louis, MO), dissolved in dimethylsulfoxide (DMSO) at a concentration of 100 mM, and stored as aliquots at −20°C. The RAR-α agonist, AM80, and the RAR-α antagonist, BMS 195614, were purchased from Tocris Bioscience (Ellisville, MO) and used at the concentrations indicated.
Bone marrow transplantation
Bone marrow (BM) was flushed from donor femurs and tibias with Dulbecco’s modified Eagle medium (Gibco-BRL, Carlsbad, CA) and passed through sterile mesh filters to obtain single-cell suspensions. Host mice were conditioned with total body irradiation administered as a single exposure at a dose rate of 74 cGy using a Shepherd Mark I cesium irradiator (J. L. Shepherd and Associates, San Fernando, CA). Irradiated recipients received a single intravenous injection of BM with or without added spleen cells in the lateral tail vein.
Leukemia model
A20 murine lymphoma cells of Balb/c background (H-2d) were obtained from the American Type Culture Collection and transfected with firefly luciferase as previously described.29 For in vivo bioluminescence imaging (BLI), mice were given an intraperitoneal injection of luciferin (150 mg/kg body weight) and then anesthetized with isoflurane gas using a Xenogen XGI gas anesthesia system (Perkin Elmer, Waltham, MA). The mice were imaged using the In Vivo Imaging System to assess bioluminescence 10 minutes after injection of the substrate. Imaging data were analyzed with Living Image software (Xenogen).
Cell sorting and flow cytometry
Spleen cells were collected from naive mice or bone marrow transplantation (BMT) recipients and sorted on a FACSAria (Becton Dickinson, Mountain View, CA). Sort purity for these studies consistently averaged 98% to 99%. Cells were isolated from spleen, lymph nodes, and GVHD target organs (liver, lung, and colon) of transplant recipients as described previously23 and were labeled with monoclonal antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE-Cy5.5, or allophycocyanin (APC). FITC-anti-CD11c (clone HL3, hamster immunoglobulin (Ig)-G), PE-anti-CD11b (clone MV70, rat IgG2b), FITC-anti-H-2Kb (clone AF6-88.5, mouse IgG2a), PE-Cy5-anti-CD4 (clone RM4-5, rat IgG2a), PE-Cy5-anti-CD8 (clone 543-6.7, rat IgG2a), PE-anti-TCRβ (clone H57-597, hamster IgG), APC-anti-CD8a (clone 53-6.7, rat IgG2a), PE-anti-CD4 (clone GK1.5, rat IgG2b), FITC-anti-CD8a (clone 53-6.7, rat IgG2a), FITC-anti- interferon (IFN)-γ (clone XMG1.2, rat IgG1), and PE-anti-IL-17 (clone TC11-18H10, rat IgG1) were purchased from BD Biosciences (Franklin Lakes, NJ). FITC-anti-CCR9 (clone CW-1.2, mouse IgG2a), APC-anti-CCR9 (clone CW-1.2, mouse IgG2a), and PE-anti-α4β7 (clone DATK32, rat IgG2a) were obtained from eBioscience (San Diego, CA). Intracellular cytokine staining for IFN-γ and IL-17 was performed as described.23 In some experiments, cells were labeled with CellTrace violet (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Cells were analyzed on a FACSCalibur or LSRII flow cytometer with CellQuest or FACSDiva software (Becton-Dickinson). Data were analyzed using FlowJo software (TreeStar, Ashland, Oregon).
Histological analysis
Representative samples of liver, colon, tongue, and lung were obtained from transplant recipients and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-μm–thick sections, and stained with hematoxylin and eosin. One to 2 sections per organ were evaluated. A semiquantitative scoring system was used to account for histological changes in the colon, liver, and lung as previously described.30,31 The scoring system used to define the severity of GVHD in the tongue denoted 0 as normal, 1 as mononuclear cell infiltration into the dermis and stroma, 2 as inflammatory cells extending into the epithelium, and 3 as blistering or ulceration present in the epidermis. All slides were coded and read in a blinded fashion. Images were visualized using a Nikon Eclipse E400 microscope and a Nikon Plan APO 10×/0.45 objective lens (Nikon, Tokyo, Japan). Image acquisition was performed with a Zeiss Axiom camera and Axiovision 3.0.6 SP2 software (Zeiss, Berlin, Germany).
Mixed lymphocyte culture
B6 splenocytes (adjusted to yield a T-cell dose of 1 × 105 cells/well) were cocultured with 5 × 104 Balb/c dendritic cells in 96-well U-bottomed plates at 37°C for 4 days. Isolation of CD11c+ dendritic cells using the magnetic cell separation system (Miltenyi Biotech, Auburn, CA) has been described.32 One microcurie (0.037 MBq) of 3H-thymidine was added to triplicate wells for the final 12 to 18 hours prior to harvest. Thymidine incorporation was assessed using a Wallac 1450 Microbeta liquid scintillation counter (Perkin Elmer, Shelton, CT).
Real-time quantitative polymerase chain reaction
Real-time quantitative polymerase chain reaction (q-PCR) was performed using a QuantiTect SYBR Green PCR kit (Qiagen) and run in a CFX C1000 real-time thermal cycler (Bio-Rad, Hercules, CA). The 18S reference gene was amplified using a QuantiTect Primer assay kit (Qiagen, Valencia, CA). The primers for RAR-α have been reported33 and were purchased from Integrated DNA Technologies (Coralville, IA). Specificity for all q-PCR reactions was verified by melting curve analysis. To calculate fold change in gene expression, the average ΔΔCq values from triplicate wells were combined from separate experiments.
Quantification of RA in tissues by mass spectrometry
Excised tissues were weighed and all-trans RA-d5 (2 ng) was added to each sample as an internal standard. The tissues were homogenized in acetonitrile and prepared as previously described.34 RA was determined by liquid chromatography–electrospray ionization–mass spectrometry (Agilent 1100 LC-MSD, SL model). The samples were separated on an ACE 5 C18-PFP column, 250 × 2.1 mm 5μm (MAC MOD, Chadds Ford, PA) using water and acetonitrile containing 0.1% formic acid as a mobile phase. The concentrations of RA were calculated by comparing the ratio of peak areas to the standard curve.
Statistics
Data analysis was performed using Prism software (GraphPad, La Jolla, CA). Survival comparisons were performed using the log-rank test. Other differences between experimental groups were analyzed using a 2-tailed unpaired Student t test. A P value ≤ 0.05 was deemed to be significant in all experiments.
Results
Administration of RA selectively intensifies GVHD of the colon
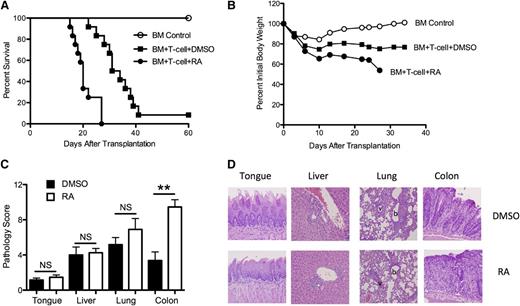
Initial experiments were performed to determine whether exogenous RA administration had any salutary effect on the severity of acute GVHD. Lethally irradiated Balb/c mice were transplanted with B6 BM alone or together with B6 spleen cells to induce GVHD. Cohorts of mice that received adjunctive spleen cells were then administered either DMSO (vehicle) or RA every other day beginning on the day of transplantation through day 20. The dose, route, and administration schedule were derived from previously published studies that had demonstrated potent immune effects in vivo.22,35 Unexpectedly, we observed significantly accelerated GVHD-associated mortality in RA-treated mice compared with DMSO-treated animals (P < .0001; Figure 1A). This was accompanied by more weight loss over the first 4 weeks, which corresponded to the period of time when both groups could be comparably assessed given the early mortality in RA-treated mice (Figure 1B). Histological examination of GVHD target organs (colon, liver, and lung) and the tongue, which served as a surrogate for skin damage,36 was performed 5 weeks after transplantation in separate experiments. There was a significant increase in pathologic damage in the colons of RA-treated mice compared with DMSO-treated animals (9.7 ± 0.8 vs 3.3 ± 0.9, P < .001), whereas there was no significant difference in pathology scores in the liver, tongue, or lung (Figure 1C-D). Administration of RA to animals reconstituted with BM only resulted in no mortality, and pathological analysis showed no evidence of GVHD in the colon at 90 days post transplantation (data not shown). Thus, the detrimental effect of RA was dependent upon the presence of alloreactive donor T cells. These studies demonstrate that exogenous RA administration exacerbates GVHD lethality and augments GVHD-associated damage within the colon microenvironment.
Administration of exogenous RA selectively intensifies GVHD of the colon. Lethally irradiated Balb/c mice received a transplant of 10 × 106 B6 BM alone (○) or together with B6 spleen cells (adjusted to yield a dose of 0.6 × 106 T cells). Cohorts of mice that received adjunctive spleen cells were then treated by intraperitoneal injection with either DMSO (▪) or RA (●)(450 μg/mouse) every other day beginning on the day of transplantation until day 20. (A) Overall survival. Data are cumulative results of 3 experiments (n = 6 for BM control group, n = 12 for both DMSO- and RA-treated groups). (B) Mean percent initial body weight of recipient mice transplanted as in panel A over the first 35 days. (C) Lethally irradiated Balb/c mice were transplanted with B6 BM and spleen cells as above with the exception that the spleen cell dose was adjusted to yield a dose of 0.3 × 106 T cells. Groups were then treated with either DMSO or RA. Mice were killed 5 weeks after transplantation. Pathological damage in the tongue, liver, lung, and colon was examined using a semiquantitative scoring system as described in the Material and Methods section. Data are presented as the mean (± standard error of the mean) and are the cumulative results from 3 experiments (n = 12 for both groups). Statistics: **P ≤ .01. (D) Histology of tongue, liver, lung, and colon from representative Balb/c recipients transplanted with B6 BM and spleen cells and treated with either DMSO or RA. Tongues in DMSO- and RA-treated mice reveal lymphocytic infiltration into the epidermis. Livers in the 2 groups show mononuclear cell infiltration in the portal triads, while lungs demonstrate both perivascular cuffing attributable to mononuclear cells and associated interstitial inflammation. Colon in DMSO-treated animals demonstrates loss of mucin and lamina propria inflammation but preservation of crypt cell architecture. Conversely, RA-treated mice show extensive loss of crypts with goblet cell depletion and inflammation extending into the muscle. Original magnification is 200× for liver, colon, and tongue images and 100× for lung images.
Administration of exogenous RA selectively intensifies GVHD of the colon. Lethally irradiated Balb/c mice received a transplant of 10 × 106 B6 BM alone (○) or together with B6 spleen cells (adjusted to yield a dose of 0.6 × 106 T cells). Cohorts of mice that received adjunctive spleen cells were then treated by intraperitoneal injection with either DMSO (▪) or RA (●)(450 μg/mouse) every other day beginning on the day of transplantation until day 20. (A) Overall survival. Data are cumulative results of 3 experiments (n = 6 for BM control group, n = 12 for both DMSO- and RA-treated groups). (B) Mean percent initial body weight of recipient mice transplanted as in panel A over the first 35 days. (C) Lethally irradiated Balb/c mice were transplanted with B6 BM and spleen cells as above with the exception that the spleen cell dose was adjusted to yield a dose of 0.3 × 106 T cells. Groups were then treated with either DMSO or RA. Mice were killed 5 weeks after transplantation. Pathological damage in the tongue, liver, lung, and colon was examined using a semiquantitative scoring system as described in the Material and Methods section. Data are presented as the mean (± standard error of the mean) and are the cumulative results from 3 experiments (n = 12 for both groups). Statistics: **P ≤ .01. (D) Histology of tongue, liver, lung, and colon from representative Balb/c recipients transplanted with B6 BM and spleen cells and treated with either DMSO or RA. Tongues in DMSO- and RA-treated mice reveal lymphocytic infiltration into the epidermis. Livers in the 2 groups show mononuclear cell infiltration in the portal triads, while lungs demonstrate both perivascular cuffing attributable to mononuclear cells and associated interstitial inflammation. Colon in DMSO-treated animals demonstrates loss of mucin and lamina propria inflammation but preservation of crypt cell architecture. Conversely, RA-treated mice show extensive loss of crypts with goblet cell depletion and inflammation extending into the muscle. Original magnification is 200× for liver, colon, and tongue images and 100× for lung images.
RA enhances gut-homing molecule expression on donor T cells in mesenteric lymph nodes
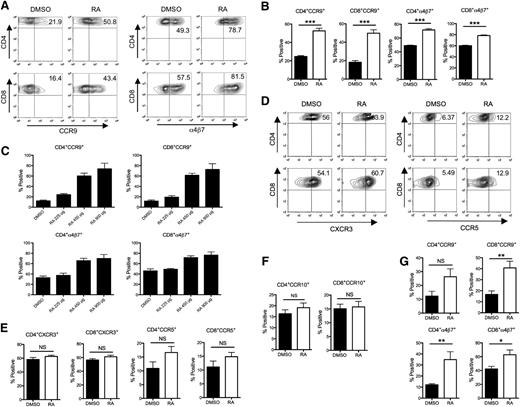
The acquisition of tissue-homing molecule expression on T cells after activation in secondary lymphoid tissues is important in determining the subsequent migration pattern of effector T cells into nonlymphoid tissues.37,38 Recent studies have demonstrated that an important biological function of RA is to induce the expression of gut-homing molecules, α4β7 and CCR9, on T cells under steady-state conditions.24 Since we observed a significant enhancement of colonic damage in RA-treated mice, we sought to determine whether RA exposure resulted in increased expression of these molecules. We first addressed this question using a mixed lymphocyte culture to mimic the in vivo alloimmune response. Culture in the presence of physiological concentrations of RA (10 nM) resulted in increased T-cell proliferation (supplemental Figure 1A). There was also decreased expression of CD62L and increased expression of CD25 on CD4+ and CD8+ T cells, indicative of an activated T-cell phenotype (supplemental Figure 1B). More importantly, in the presence of RA, there was a dose-dependent increase in the expression of CCR9 and α4β7 on CD4+ and CD8+ T cells (supplemental Figure 1C). To determine whether RA affects T-cell proliferation in the absence of alloreactivity, we performed syngeneic BMT and examined early T-cell division in syngeneic BMT recipients. Recipients of syngeneic marrow grafts that were administered RA had no increase in donor T-cell proliferation, indicating that RA did not enhance T-cell division under conditions of homeostatic expansion (supplemental Figure 2).
RA enhances the expression of α4β7 and CCR9 on donor T cells in mesenteric lymph nodes. Lethally irradiated Balb/c mice were transplanted with B6 BM and spleen cells and treated with either DMSO or RA. Mice from both groups were killed at day 7 after BMT. The expression of integrin and chemokine receptors on donor T cells isolated from different secondary lymphoid tissues of recipient mice was examined by gating on donor-derived H-2Kb+ CD4+ or H-2Kb+ CD8+ cells. (A) Representative contour plots for CCR9 and α4β7 expression on donor T cells isolated from MLNs. (B) Mean percent positive (± SEM) donor T cells expressing CCR9 and α4β7 isolated from MLNs (n = 6-8 per group). Statistics: ***P ≤ .001. (C) Recipient mice were treated with either DMSO or escalating doses of RA, the expression of CCR9 and α4β7 on donor T cells from the MLNs of recipient mice 7 days after BMT is depicted (n = 3 per group). Data are from 1 of 2 representative experiments. (D) Representative contour plots for CXCR3 and CCR5 expression on donor T cells isolated from spleen. (E) Mean percent positive (± SEM) donor T cells expressing CXCR3 and CCR5 isolated from spleen (n = 6-8 per group). (F, G) Mean percent positive (± SEM) donor T cells expressing CCR10 (F) and gut-homing molecules (G) isolated from peripheral lymph nodes (n = 6-8 per group). Data are combined results from two independent experiments. Statistics: *P ≤ .05, **P < .01.
RA enhances the expression of α4β7 and CCR9 on donor T cells in mesenteric lymph nodes. Lethally irradiated Balb/c mice were transplanted with B6 BM and spleen cells and treated with either DMSO or RA. Mice from both groups were killed at day 7 after BMT. The expression of integrin and chemokine receptors on donor T cells isolated from different secondary lymphoid tissues of recipient mice was examined by gating on donor-derived H-2Kb+ CD4+ or H-2Kb+ CD8+ cells. (A) Representative contour plots for CCR9 and α4β7 expression on donor T cells isolated from MLNs. (B) Mean percent positive (± SEM) donor T cells expressing CCR9 and α4β7 isolated from MLNs (n = 6-8 per group). Statistics: ***P ≤ .001. (C) Recipient mice were treated with either DMSO or escalating doses of RA, the expression of CCR9 and α4β7 on donor T cells from the MLNs of recipient mice 7 days after BMT is depicted (n = 3 per group). Data are from 1 of 2 representative experiments. (D) Representative contour plots for CXCR3 and CCR5 expression on donor T cells isolated from spleen. (E) Mean percent positive (± SEM) donor T cells expressing CXCR3 and CCR5 isolated from spleen (n = 6-8 per group). (F, G) Mean percent positive (± SEM) donor T cells expressing CCR10 (F) and gut-homing molecules (G) isolated from peripheral lymph nodes (n = 6-8 per group). Data are combined results from two independent experiments. Statistics: *P ≤ .05, **P < .01.
To further evaluate the acquisition of gut-homing molecule expression by RA exposure in vivo, DMSO- or RA-treated animals were killed on day 7 after transplantation and examined for the expression of tissue-homing molecules on donor T cells in secondary lymphoid tissues. Expression of CCR9 and α4β7 was significantly higher on donor T cells (both CD4+ and CD8+) isolated from the MLNs of RA-treated mice in comparison with DMSO-treated animals (Figure 2A-B). The augmentation of CCR9 and α4β7 expression by RA was also observed to be dose dependent (Figure 2C). Expression of other chemokine receptors that have been implicated in GVHD pathogenesis, such as CCR5, CXCR3, and CCR1039,40 in other secondary lymphoid tissues, was not affected by RA treatment (Figure 2D-F). Notably, we observed that there was also an increase in the percentage of donor T cells that expressed CCR9 and α4β7 in peripheral lymph nodes, suggesting that exogenous RA could affect expression of gut-homing molecules at nodal sites distant from the mesentery (Figure 2G). Collectively, these data indicated that RA treatment enhances the priming of gut-homing donor T cells in the MLNs but does not affect the acquisition of chemokine receptors on T cells designated for other GVHD target organs.
Effect of RA administration on proinflammatory donor T cells and Tregs within the colon microenvironment
Since we observed a significantly higher number of T cells expressing gut-homing molecules in MLNs after RA administration, we sought to determine if this led to increased recruitment of proinflammatory donor T cells into the colon in the later stage of GVHD. To examine the effect of RA more specifically in GVHD target tissues, we isolated mononuclear cells from the spleen, liver, lung, and colon of recipient mice treated with either DMSO or RA 3 weeks after transplantation. There was a significant 8-fold increase in the absolute numbers of both CD4+IFN-γ+ (TH1; mean 0.08 × 104 vs 0.01 × 104) and CD4+IL-17+ (TH17; mean 0.42 × 103 vs 0.05 × 103) in the colon of RA-treated mice vs DMSO-treated animals, whereas there were no statistical differences in TH1 or TH17 cells in the spleen, liver, and lung (Figure 3A-B). The absolute number of CD8+CD103+ cells, which has been shown to represent a specific gut-destructive T-cell population,41 was also significantly augmented (9-fold) in the colons of RA-treated mice (mean 1.0 × 103 vs 0.11 × 103; Figure 3C). We also examined the effects of RA on Treg reconstitution using Foxp3EGFP reporter mice as donors for transplantation. We observed that there was a significant increase in the absolute number of Tregs in the colon of RA-treated mice as well (Figure 3D). However, the increase in Tregs (3-fold, mean 0.022 × 104 vs 0.07 × 104) was noted to be more modest than was observed for TH1, TH17, and CD8+ T cells. Notably, there was a corresponding increase in the percentage of CD4+ and CD8+ T cells that expressed α4β7 in the colon, indicating that these cells are indeed able to traffic to the colon. Since CCR9 has been shown to be important in directing T-cell trafficking into the small intestines, we examined this tissue site and observed an increased percentage of CCR9-expressing T cells in RA-treated mice (Figure 3E). Collectively, these data indicate that RA administration results in a significant increase in the absolute number of proinflammatory donor T cells in the colon without a proportional increase in the Treg compartment, resulting in a more pronounced imbalance between the effector and regulatory arms of the immune system.
Effect of exogenous RA administration on effector and regulatory T-cell infiltration into the colon. Lethally irradiated Balb/c mice were transplanted with 10 × 106 B6 BM together with B6 spleen cells (adjusted to yield a T-cell dose of 0.6 × 106). Cohorts of mice were then treated with either DMSO (n = 6 to 8/group, black bars) or RA (n = 6 to 8/group, white bars). (A) Absolute cell number of CD4+IFN-γ+ or (B) CD4+IL-17+ T cells in the spleen, liver, lung, and colon of animals treated with either DMSO or RA and then killed 19 to 21 days after transplantation. (C) Absolute cell number of CD8+CD103+ cells in the colon of recipient mice. Data are presented as the mean (± standard error of the mean [SEM]) and are the cumulative results from 2 independent experiments. (D) Lethally irradiated Balb/c mice were transplanted with 10 × 106 Foxp3EGFP BM and spleen cells (adjusted to yield a T-cell dose of 0.6 × 106). Cohorts of mice were then treated with either DMSO (n = 8) or RA (n = 8). Mice in both groups were killed 19 to 21 days after transplantation. The absolute cell number of Foxp3+ (GFP+) Tregs in the spleen, liver, and colon of mice is depicted. Data are presented as the mean (± SEM) and are the cumulative results from 2 independent experiments. (E) Percentage of CD4+ and CD8+ T cells that expressed either α4β7 in the colon or CCR9 in the small intestines of transplant recipients that were treated with either DMSO or RA. Animals were killed 19 to 21 days post transplantation. Data are presented as the mean (± SEM) and are the cumulative results from 2 independent experiments. Statistics: *P ≤ .05, **P < .01, ***P < .001.
Effect of exogenous RA administration on effector and regulatory T-cell infiltration into the colon. Lethally irradiated Balb/c mice were transplanted with 10 × 106 B6 BM together with B6 spleen cells (adjusted to yield a T-cell dose of 0.6 × 106). Cohorts of mice were then treated with either DMSO (n = 6 to 8/group, black bars) or RA (n = 6 to 8/group, white bars). (A) Absolute cell number of CD4+IFN-γ+ or (B) CD4+IL-17+ T cells in the spleen, liver, lung, and colon of animals treated with either DMSO or RA and then killed 19 to 21 days after transplantation. (C) Absolute cell number of CD8+CD103+ cells in the colon of recipient mice. Data are presented as the mean (± standard error of the mean [SEM]) and are the cumulative results from 2 independent experiments. (D) Lethally irradiated Balb/c mice were transplanted with 10 × 106 Foxp3EGFP BM and spleen cells (adjusted to yield a T-cell dose of 0.6 × 106). Cohorts of mice were then treated with either DMSO (n = 8) or RA (n = 8). Mice in both groups were killed 19 to 21 days after transplantation. The absolute cell number of Foxp3+ (GFP+) Tregs in the spleen, liver, and colon of mice is depicted. Data are presented as the mean (± SEM) and are the cumulative results from 2 independent experiments. (E) Percentage of CD4+ and CD8+ T cells that expressed either α4β7 in the colon or CCR9 in the small intestines of transplant recipients that were treated with either DMSO or RA. Animals were killed 19 to 21 days post transplantation. Data are presented as the mean (± SEM) and are the cumulative results from 2 independent experiments. Statistics: *P ≤ .05, **P < .01, ***P < .001.
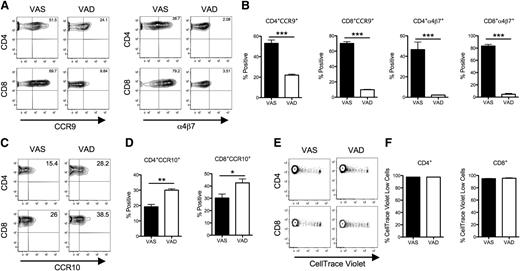
Vitamin-A deficiency in recipient mice impairs the acquisition of gut-homing molecule expression on donor T cells after allogeneic BMT
Since exogenous RA administration augmented the generation of gut-homing T cells, we examined whether depletion of RA would result in a corresponding reduction in these cells as additional confirmation for the role of this metabolite in GVHD. To address this question, we generated vitamin A–deficient mice and used them as recipients in this study. Due to superior breeding on a vitamin A–restricted diet, we used Balb/c Rag mice, not Balb/c mice. Lethally irradiated VAS or VAD Balb/c Rag mice were transplanted with B6 BM and spleen T cells to induce GVHD. Three days after transplantation, a sizable proportion of donor T cells from the MLNs of VAS mice expressed α4β7 and CCR9, whereas expression of these gut-homing receptors was significantly reduced on donor CD4+ and CD8+ T cells from VAD mice (Figure 4A-B). Interestingly, expression of the skin-homing molecule CCR10 in the peripheral lymph nodes (PLNs) was increased in the VAD group (Figure 4C-D), suggesting that vitamin A may have divergent actions on chemokine receptor expression. To determine whether vitamin A deficiency affected alloreactive T-cell proliferation, B6 donor T cells were labeled with CellTrace-violet dye and adoptively transferred along with unlabeled BM cells into lethally irradiated VAS or VAD recipients. Splenic donor CD4+ and CD8+ T cells were then examined 3 days post transplantation, a point at which prior studies have shown that robust cell division occurs in the spleen.42 There was no significant difference in the percentage of CellTrace-violet low-donor CD4+ and CD8+ T cells between the 2 groups (Figure 4E-F), indicating that lack of vitamin A did not appear to affect T-cell proliferation. Consistent with an earlier report by Koenecke and colleagues,43 we also observed that VAD recipients had more severe GVHD in the liver and increased splenic cellularity (supplemental Figure 3). These data indicate that vitamin A deficiency in recipient mice impairs the acquisition of gut-homing molecules on donor T cells in MLNs and further reinforces a role for RA in imprinting gut-homing donor T cells during GVHD.
Vitamin A deprivation in recipient mice impairs the acquisition of gut-homing molecule expression on donor T cells after BMT. Lethally irradiated Balb/c Rag mice fed with either VAS or VAD diets were transplanted with B6 BM and CellTrace-violet–labeled splenic T cells (adjusted to yield a T-cell dose of 1 × 106). Mice from both groups were killed on day 3 after BMT. (A) Representative contour plots of CCR9 and α4β7 expression on donor T cells isolated from the MLNs of recipient mice after gating on H-2Kb+ CD4+ or H-2Kb+ CD8+ cells. (B) The mean percentage positive donor T cells (± SEM) expressing CCR9 and α4β7 from 4 mice per group. Data are from 1 of 2 representative experiments. Statistics: ***P ≤ .001. (C) Representative contour plots of CCR10 expression on donor T cells isolated from the PLNs of recipient mice. (D) The mean percentage positive donor T cells (± SEM) expressing CCR10 from 4 mice per group. Data are from 1 of 2 representative experiments. Statistics: *P ≤ .05, **P < .01. (E) Representative example of CellTrace-violet-labeled donor H-2Kb+ CD4+ or CD8+ T-cell proliferation in the spleen of VAS and VAD recipients 3 days after transplantation. (F) Mean (± SEM) percentage of proliferating (CellTrace-violet low) splenic donor T cells (n = 4 mice per group). One of 2 representative experiments is shown.
Vitamin A deprivation in recipient mice impairs the acquisition of gut-homing molecule expression on donor T cells after BMT. Lethally irradiated Balb/c Rag mice fed with either VAS or VAD diets were transplanted with B6 BM and CellTrace-violet–labeled splenic T cells (adjusted to yield a T-cell dose of 1 × 106). Mice from both groups were killed on day 3 after BMT. (A) Representative contour plots of CCR9 and α4β7 expression on donor T cells isolated from the MLNs of recipient mice after gating on H-2Kb+ CD4+ or H-2Kb+ CD8+ cells. (B) The mean percentage positive donor T cells (± SEM) expressing CCR9 and α4β7 from 4 mice per group. Data are from 1 of 2 representative experiments. Statistics: ***P ≤ .001. (C) Representative contour plots of CCR10 expression on donor T cells isolated from the PLNs of recipient mice. (D) The mean percentage positive donor T cells (± SEM) expressing CCR10 from 4 mice per group. Data are from 1 of 2 representative experiments. Statistics: *P ≤ .05, **P < .01. (E) Representative example of CellTrace-violet-labeled donor H-2Kb+ CD4+ or CD8+ T-cell proliferation in the spleen of VAS and VAD recipients 3 days after transplantation. (F) Mean (± SEM) percentage of proliferating (CellTrace-violet low) splenic donor T cells (n = 4 mice per group). One of 2 representative experiments is shown.
RAR-α is critical for mediating the effects of RA in alloreactive T cells
Two distinct families of nuclear receptors for RA have been identified: the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs), both of which have 3 isoforms known as α, β, and γ.44 Since previous studies indicated a dominant role of RAR-α in mediating the effects of RA on T cells during adaptive immune responses,21,28 we focused our studies on this RAR subfamily. We used a pharmacologic agonist and antagonist specific for RAR-α to dissect the role of this pathway in mediating the effects of RA. B6 T cells were cocultured with Balb/c dendritic cells (DCs) in the presence or absence of the RAR-α agonist, AM80. We found that AM80 significantly enhanced expression of the gut-homing molecules CCR9 and α4β7 on both CD4+ and CD8+ alloreactive T cells (Figure 5A-B), indicating that direct targeting of the RAR-α signaling pathway can mimic the effect of RA on alloreactive T cells. To further confirm the involvement of RAR-α, B6 spleen cells were cocultured with purified Balb/c DCs in the presence of RA alone or together with an RAR-α–specific antagonist, BMS195614. We observed that BMS195614 significantly inhibited the upregulation of CCR9 and α4β7 on CD4+ and CD8+ T cells that is induced by RA (Figure 5C). Furthermore, addition of BMS195614 resulted in increased expression of CD62L and decreased expression of CD25, indicating that RAR-α blockade led to a reduction in the percentage of T cells with an activated phenotype (Figure 5D). Finally, BMS195614 abrogated the RA-induced increase in T-cell proliferation (Figure 5E). These studies demonstrate that an RAR-α agonist could replicate the augmentation of gut-homing molecule expression observed with RA and that this effect could be blocked by specific antagonism of the RAR-α pathway.
RAR-α signaling is important in mediating the effects of RA on T cells. B6 spleen cells (adjusted to 1 × 105 T cells/well) were cocultured with 5 × 104 Balb/c dendritic cells in the presence or absence of 1 μM AM80, an RAR-α agonist. Four days later, cells were harvested for flow cytometric analysis. (A) Representative contour plots of α4β7 and CCR9 expression on CD4+ and CD8+ T cells. (B) Mean (± standard error of the mean [SEM]) percentage of CD4+ and CD8+ T cells that expressed α4β7 and CCR9. Data are cumulative results from 4 independent experiments. Statistics: *P ≤ .05, ***P < .001. (C, D) B6 spleen cells (adjusted to 1 × 105 T cells/well) were cocultured with 5 × 104 Balb/c dendritic cells in the presence of 10 nM exogenous RA. An RAR-α antagonist, BMS195614 (1 μM), was added to some cultures 1 hour prior to the addition of RA. Cells were analyzed after 4 days in culture. Representative contour plots of CCR9, α4β7, CD62L, and CD25 expression on CD4+ and CD8+ T cells is depicted. Data are from 1 of 4 representative experiments with similar results. (E) Cells were cultured as in panels C and D with the exception of 3H-thymidine was added for the last 18 hours to assess proliferation. Data are presented as mean counts per minute ± SEM and are representative of 1 of 2 experiments with similar results. Statistics: *P ≤ .05
RAR-α signaling is important in mediating the effects of RA on T cells. B6 spleen cells (adjusted to 1 × 105 T cells/well) were cocultured with 5 × 104 Balb/c dendritic cells in the presence or absence of 1 μM AM80, an RAR-α agonist. Four days later, cells were harvested for flow cytometric analysis. (A) Representative contour plots of α4β7 and CCR9 expression on CD4+ and CD8+ T cells. (B) Mean (± standard error of the mean [SEM]) percentage of CD4+ and CD8+ T cells that expressed α4β7 and CCR9. Data are cumulative results from 4 independent experiments. Statistics: *P ≤ .05, ***P < .001. (C, D) B6 spleen cells (adjusted to 1 × 105 T cells/well) were cocultured with 5 × 104 Balb/c dendritic cells in the presence of 10 nM exogenous RA. An RAR-α antagonist, BMS195614 (1 μM), was added to some cultures 1 hour prior to the addition of RA. Cells were analyzed after 4 days in culture. Representative contour plots of CCR9, α4β7, CD62L, and CD25 expression on CD4+ and CD8+ T cells is depicted. Data are from 1 of 4 representative experiments with similar results. (E) Cells were cultured as in panels C and D with the exception of 3H-thymidine was added for the last 18 hours to assess proliferation. Data are presented as mean counts per minute ± SEM and are representative of 1 of 2 experiments with similar results. Statistics: *P ≤ .05
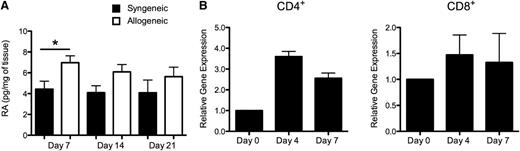
Endogenous RA production and gene expression of RAR-α in donor CD4+ T cells are enhanced after allogeneic BMT
While the above studies established that exogenous RA significantly exacerbated GVHD within the colon microenvironment, it is unclear how endogenous RA production and RAR-α expression on donor T cells contribute to the pathophysiology of GVHD. To address these questions, we measured endogenous RA levels in the colons of mice undergoing either syngeneic or allogeneic BMT. These studies revealed that RA was significantly increased in the colon tissue of allogeneic transplant recipients compared with syngeneic transplant recipients early after BMT (day 7; Figure 6A). Thereafter, there was a trend toward higher RA levels in recipients of allogeneic vs syngeneic marrow grafts; however, this did not reach statistical significance at these later time points. Given prior results supporting a role for the RAR-α signaling pathway in mediating the effects of RA (Figure 5), we examined whether RAR-α gene expression levels were augmented in donor T cells during GVHD. Real time q-PCR analysis revealed a mean 3.5-fold increase in RAR-α expression in sort-purified donor splenic CD4+ T cells 4 days post transplantation when compared with purified naive CD4+ T cells obtained from original donor animals (Figure 6B). Gene expression levels were also noted to be higher 7 days after BMT, although somewhat less than at the earlier time point. In contrast, mRNA levels of RAR-α in purified CD8+ T cells obtained from the spleens of GVHD animals did not significantly differ from levels observed in naive CD8+ T cells from original donor mice (Figure 6B). Collectively, these results indicate that endogenous RA levels are increased early during GVHD in the colon and that there is also concomitant increased expression of RAR-α in donor CD4+ T cells.
Endogenous RA production and RAR signaling on donor T cells are enhanced after allogeneic BMT. (A) Lethally irradiated Balb/c mice were transplanted with Balb/c BM (10 × 106) and spleen cells (0.4–0.5 × 106, syngeneic) or B6 BM and an equivalent number of B6 spleen cells (allogeneic). Cohorts of animals were killed weekly, and colon tissue was analyzed for RA levels by mass spectrometric analysis as described in the section titled “Quantification of RA in tissues by mass spectrometry.” Data are derived from 9 to 10 mice per group and are cumulative results from 2 independent experiments. Statistics: *P ≤ .05. (B) Lethally irradiated Balb/c mice were transplanted with B6 BM and spleen cells to induce GVHD. RNA was extracted from sort-purified CD4+ and CD8+ T cells isolated from spleens of allogeneic transplant recipients at the indicated time points after BMT, and gene expression of RAR-α was analyzed by real-time q-PCR as described in the section titled “Real-time q-PCR.” Data are normalized for 18S ribosomal RNA and presented as fold increase over RAR-α expression in sort-purified original naive CD4+ or CD8+ donor T cells used for transplantation. Data are derived from 2 independent experiments and are presented as the mean ± standard error of the mean.
Endogenous RA production and RAR signaling on donor T cells are enhanced after allogeneic BMT. (A) Lethally irradiated Balb/c mice were transplanted with Balb/c BM (10 × 106) and spleen cells (0.4–0.5 × 106, syngeneic) or B6 BM and an equivalent number of B6 spleen cells (allogeneic). Cohorts of animals were killed weekly, and colon tissue was analyzed for RA levels by mass spectrometric analysis as described in the section titled “Quantification of RA in tissues by mass spectrometry.” Data are derived from 9 to 10 mice per group and are cumulative results from 2 independent experiments. Statistics: *P ≤ .05. (B) Lethally irradiated Balb/c mice were transplanted with B6 BM and spleen cells to induce GVHD. RNA was extracted from sort-purified CD4+ and CD8+ T cells isolated from spleens of allogeneic transplant recipients at the indicated time points after BMT, and gene expression of RAR-α was analyzed by real-time q-PCR as described in the section titled “Real-time q-PCR.” Data are normalized for 18S ribosomal RNA and presented as fold increase over RAR-α expression in sort-purified original naive CD4+ or CD8+ donor T cells used for transplantation. Data are derived from 2 independent experiments and are presented as the mean ± standard error of the mean.
Recipients of RAR-α–deficient T cells are protected from GVHD and retain the ability to mount a graft-versus-leukemia response
To further define the role of the RAR-α signaling pathway in GVHD pathogenesis, we examined the ability of RAR-α–deficient T cells, which lack the capacity to respond to endogenous RA, to cause GVHD. BM and splenocytes from RAR-α–deficient (RAR-α─/─) mice or wild-type (WT, RAR-α+/+) littermates were transplanted into lethally irradiated Balb/c mice. Recipients of RAR-α–deficient marrow grafts had decreased GVHD-associated mortality when compared with the recipients of WT T cells (93% vs 36%, P = .002; Figure 7A). Moreover, mice that received RAR-α─/─ T cells had significantly less GVHD-associated weight loss (data not shown). Moreover, histological examination of GVHD target organs 10 to 12 days after transplantation revealed a significant reduction in pathologic damage in the colon of mice that received RAR-α─/─ T cells compared with control mice (2.1 ± 0.7 vs 8.4 ± 1.0, P < .001; Figure 7B), whereas there was no difference in the liver. We did observe more pathological damage in the lung in recipients of RAR-α─/─ grafts, which was due exclusively to increased perivascular cuffing without any parenchymal damage. Early post transplantation, there was a significant decrease in the percentage of TH1 cells, but not TH17 cells, in the spleens of animals reconstituted with RAR-α─/─ marrow grafts (supplemental Figure 4). Quantification of donor T cells in GVHD target organs revealed a significant and selective reduction in the absolute number of donor CD4+ and CD8+ T cells in the colons of recipients of RAR-α─/─ T cells. There was a corresponding increase in the absolute number of donor CD4+ and CD8+ T cells in the lung and spleen (Figure 7C-D). The observed differences in GVHD-induced mortality could not be ascribed to any preexisting differences in the T-cell compartment of the donor mice because there was no difference in the percentage of CD4+, CD8+, and CD4+Foxp3+ T cells in the spleen between naive RAR-α─/─ and WT littermate controls (supplemental Figure 5A-B). There was also no difference in proliferation of CD4+ or CD8+ T cells in response to alloantigen between the 2 donor types (supplemental Figure 5C). These results demonstrate that abrogation of RA signaling through RAR-α in donor T cells markedly diminished their capacity to cause lethal GVHD and that this was attributable to selective protection in the colon. Despite the reduction in GVHD, animals transplanted with marrow grafts from RAR-α─/─ donors retained their ability to mount a graft-versus-leukemia (GVL) response and had significantly longer survival when compared with leukemia or GVHD control mice (Figure 7E). Moreover, bioluminescence imaging studies confirmed that these animals were free of tumor at the conclusion of these studies.
Recipients of RAR-α–deficient T cells are protected from lethal GVHD yet are able to mount a GVL response. Lethally irradiated Balb/c mice were transplanted with BM cells (5 × 106) from either RAR-α─/─ (□) or wild type (WT) littermate controls (○) or BM and spleen cells (adjusted to yield a T-cell dose of 0.7−1.0 × 106 T cells) from either RAR-α─/─ (▪) or WT littermate controls (●). (A) Overall survival. Data are cumulative results from 3 independent experiments (n = 3 for BM control groups, n = 14 for each BM + T-cell group). (B) Lethally irradiated Balb/c mice were transplanted with BM (5 × 106) and spleen cells (adjusted to yield a T-cell dose of 2.1 × 106) from either RAR-α─/─ (n = 9) or WT littermate controls (n = 5). Mice were killed at days 10 to 12 after BMT and pathological damage in the liver, lung, and colon was examined. Data are derived from 2 independent experiments and are presented as the mean (± standard error of the mean [SEM]). Representative photomicrographs of the colon from mice transplanted with WT vs RAR-α─/─ marrow grafts are shown. Colon from recipients of WT grafts show loss of mucin and destruction of crypt architecture, while animals reconstituted with grafts from RAR-α─/─ mice have normal-appearing crypts. Statistics: *P ≤ .05, ***P ≤ .001. (C,D) The absolute number of donor-derived CD4+ (C) and CD8+ cells (D) in the liver, spleen, lung, and colon is shown for each group. Data are derived from 2 independent experiments and are presented as the mean (± SEM). (E) Lethally irradiated Balb/c mice were administered 0.6 × 106 A20 cells transfected with firefly luciferase (A20-luc) and then transplanted with WT BM alone (n = 5), WT BM plus spleen cells (adjusted to yield a dose of 1.2 × 106 T cells; n = 5), or RAR-α─/─ BM plus spleen cells (adjusted to yield an equivalent T cell dose; n = 5). Overall survival and whole body distribution of A20-luc cells using in vivo BLI is depicted.
Recipients of RAR-α–deficient T cells are protected from lethal GVHD yet are able to mount a GVL response. Lethally irradiated Balb/c mice were transplanted with BM cells (5 × 106) from either RAR-α─/─ (□) or wild type (WT) littermate controls (○) or BM and spleen cells (adjusted to yield a T-cell dose of 0.7−1.0 × 106 T cells) from either RAR-α─/─ (▪) or WT littermate controls (●). (A) Overall survival. Data are cumulative results from 3 independent experiments (n = 3 for BM control groups, n = 14 for each BM + T-cell group). (B) Lethally irradiated Balb/c mice were transplanted with BM (5 × 106) and spleen cells (adjusted to yield a T-cell dose of 2.1 × 106) from either RAR-α─/─ (n = 9) or WT littermate controls (n = 5). Mice were killed at days 10 to 12 after BMT and pathological damage in the liver, lung, and colon was examined. Data are derived from 2 independent experiments and are presented as the mean (± standard error of the mean [SEM]). Representative photomicrographs of the colon from mice transplanted with WT vs RAR-α─/─ marrow grafts are shown. Colon from recipients of WT grafts show loss of mucin and destruction of crypt architecture, while animals reconstituted with grafts from RAR-α─/─ mice have normal-appearing crypts. Statistics: *P ≤ .05, ***P ≤ .001. (C,D) The absolute number of donor-derived CD4+ (C) and CD8+ cells (D) in the liver, spleen, lung, and colon is shown for each group. Data are derived from 2 independent experiments and are presented as the mean (± SEM). (E) Lethally irradiated Balb/c mice were administered 0.6 × 106 A20 cells transfected with firefly luciferase (A20-luc) and then transplanted with WT BM alone (n = 5), WT BM plus spleen cells (adjusted to yield a dose of 1.2 × 106 T cells; n = 5), or RAR-α─/─ BM plus spleen cells (adjusted to yield an equivalent T cell dose; n = 5). Overall survival and whole body distribution of A20-luc cells using in vivo BLI is depicted.
Discussion
Severe GVHD of the gastrointestinal tract is primarily responsible for much of the morbidity and mortality that is observed in experimental murine models and BMT patients. In this report, we identified RA as a novel and critical factor in the induction of gastrointestinal GVHD. In gain-of-function studies, we found that exposure of recipient mice to exogenous RA not only failed to ameliorate the severity of GVHD, as we had initially postulated, but selectively exacerbated colonic GVHD, resulting in significantly increased mortality in recipient mice. This observation was associated with enhanced generation of gut-tropic, donor T cells in the MLNs early after BMT and an increased influx of proinflammatory donor T cells into the gut mucosa during later stages of disease development. Conversely, vitamin A deprivation, which resulted in RA deficiency in recipient mice, inhibited the acquisition of the gut-homing molecules CCR9 and α4β7 on donor T cells after BMT, confirming a role for RA in this process. Importantly, genetic ablation of RAR-α signaling on donor T cells markedly attenuated the ability of these cells to cause GVHD lethality and, more specifically, colonic GVHD, which was associated with a significant reduction in donor CD4+ and CD8+ T-cell accumulation in this tissue. Thus, these results demonstrate that RA facilitates the initiation and progression of gastrointestinal GVHD and thereby has an important proinflammatory role in GVHD biology.
Trafficking of donor T cells after in vivo infusion is an important aspect of GVHD pathophysiology since these cells exert their pathogenic effect only after infiltrating into target organs.39 The acquisition of gut-homing molecules on donor T cells is essential for them to migrate specifically to the gut mucosa. The important role of the integrin α4β7 in the pathogenesis of gastrointestinal GVHD has been well demonstrated in experimental BMT models. Specifically, Petrovic and colleagues45 showed that recipients of sort-purified α4β7-negative donor T cells had significantly less GVHD-associated morbidity and mortality compared with recipients of α4β7-positive donor T cells. This phenomenon was associated with a selective reduction of pathological damage in the intestine and liver but not other target organs, demonstrating an important role of α4β7 in the selective migration of donor T cells to the intestinal mucosa.39,45 In addition, similar studies were performed using β7-deficient donor T cells where it was confirmed that this molecule is critically involved in the development of intestinal GVHD.46,47 Clinically, upregulation of α4β7 on peripheral blood T cells has been associated with the subsequent development of intestinal GVHD in allogeneic HSCT recipients.48 Although the importance of α4β7 in the pathogenesis of colonic GVHD has been established, it is unclear how the expression of this molecule on donor T cells is regulated during GVHD. Our studies extend these previous findings and now show that the expression of gut-homing molecules that are critically involved in the induction of intestinal GVHD is regulated by RA.
The observation that RA dose-dependently augmented the induction of gut-homing molecule expression on donor T cells indicates that the bioavailability of RA in vivo during donor T-cell activation profoundly influences the generation of donor T cells that are destined to migrate to the gut and initiate colonic GVHD. Notably, RA treatment did not affect the expression of other chemokine receptors mediating T-cell migration to other tissues. For example, the expression of CCR10, which directs T-cell migration into the skin, was not altered on donor T cells isolated from PLNs after RA exposure. The same was true for the expression of CCR5 and CXCR3 on alloreactive T cells primed at other secondary lymphoid tissues such as spleen. Interestingly, RA administration was associated with increased expression of α4β7 and CCR9 on T cells in peripheral lymph nodes, raising the possibility that RA may be able to direct T cells into the GI tract from distal nodal sites as well. Thus, RA appears to play a dominant and selective role in regulating gut immunity during the alloimmune response, although we cannot exclude the possibility that other chemokine receptor expression could also be regulated by RA after prolonged exposure. It was noteworthy that we observed an increased number of donor T cells in the spleen and lung in recipients of RAR-α─/─ grafts. While the splenic data, in particular, may have been attributable to enhanced T-cell reconstitution in mice with reduced GVHD, an alternative possibility is that altered trafficking away from the colon led to higher levels of donor T cells in the circulation and was manifested as augmented perivascular cuffing in the lung and T-cell recovery in the spleen.
RA has been generally accepted to have an immunoregulatory role because of its ability to drive T-cell differentiation toward the Treg pathway17,-19 and has been proposed as a crucial factor in the induction of oral tolerance and the maintenance of intestinal homeostasis.11 However, emerging evidence suggests that RA is also essential for the activation and function of effector T cells and may play a proinflammatory role under certain pathogenic conditions.28,49,50 For example, RA was shown to promote TH1 polarization and inhibit Treg induction in the presence of the proinflammatory cytokine IL-15 in a murine celiac disease model.50 Therefore, RA can function as either an antiinflammatory or a proinflammatory molecule that appears to depend, in part, upon the existing cytokine milieu. It is noteworthy that we did observe an increase in the absolute number of Tregs in the colons of animals that were treated with exogenous RA. However, the magnitude of Treg expansion was substantially less than that observed for the corresponding proinflammatory effector T-cell populations and was insufficient to prevent increased pathological damage within the colon. Thus, we infer from these data that the proinflammatory effects of RA outweighed any salutary effects on Treg reconstitution during GVHD.
Despite the well-known effect of RA in imprinting gut-homing molecules on T cells under steady-state conditions, our studies demonstrate that this functional property of RA is indispensable even in a systemic inflammatory disease such as GVHD. The finding that imprinting of gut-homing molecules on donor T cells was significantly impaired in vitamin A–deficient mice indicates that although irradiation and the alloimmune response induce the secretion of many proinflammatory cytokines, RA plays a nonredundant role in generating gut-homing donor T cells during GVHD. These results are consistent with a recent publication by Koenecke and colleagues43 who showed that vitamin A deficiency resulted in reduced expression of CCR9 and α4β7 on donor T cells and accumulation of these cells in the intestines during GVHD. Rodent models of BMT use highly inbred experimental mice that are typically fed with commercially available diets that are well supplemented with vitamin A. This is in contrast to humans who consume highly variable diets and who therefore may have more variability in nutritional status and hence vitamin A levels. Our results suggest that recipient vitamin A levels could be a heretofore unrecognized variable that influences the severity of GVHD in the gastrointestinal tract after allogeneic BMT. Future studies will be of interest to determine if there is a correlation between recipient vitamin A levels and the severity of GVHD within this tissue site.
We observed that RAR-α signaling was important in mediating the effects of RA. The concomitant increase in RAR-α gene expression in donor CD4 T cells and endogenous RA production in the colon early after allogeneic BMT suggest that RA acts directly on T cells to modulate their function through RAR-α. However, since RAR is broadly expressed by many cell types, it is formally possible that RA can act on other cells and modulate T-cell phenotype and function indirectly. For example, it has been shown that nonmucosal dendritic cells can take up and deliver RA to T cells in order to upregulate gut-homing molecule expression.51 Therefore, it is possible that RA modulates donor T-cell function indirectly through DCs. In addition, our data do not exclude that other RAR isoforms, namely, RAR-β and RAR-γ, may also participate in mediating RA signaling and contribute to the pathogenesis of acute GVHD in the gastrointestinal tract. Nonetheless, these results suggest that blockade of RAR-α signaling with small molecule inhibitors or through other approaches may be a clinically relevant strategy for reducing the priming of gut-homing donor T cells and selectively prevent or mitigate GVHD in the colon. We should note, however, that our results differ somewhat from those reported by Nishimori and colleagues52 who noted that administration of AM80, a synthetic retinoid that binds to both RARα and RARβ, resulted in a reduction in GVHD severity in the skin and did not exacerbate pathological damage in the colon. Of note, the authors employed a murine model of chronic GVHD in which cutaneous and not visceral manifestations of GVHD are most pronounced, as opposed to the acute model used in the current studies. Thus, it is formally possible that the effects of RAR-α signaling blockade may vary to some extent in the setting of acute vs chronic GVHD. Further studies will be necessary to determine if this is indeed the case.
In summary, our studies identify RA as a critical molecule that plays a major role in the regulation of gut-homing molecule expression and, by extension, the pathophysiology of GVHD in the gastrointestinal tract. Manipulation of the RA signaling pathway may therefore represent a novel strategy for mitigating the severity of colonic GVHD and reducing overall mortality. Finally, our results suggest that the vitamin A status of the recipient can significantly affect the ability of T cells to traffic into the colon. Consequently, determination and/or modification of vitamin A levels in patients may be useful for the prognostication of GVHD severity in the colon and may represent a less-toxic, nonpharmacological approach by which GVHD can be successfully modulated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kasem Nithipatikom for assistance with the mass spectrometry analysis. The authors also thank Dr Pierre Chambon for providing RAR-α deficient mice.
This research was supported by the Amy Strelzer Manasevit Research Program, which is funded through the Be The Match Foundation and the National Marrow Donor Program, and an Advancing a Healthier Wisconsin award and a seed grant from the MCW Cancer Center (X.C.). This work was also supported in part by the National Institutes of Health (grants HL64603, HL081650, and DK083358) and by awards from the Midwest Athletes Against Childhood Cancer Fund (W.R.D.).
Authorship
Contribution: X.C. designed and performed research, analyzed and interpreted data, generated the figures, and wrote the manuscript; J.D. performed research; R.K. performed pathological analysis of all tissue samples; and W.R.D. designed experiments, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiao Chen, Division of Hematology and Oncology, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: xchen@mcw.edu.

![Figure 3. Effect of exogenous RA administration on effector and regulatory T-cell infiltration into the colon. Lethally irradiated Balb/c mice were transplanted with 10 × 106 B6 BM together with B6 spleen cells (adjusted to yield a T-cell dose of 0.6 × 106). Cohorts of mice were then treated with either DMSO (n = 6 to 8/group, black bars) or RA (n = 6 to 8/group, white bars). (A) Absolute cell number of CD4+IFN-γ+ or (B) CD4+IL-17+ T cells in the spleen, liver, lung, and colon of animals treated with either DMSO or RA and then killed 19 to 21 days after transplantation. (C) Absolute cell number of CD8+CD103+ cells in the colon of recipient mice. Data are presented as the mean (± standard error of the mean [SEM]) and are the cumulative results from 2 independent experiments. (D) Lethally irradiated Balb/c mice were transplanted with 10 × 106 Foxp3EGFP BM and spleen cells (adjusted to yield a T-cell dose of 0.6 × 106). Cohorts of mice were then treated with either DMSO (n = 8) or RA (n = 8). Mice in both groups were killed 19 to 21 days after transplantation. The absolute cell number of Foxp3+ (GFP+) Tregs in the spleen, liver, and colon of mice is depicted. Data are presented as the mean (± SEM) and are the cumulative results from 2 independent experiments. (E) Percentage of CD4+ and CD8+ T cells that expressed either α4β7 in the colon or CCR9 in the small intestines of transplant recipients that were treated with either DMSO or RA. Animals were killed 19 to 21 days post transplantation. Data are presented as the mean (± SEM) and are the cumulative results from 2 independent experiments. Statistics: *P ≤ .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/19/10.1182_blood-2012-08-445130/3/m_3970f3.jpeg?Expires=1769159128&Signature=4DVnjv8QT85-70OOoSQIEDcZDjJvwJtrJ7G2ZHGYk1hl9kpyLC57UwgkRiFWDnITDtZG4FNrkMXONgNxSfyk~X27qCkEKGKMHb2KRn3BLFLOCnRVEpKFO~wxHD6zL-8BvHn8CXKbnAmTJ49ukdtpTz9el7dNUcDgAAdmfe1CMqqHIl7OKogFLcJvzRzQWLgOlCGLe~nnwEfnzJZmzzdyaRj6E3DCtIlUiWwOwAXZdENWCDtXlU~~l8hPtkw0YXj1CcDdVKRpbZKf6DIH-Zb-e~hvFJBUIfJh6itz5tiubuix5xzcGZYxPy7hB6Jz1Qh76thTVkEyrLjtwSTJ6QfYcQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. RAR-α signaling is important in mediating the effects of RA on T cells. B6 spleen cells (adjusted to 1 × 105 T cells/well) were cocultured with 5 × 104 Balb/c dendritic cells in the presence or absence of 1 μM AM80, an RAR-α agonist. Four days later, cells were harvested for flow cytometric analysis. (A) Representative contour plots of α4β7 and CCR9 expression on CD4+ and CD8+ T cells. (B) Mean (± standard error of the mean [SEM]) percentage of CD4+ and CD8+ T cells that expressed α4β7 and CCR9. Data are cumulative results from 4 independent experiments. Statistics: *P ≤ .05, ***P < .001. (C, D) B6 spleen cells (adjusted to 1 × 105 T cells/well) were cocultured with 5 × 104 Balb/c dendritic cells in the presence of 10 nM exogenous RA. An RAR-α antagonist, BMS195614 (1 μM), was added to some cultures 1 hour prior to the addition of RA. Cells were analyzed after 4 days in culture. Representative contour plots of CCR9, α4β7, CD62L, and CD25 expression on CD4+ and CD8+ T cells is depicted. Data are from 1 of 4 representative experiments with similar results. (E) Cells were cultured as in panels C and D with the exception of 3H-thymidine was added for the last 18 hours to assess proliferation. Data are presented as mean counts per minute ± SEM and are representative of 1 of 2 experiments with similar results. Statistics: *P ≤ .05](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/19/10.1182_blood-2012-08-445130/3/m_3970f5.jpeg?Expires=1769159128&Signature=A6-WIEoJ7wf07wizQXO8QFk3P1S6FKH3oQiTdvGqF30cdo-UFWEskvNPExKc8MjvmEzEmumW1D9AUHFXOEefjzqd2jD2MrGCgcLcn8pdm2-2B0IFfRBYqsK3f60j8TedHUfCvv-e-p-HwORSArMCkVQC4WQCAEa1yX6awMVmPzZ-S9kCIy1cc5SA519ewgEYjGAXvZfNIhDU75s1CKk5klHDXcSMM63ZJ323Qn6QGjtaW-CATi88kec277ffeOsUthubo4SCVWezCMMxlaDAq~QnMBnwVPz5PAZhnwaolR~popVm-W4hTnNE3Qs~dOrKNFN4rqmwuisNlqZdjVjZOA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Recipients of RAR-α–deficient T cells are protected from lethal GVHD yet are able to mount a GVL response. Lethally irradiated Balb/c mice were transplanted with BM cells (5 × 106) from either RAR-α─/─ (□) or wild type (WT) littermate controls (○) or BM and spleen cells (adjusted to yield a T-cell dose of 0.7−1.0 × 106 T cells) from either RAR-α─/─ (▪) or WT littermate controls (●). (A) Overall survival. Data are cumulative results from 3 independent experiments (n = 3 for BM control groups, n = 14 for each BM + T-cell group). (B) Lethally irradiated Balb/c mice were transplanted with BM (5 × 106) and spleen cells (adjusted to yield a T-cell dose of 2.1 × 106) from either RAR-α─/─ (n = 9) or WT littermate controls (n = 5). Mice were killed at days 10 to 12 after BMT and pathological damage in the liver, lung, and colon was examined. Data are derived from 2 independent experiments and are presented as the mean (± standard error of the mean [SEM]). Representative photomicrographs of the colon from mice transplanted with WT vs RAR-α─/─ marrow grafts are shown. Colon from recipients of WT grafts show loss of mucin and destruction of crypt architecture, while animals reconstituted with grafts from RAR-α─/─ mice have normal-appearing crypts. Statistics: *P ≤ .05, ***P ≤ .001. (C,D) The absolute number of donor-derived CD4+ (C) and CD8+ cells (D) in the liver, spleen, lung, and colon is shown for each group. Data are derived from 2 independent experiments and are presented as the mean (± SEM). (E) Lethally irradiated Balb/c mice were administered 0.6 × 106 A20 cells transfected with firefly luciferase (A20-luc) and then transplanted with WT BM alone (n = 5), WT BM plus spleen cells (adjusted to yield a dose of 1.2 × 106 T cells; n = 5), or RAR-α─/─ BM plus spleen cells (adjusted to yield an equivalent T cell dose; n = 5). Overall survival and whole body distribution of A20-luc cells using in vivo BLI is depicted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/19/10.1182_blood-2012-08-445130/3/m_3970f7.jpeg?Expires=1769159128&Signature=FqPchMtY0os6Mpi~j8zcmH5FcrZJsMJVSabqPKgyMBtYkKojQKiJth~qzhEdi6rYEckhHeWbVnEdvQLpOpLIP8COTXmsML1FsJlWn2~3KxDi35pxWGoF-BcpQEgiHW~rXn7pZK8IvkoUCXuWS7H77qMouiGvp8LLgpVF8Kf9QcheO82CALo9-94a6vW-yai51lK3FF9QkWm8fV3GuQUebirOTmGlk3ogN-upKAKHOD9NguxBFJMHtifBxrWmNOEfnvwYcEvkIz3hjjjdZKJQk4cnjCVMhDRQSKeaUsioXrsn1xrQAspjwsfYArhjwPv8oULMLhwfjCkgWoLSPQbezQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal