Key Points
FXI must be a dimer for normal activation by fXIIa but not for activation by thrombin or autoactivation.
Poly-P is a cofactor for activation of coagulation fXI by fXIIa and thrombin and supports fXI autoactivation.
Factor XI (fXI) is a homodimeric zymogen that is converted to a protease with 1 (1/2-fXIa) or 2 (fXIa) active subunits by factor XIIa (fXIIa) or thrombin. It has been proposed that the dimeric structure is required for normal fXI activation. Consistent with this premise, fXI monomers do not reconstitute fXI-deficient mice in a fXIIa-dependent thrombosis model. FXI activation by fXIIa or thrombin is a slow reaction that can be accelerated by polyanions. Phosphate polymers released from platelets (poly-P) can enhance fXI activation by thrombin and promote fXI autoactivation. Poly-P increased initial rates of fXI activation 30- and 3000-fold for fXIIa and thrombin, respectively. FXI monomers were activated more slowly than dimers by fXIIa in the presence of poly-P. However, this defect was not observed when thrombin was the activating protease, nor during fXI autoactivation. The data suggest that fXIIa and thrombin activate fXI by different mechanisms. FXIIa may activate fXI through a trans-activation mechanism in which the protease binds to 1 subunit of the dimer, while activating the other subunit. For activation by thrombin, or during autoactivation, the data support a cis-activation mechanism in which the activating protease binds to and activates the same fXI subunit.
Introduction
Factor XI (fXI), the zymogen of factor XIa (fXIa), is a dimer of identical 80-kDa subunits.1,,,-5 This configuration is unique among coagulation proteases. The fXI gene arose from a duplication of the gene for the monomeric zymogen prekallikrein (PK).6,7 FXI and PK polypeptides contain 4 apple domains (A1–A4) and a trypsin-like protease domain.4,,,-8 In fXI, the A4 domain forms an interface with the A4 domain of a second subunit.5,9,10 The dimeric structure is conserved, indicating it serves an important functional role.6,11 Wu et al observed that fXI monomers are converted to fXIa significantly more slowly than fXI dimers and proposed that the dimeric structure supports a trans-activation mechanism in which an activating protease binds to 1 fXI subunit within a dimer while activating the opposite subunit.9
Activation of each fXI subunit involves cleavage of the Arg369-Ile370 bond; a reaction catalyzed by factor XIIa (fXIIa)1,2 or various forms of thrombin.12,-14 Activated fXI may have 1 (1/2-fXIa) or 2 (fXIa) activated subunits (Figure 1).15 The slow rates of activation by fXIIa or thrombin in purified protein systems indicate that a cofactor is involved in activation in vivo. Polyanions such as dextran sulfate (DS) and heparin enhance fXI activation by fXIIa, thrombin, and fXIa (autoactivation).9,13,16 Recently, Choi et al showed that phosphate polymers (poly-P) released from platelet-dense granules may be a physiologic counterpart to DS or heparin, enhancing fXI activation by thrombin and promoting fXI autoactivation.17
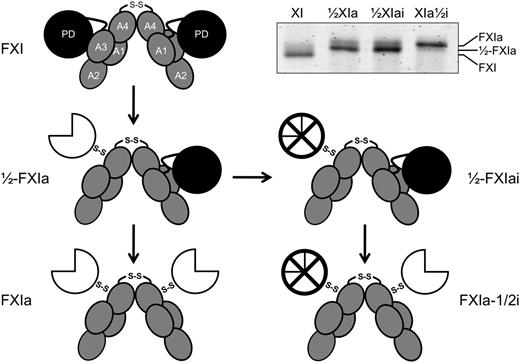
Schematic diagrams of species generated during fXI activation. The gray ellipses represent the 4 apple domains (A1–A4) and the black circles represent the protease domains (PD) of zymogen fXI. The 2 subunits of the fXI dimer are connected by a hydrophobic interface involving the A4 domains and a disulfide bond involving Cys321. Activation of 1 fXI subunit by cleavage of the Arg369-Ile370 bond results in a species with 1 active site, referred to as 1/2-fXIa. The white three-quarter circle represents the activated protease domain. Subsequent cleavage of the Arg369-Ile370 bond on the second subunit results in generation of fXIa. fXI, 1/2-fXIa, and fXIa migrate slightly differently on SDS-PAGE (inset), facilitating their identification and purification. The preparation of 1/2-fXIa shown in the inset has approximately 10% contamination with fXIa (upper of the 2 bands). Inhibition of the active protease domain (⊗) of 1/2-fXIa yields a species called 1/2-fXIai. Activation of the second subunit of 1/2-fXIai results in a species with 2 activated subunits, 1 of which is inhibited and called fXIa-1/2i.
Schematic diagrams of species generated during fXI activation. The gray ellipses represent the 4 apple domains (A1–A4) and the black circles represent the protease domains (PD) of zymogen fXI. The 2 subunits of the fXI dimer are connected by a hydrophobic interface involving the A4 domains and a disulfide bond involving Cys321. Activation of 1 fXI subunit by cleavage of the Arg369-Ile370 bond results in a species with 1 active site, referred to as 1/2-fXIa. The white three-quarter circle represents the activated protease domain. Subsequent cleavage of the Arg369-Ile370 bond on the second subunit results in generation of fXIa. fXI, 1/2-fXIa, and fXIa migrate slightly differently on SDS-PAGE (inset), facilitating their identification and purification. The preparation of 1/2-fXIa shown in the inset has approximately 10% contamination with fXIa (upper of the 2 bands). Inhibition of the active protease domain (⊗) of 1/2-fXIa yields a species called 1/2-fXIai. Activation of the second subunit of 1/2-fXIai results in a species with 2 activated subunits, 1 of which is inhibited and called fXIa-1/2i.
Here, we show that dimeric fXI, but not monomeric fXI, reconstitutes the wild-type phenotype in fXI-deficient mice in a fXIIa-dependent thrombosis model and that fXI must be a dimer for normal activation by fXIIa in the presence or absence of poly-P. FXI activation by thrombin or autoactivation in the presence of poly-P, however, does not require fXI to be a dimer. The results suggest that fXI is activated by fXIIa and thrombin through different mechanisms, with the dimeric structure perhaps playing a more significant role in fXIIa-dependent processes, such as thrombosis.
Methods
Recombinant fXI
Complementary DNAs for wild-type fXI (fXIWT), fXI with serine replacing Cys321 (fXIC321S), or fXIC321S with alanine replacing Leu284 or Ile290 (fXIC321S,L284A, fXIC321S,I290A) in vector pJVCMV were expressed in HEK293 and purified by antibody-affinity chromatography, as described.15 FXI was treated with 500 µM diisopropylfluorophosphate (DIP) to inhibit traces of fXIa, then dialysis into 50 mM Tris-hydrogen chloride pH 7.4, 100 mM sodium chloride (NaCl; Tris-buffered saline [TBS]). FXI (20 μg) was passed over a Superose-12 column equilibrated with 50 mM sodium phosphate, pH 7.3, 150 mM NaCl.18 Retention volumes were compared with protein standards and with plasma fXI and PK.18 FXI binding to immobilized poly-P was determined by surface plasmon resonance (SPR), as described.17
Expression of fXI in mice by hydrodynamic tail-vein injection
The C57Bl/6 fXI–deficient (fXI−/−) mice (24–27 g) were anesthetized with 50 mg/kg intraperitoneal pentobarbital.19,,-22 pJVCMV/fXI constructs (15 μg) in 2 mL lactated Ringer’s solution were infused over 20 seconds into the tail vein. Plasma was prepared from 25 μL blood samples collected at various times post-hydrodynamic tail-vein injection (HTI), and fXI concentration was determined by enzyme-linked immunosorbent assay (ELISA). Western blots of plasma fXI (1 μL per lane) were prepared using anti-fXI immunoglobulin G 14E11.11
Carotid artery thrombosis model
The fXI−/− mice underwent HTI with pJVCMV/fXI constructs.11 Twenty-four hours post-HTI, mice were anesthetized, and the right common carotid artery was fitted with a Doppler flow probe (model 0.5 VB; Transonic System). Thrombus formation was induced by applying 3.5% ferric chloride (FeCl3) to the artery, as described.11 Flow was monitored for 30 minutes. After the experiment, blood was collected and plasma fXI concentration was determined by ELISA.
Polyphosphate
Sodium phosphate glass from Sigma-Aldrich. Poly-P (75 to 100 phosphate units) was prepared by gel electrophoresis, as described,23 except that a model 491 Prep cell (Biorad) was used for continuous elution electrophoresis. Poly-P concentrations are given in μM phosphate groups.
Plasma fXI and fXIa species with 1 active site
FXI purified from human plasma (HP; 600 nM) was incubated with thrombin (900 nM) for 6 hours at 37°C. Reactions were terminated with 20 µM hirudin, applied to a soybean trypsin inhibitor-agarose column, and eluted with TBS containing 200 mM benzamidine, 1 mM EDTA. Eluate contained 1/2-fXIa (Figure 1).15 1/2-fXIa (500 nM) in TBS was incubated with 500 µM DIP, irreversibly inhibiting the active site(s) of 1/2-fXIa and contaminating fXIa (confirmed by chromogenic assay). The inhibited preparation is referred to as 1/2-fXIai (Figure 1).
FXI Activation
fXI (200 nM subunits [100 nM dimer, 200 nM monomer]) was incubated with fXIIa (5 to 100 nM) or α-thrombin (50 nM) in 50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4, and 100 mM NaCl with 0.1% BSA at 37°C. For fXI (120 nM subunits) activation in the presence of poly-P (4 μM), 3 nM fXIIa, or α-thrombin was used. At various times, 1.5 μM corn trypsin inhibitor (fXIIa) or hirudin (α-thrombin) was added to aliquots to terminate activation, then mixed with an equal volume of 1 mM L-pyroglutamyl-l-prolyl-l-arginine-p-nitroanilide (S-2366; DiaPharma). p-nitroaniline (pNA) generation was monitored at 405 nm, and activation rates were determined by linear regression of time dependences of fXIa active site generation. Second-order rate constants kcat/Km (M-1s−1) were obtained by dividing initial rates (v0) by protease and substrate concentrations and by measuring initial slopes of fXIIa or fXI concentration dependences of v0. FXIWT activation by fXIIa (5 to 100 nM) was modeled by numerical integration using KinTek Explorer version 2.5 software.24 For fXIWT activation by thrombin in the presence of poly-P, a fXI concentration dependence (30–480 nM subunits) was established to define the extent of saturation.
FXI autoactivation was induced by incubating fXI (120 nM subunits) with DS (1 µg/mL, 500 000 D) or poly-P (4 μM) in HBSA at 37°C. At various times, 18 μL samples were mixed with an equal volume of 1 mM S-2366, 4 µg/mL Polybrene. Conversion of fXI to fXIa was followed by monitoring ΔOD405 nm. FXI activation was also analyzed by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE). FXI (120 nM subunits) in HBS containing 1 mg/mL PEG 8000 was incubated at 37°C with 1 µg/mL DS. At various times, aliquots were removed and placed into nonreducing sample buffer, size-fractionated on 7.5% polyacrylamide-SDS gels, and stained with GelCode Blue (Pierce). Gels were imaged with an Odyssey infrared imaging system.
FXI activity in plasma
FXI-deficient plasma (30 μL, George King) was mixed with 30 μL fXI (0.3–30 nM) in TBS with 0.1% BSA (TBSA). Contact activation was initiated with 30 μL PTT (partial thromboplastin time)-A reagent (Diagnostica Stago, undiluted, or diluted 1:256 in TBSA with 60 μM phosphatidylcholine–phosphatidylserine [PC–PS] vesicles) or 16 μM poly-P (with 60 μM PC–PS). After incubation at 37°C (5 minutes for PTT reagent, 1 minute for 1:256 PTT reagent or poly-P), 30 μL of 25 mM calcium chloride (CaCl2) was added, and time to clot formation was determined on an ST4 fibrometer (Diagnostica Stago). In separate experiments, 30 μL fXI (5 μg/mL) in TBSA was mixed with equal volumes of fXI-deficient plasma containing 8 μM corn trypsin inhibitor, 60 μM PC–PS, and 16 μM poly-P. β-thrombin (1.5 nM) was added and incubated for 1 minute at 37°C. Clotting was initiated with 30 μL of 25 mM CaCl2.
Results
FXI Monomers
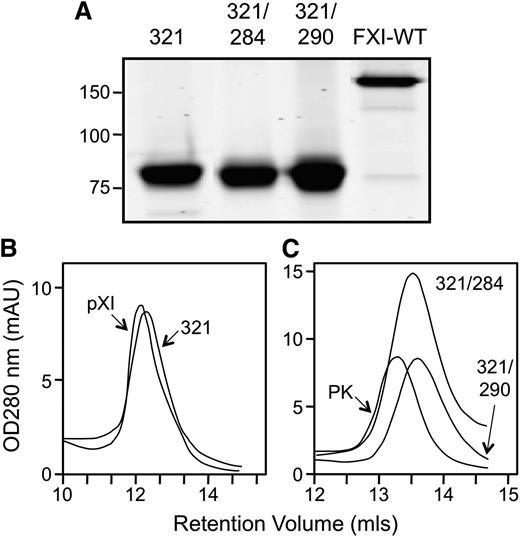
The interface between fXI subunits has been studied in detail.1,5,9,10,25 While Cys321 forms a disulfide bond connecting the A4 domains,4,5,25 fXI is a dimer in the absence of this bond.18,25 Leu284, Ile290, and Tyr329 form the core of the dimer interface.5,9,10 Wu et al showed that fXI with serine replacing Cys321 and alanine replacing Leu284 or Ile290 is a monomer.9 FXIWT, as expected, is a 160-kDa dimer on SDS-PAGE (Figure 2A), while fXIC321S, fXIC321S,L284A, and fXIC321S,I321A migrate as 80-kDa monomers because they lack Cys321. On size exclusion chromatography, peak retention volumes for fXIWT (11.7 mL) and fXIC321S (12.1 mL; Figure 2B) are similar, indicating fXIC321S is a dimer despite missing Cys321. In contrast, retention volumes for fXI C321S, L284A (13.5 mL) and fXIC321S,I290A (13.7 mL) are similar to PK (13.3 mL), a monomeric homolog of fXI (Figure 2C). The expression constructs for these fXI species were used to induce transient expression of human fXI in fXI−/− mice.
Recombinant fXI. (A) Recombinant fXIC321S (321), fXIC321S,L284A (321/284), and fXIC321S,I290A (321/290) appear as 80-kDa monomers on 7.5% nonreducing SDS-PAGE because they lack the Cys321-Cys321 interchain disulfide bond that connects the 2 subunits of the dimer in fXIWT (160-kDa band at right). Positions of molecular mass standards (kDa) are indicated at the left of the panel. Size-exclusion chromatography of (B) plasma fXI (pXI) and fXIC321S (321) or (C) fXIC321S,L284A (321/284), fXIC321S,I290A (321/290) and plasma PK. Shown are continuous readouts of optical density (280 nm) of solutions exiting a Superose-12 size exclusion column (flow rate 1.0 mL/min). Protein retention volumes in milliliters are indicated below the curves.
Recombinant fXI. (A) Recombinant fXIC321S (321), fXIC321S,L284A (321/284), and fXIC321S,I290A (321/290) appear as 80-kDa monomers on 7.5% nonreducing SDS-PAGE because they lack the Cys321-Cys321 interchain disulfide bond that connects the 2 subunits of the dimer in fXIWT (160-kDa band at right). Positions of molecular mass standards (kDa) are indicated at the left of the panel. Size-exclusion chromatography of (B) plasma fXI (pXI) and fXIC321S (321) or (C) fXIC321S,L284A (321/284), fXIC321S,I290A (321/290) and plasma PK. Shown are continuous readouts of optical density (280 nm) of solutions exiting a Superose-12 size exclusion column (flow rate 1.0 mL/min). Protein retention volumes in milliliters are indicated below the curves.
FXI monomers in a model of arterial thrombosis
FXI-deficient (fXI−/−) mice are resistant to injury-induced arterial thrombosis.11,26,27 Infusion of human plasma fXI into fXI−/− mice restores the wild-type phenotype.26,27 FXI appears to be activated during thrombosis in mice by fXIIa.11,28,29 We compared the capacities of fXIWT, fXIC321S,L284A, and fXIC321S,I290A to reconstitute fXI−/− mice in a model of FeCl3-induced carotid artery thrombosis.11,27 FXIWT expressed in HEK293 cells has a plasma half-life of <20 minutes after infusion into fXI−/− mice compared with plasma fXI (>3 hours), probably due to glycosylation differences, making it difficult to establish stable fXI concentrations. Instead, we expressed human fXI in fXI−/− mice using HTI.19,-21 Expression constructs infused into the tail vein primarily transfect hepatocytes. Infusion of 15 μg pJVCMV/fXIWT construct into fXI−/− mice resulted in peak fXI expression 12 to 24 hours post-infusion (Figure 3A), with plasma levels comparable to normal human plasma (15–45 nM; Figure 3B). Larger amounts of DNA did not result in further increases in plasma fXI, suggesting maximum expression was achieved. fXI plasma concentrations for fXIWT, fXIC321S,L284A, and fXIC321S,I290A were similar (Figure 3B). Western blots of mouse plasma 24 hours post-infusion confirmed the presence of human fXI species of appropriate size (Figure 3C). The PTT of plasmas from mice expressing fXIWT, fXIC321S,L284A, and fXIC321S,I290A (25–33 seconds) were similar to results for plasma from wild-type mice (fXI−/− plasma PTTs were >45 seconds). Carotid arteries of wild-type C57Bl/6 mice occlude within 15 minutes after exposure to 3.5% FeCl3, while arteries of fXI−/− mice remain patent.11,26 All fXI−/− mice expressing fXIWT developed occlusion within 15 minutes of FeCl3 exposure, while arteries of mice treated with pJVCMV control remained patent (Figure 3D). Arteries of 70% of fXI−/− mice expressing fXIC321S,L284A and 100% expressing fXIC321S,I290A remained patent (Figure 3D). These results demonstrate that fXI monomers are synthesized in vivo but function poorly in the thrombosis model. The following experiments were designed to compare the importance of the fXI dimer with fXI activation by fXIIa and α-thrombin.
FeCl3-induced carotid artery occlusion in fXI-deficient mice expressing human fXI. (A) FXI−/− mice underwent HTI with human fXIWT cDNA in expression vector pJVCMV. Shown are concentrations of human fXI in mouse plasma at various times post-HTI. (B) Concentrations of fXI levels in plasma from fXI−/− mice 24 hours post-HTI with constructs for human fXIWT (WT), fXIC321S,L284A (321/284), or fXIC321S,I290A (321/290). Normal human plasma is shown for comparison. For panels A and B, error bars represent 1 standard deviation. (C) Western blot of mouse plasmas 24 hours post-HTI with constructs for human fXIWT, fXIC321S,L284A, or empty vector control (pJV). A sample of pure human fXI (XI) and a sample of fXI-deficient mouse plasma (XIdp) are shown for comparison. Positions of molecular mass standard (kDa) are shown at the left of the blot. Positions for fXI dimer (D) and monomer (M) are indicated on the right. (D) Groups of 10 fXI−/− mice underwent HTI with constructs for fXIWT, fXIC321S,L284A, fXIC321S,I290A, or empty vector control (pJV). Twenty-four hours later, the animals were tested in a carotid artery thrombosis model in which thrombus formation is induced by exposing the vessel to 3.5% FeCl3. Each bar indicates the percent of mice in each group with occluded arteries 30 minutes after applying FeCl3. Plasma fXI levels of the mice in this study are shown in panel B.
FeCl3-induced carotid artery occlusion in fXI-deficient mice expressing human fXI. (A) FXI−/− mice underwent HTI with human fXIWT cDNA in expression vector pJVCMV. Shown are concentrations of human fXI in mouse plasma at various times post-HTI. (B) Concentrations of fXI levels in plasma from fXI−/− mice 24 hours post-HTI with constructs for human fXIWT (WT), fXIC321S,L284A (321/284), or fXIC321S,I290A (321/290). Normal human plasma is shown for comparison. For panels A and B, error bars represent 1 standard deviation. (C) Western blot of mouse plasmas 24 hours post-HTI with constructs for human fXIWT, fXIC321S,L284A, or empty vector control (pJV). A sample of pure human fXI (XI) and a sample of fXI-deficient mouse plasma (XIdp) are shown for comparison. Positions of molecular mass standard (kDa) are shown at the left of the blot. Positions for fXI dimer (D) and monomer (M) are indicated on the right. (D) Groups of 10 fXI−/− mice underwent HTI with constructs for fXIWT, fXIC321S,L284A, fXIC321S,I290A, or empty vector control (pJV). Twenty-four hours later, the animals were tested in a carotid artery thrombosis model in which thrombus formation is induced by exposing the vessel to 3.5% FeCl3. Each bar indicates the percent of mice in each group with occluded arteries 30 minutes after applying FeCl3. Plasma fXI levels of the mice in this study are shown in panel B.
Activation of fXI in the absence of a polyanion
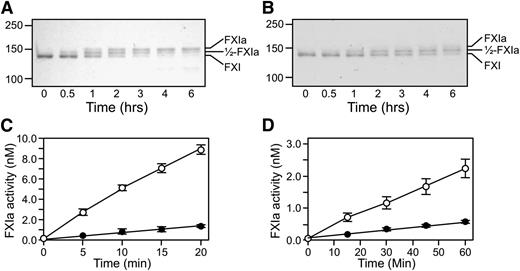
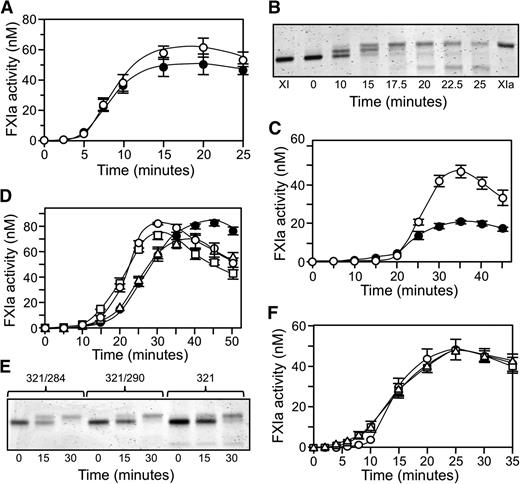
Conversion of fXI to fXIa proceeds through a species with 1 activated subunit (1/2-fXIa).15 fXI, 1/2-fXIa, and fXIa migrate slightly differently on nonreducing SDS-PAGE (Figure 1 and 4) despite similar molecular masses, which is consistent with activation-induced conformational changes.30 FXI activation by fXIIa (Figure 4A) or α-thrombin (Figure 4B) is a slow process in the absence of a polyanion. Rates of conversion of plasma fXI to 1/2-fXIa were measured at early time points prior to appearance of fully activated fXIa (Figure 4C and 4D). Activation of the first fXI subunit was 60-fold faster with fXIIa (15 000 M−1.sec−1) than with α-thrombin (250 M−1.sec−1). Activation of the second subunit (1/2-fXIa to fXIa) was assessed with 1/2-fXIa in which the activated subunit is irreversibly blocked (1/2-fXIai; as seen in Figure 1). Cleavage was 40-fold faster with fXIIa than with α-thrombin (1200 M−1.sec−1 and 30 M−1.sec−1, respectively). Cleavage of the first subunit was 12-fold faster than cleavage of the second subunit with fXIIa and 8-fold faster with α-thrombin (Figure 4C-D), indicating that changes accompanying activation of a subunit affect activation of its partner.
Activation of plasma fXI. (A-B) Plasma fXI (600 nM) was incubated at 37°C with (A) 60 nM fXIIa or (B) 600 nM α-thrombin. Samples of each reaction were quenched in nonreducing sample buffer at the indicated times and size fractionated on 6% polyacrylamide-SDS gels. Positions for markers and protein standards for fXI, fXIa, and 1/2-fXIa are indicated. (C-D) Activation of 100 nM fXI (white circles) or 200 nM 1/2-fXIai (black circles) by (C) fXIIa (10 nM) or (D) α-thrombin (50 nM). At various times, samples were tested for enzymatic activity by measuring cleavage of the chromogenic substrate S2366 (500 μM), as described in the Methods section. Error bars represent 1 standard deviation. The concentration of 1/2-fXIai was 200 nM in the activation assay to generate sufficient signal to detect in the chromogenic substrate assay. Reactions performed with 100 nM 1/2-fXIai would have slopes 1/2 as great as those for the progress curves shown.
Activation of plasma fXI. (A-B) Plasma fXI (600 nM) was incubated at 37°C with (A) 60 nM fXIIa or (B) 600 nM α-thrombin. Samples of each reaction were quenched in nonreducing sample buffer at the indicated times and size fractionated on 6% polyacrylamide-SDS gels. Positions for markers and protein standards for fXI, fXIa, and 1/2-fXIa are indicated. (C-D) Activation of 100 nM fXI (white circles) or 200 nM 1/2-fXIai (black circles) by (C) fXIIa (10 nM) or (D) α-thrombin (50 nM). At various times, samples were tested for enzymatic activity by measuring cleavage of the chromogenic substrate S2366 (500 μM), as described in the Methods section. Error bars represent 1 standard deviation. The concentration of 1/2-fXIai was 200 nM in the activation assay to generate sufficient signal to detect in the chromogenic substrate assay. Reactions performed with 100 nM 1/2-fXIai would have slopes 1/2 as great as those for the progress curves shown.
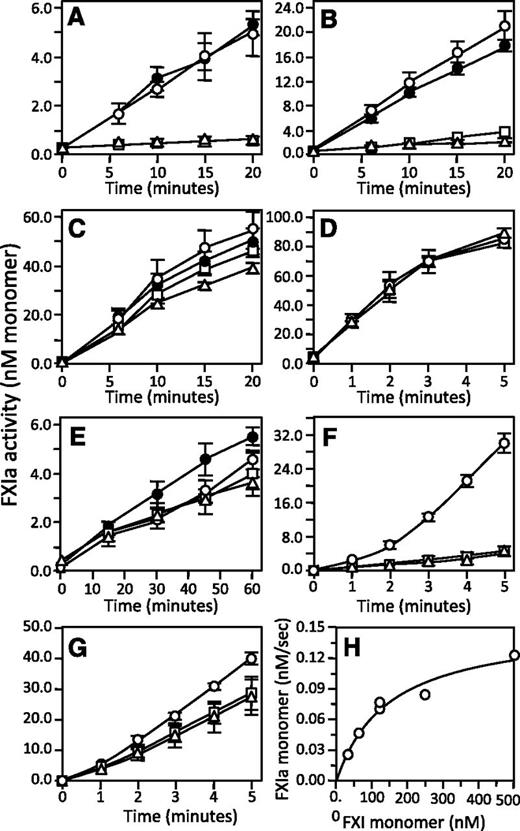
FXIC321S, L284A and fXIC321S,290A were reported to be activated more slowly than fXIWT or fXIC321S at low enzyme-to-substrate (E:S) ratios.9 We observed slower monomer activation by fXIIa at an E:S ratio of 1:20, with the discrepancy disappearing at a ratio of 1:1 (Figure 5A-D). Rates of fXIWT activation exhibited linear dependence on fXIIa concentration up to 30 nM, consistent with a second-order rate constant of 14 000 M-1.sec−1, which is in agreement with the value for conversion of plasma fXI to 1/2-fXIa. However, the rate with 100 nM fXIIa was approximately 2-fold higher than expected from this linear dependence, suggesting facilitated activation when fXIIa occupies both subunits. FXI monomers were poorer substrates at low fXIIa concentrations, but similar rates for monomer and dimer activation were observed with fXIIa ≥30 nM. This may be explained by a high-affinity fXIIa binding site on the fXI dimer that is missing on the monomer. With saturation of weaker binding sites common to monomers and dimers, activation rates become comparable. In contrast, similar activation rates were observed for fXI dimers and monomers in reactions with α-thrombin (Figure 5E), indicating that catalytic efficiencies for activation of monomer and a subunit within a dimer are comparable. However, a relatively high E:S ratio (1:4) was required, as slow activation rates precluded studying the process at lower ratios.
Activation of fXI. (A-D) fXIWT (○), fXIC321S (●), fXIC321S,L284A (□), or fXIC321S,I290A (△), 200 nM subunits for each protein, were incubated with fXIIa at (A) 5 nM, (B) 10 nM, (C) 30 nM, or (D) 100 nM concentration. (E) FXI species in panels A-D (200 nM subunits) activated by 50 nM α-thrombin. (F-G) Activation of fXIWT (○), fXIC321S,L284A (□), or fXIC321S,I290A (△), 120 nM subunits each, incubated with (F) 3 nM fXIIa or (G) 3 nM α-thrombin in the presence of 4 μM poly-P. For panels A through G, samples of reactions were tested at various times for fXIa activity by measuring cleavage of S2366 (500 μM). (H) Michaelis–Menten nonlinear, least squares analysis of the substrate concentration dependence of fXIWT subunit activation by 3 nM α-thrombin in the presence of 4 μM poly-P. For all panels, error bars show 1 standard deviation.
Activation of fXI. (A-D) fXIWT (○), fXIC321S (●), fXIC321S,L284A (□), or fXIC321S,I290A (△), 200 nM subunits for each protein, were incubated with fXIIa at (A) 5 nM, (B) 10 nM, (C) 30 nM, or (D) 100 nM concentration. (E) FXI species in panels A-D (200 nM subunits) activated by 50 nM α-thrombin. (F-G) Activation of fXIWT (○), fXIC321S,L284A (□), or fXIC321S,I290A (△), 120 nM subunits each, incubated with (F) 3 nM fXIIa or (G) 3 nM α-thrombin in the presence of 4 μM poly-P. For panels A through G, samples of reactions were tested at various times for fXIa activity by measuring cleavage of S2366 (500 μM). (H) Michaelis–Menten nonlinear, least squares analysis of the substrate concentration dependence of fXIWT subunit activation by 3 nM α-thrombin in the presence of 4 μM poly-P. For all panels, error bars show 1 standard deviation.
The effect of poly-P on fXI activation
FXI activation is accelerated by polyanions such as DS13,16 and poly-P.17 We examined fXI activation by fXIIa or α-thrombin in the presence of poly-P with a size distribution comparable to platelet poly-P. Poly-P increased the initial rate of fXI activation by fXIIa approximately 30-fold (500 000 M−1.sec−1, Figure 5F) and by α-thrombin approximately 3000-fold (700 000 M−1.sec−1, Figure 5G) compared with reactions without poly-P (Figure 4C-D). Michaelis–Menten analysis of fXIWT activation by thrombin with poly-P gave a rate of ∼700 000 M-1.sec-1 with kcat 0.05 ± 0.01 s−1 and Km 70 ± 10 nM fXI dimer (Figure 5H). While fXIIa activated fXI monomers more slowly than fXIWT in the presence of poly-P (Figure 5E), activation rates for monomers and fXIWT by α-thrombin and poly-P were comparable (Figure 5F). During the reactions, a mixture of 1/2-fXIa and fXIa is formed, and it is likely that modest fXI activation by these proteases (autoactivation) is responsible for the curvature of some time courses (see below).
FXI autoactivation
DS and poly-P induce fXI activation in the absence of fXIIa or thrombin. This occurs in a process that is probably initiated by traces of fXIa in fXI preparations and requires fXI and an activated fXI species to bind in proximity on the polyanion (a template mechanism).13,17 Published data suggest that fXI monomers do not undergo autoactivation.9 The effect of DS on plasma fXI is shown in Figure 6A. After a lag phase in which 1/2-fXIa formation predominates, there is rapid conversion to fXIa (Figure 6B). To test the possibility that an activated subunit in a dimer activates the other subunit within the same dimer, we compared fXI and 1/2-fXIai, a form of 1/2-fXIa in which the activated subunit is irreversibly inhibited. In this study, fXI and 1/2-fXIai were treated with DIP to neutralize active subunits, likely accounting for the longer lag phases than those shown in Figure 6A. DS promoted autoactivation of the unactivated subunit of 1/2-fXIai in a similar manner to conversion of fXI to fXIa (Figure 6C). The amount of fXIa activity produced from 1/2-fXIai was half that generated from fXI, consistent with the difference in the maximum number of possible activated subunits. The results suggest that fXI does not need to be a dimer to undergo autoactivation and, in fact, fXI dimers and monomers were activated at relatively similar rates with DS (Figure 6D). The gel in Figure 6E shows changes in migration that occurred as zymogen monomer is activated.
fXI autoactivation. (A) Plasma fXI (120 nM subunits) was incubated with 1 μg/mL DS (●) or 4 μM poly-P (○). At various times, fXIa generation was determined by chromogenic substrate assay. (B) Plasma fXI (120 nM subunits) was incubated with 1 μg/mL DS. Samples were collected into nonreducing sample buffer at the indicated time points and size fractionated on 6% polyacrylamide gels, then stained with GelCode Blue. (C) 120 nM subunits of plasma fXI (○) or 1/2-fXIai (●) were incubated with 1 μg/mL DS. At various times, fXIa generation was determined by chromogenic substrate assay. The fXI preparations in this panel were treated with DIP to inactivate contaminating fXIa, accounting for the longer lag phase compared with progress curves in panel A. (D) fXIWT (○), fXIC321S (●), fXIC321S,L284A (□), or fXIC321S,I290A (△), all 120 nM subunits, was incubated with 1 μg/mL DS. At various times, fXIa generation was determined by chromogenic substrate assay. (E) fXIC321S,L284A (321/284), fXIC321S,I290A (321/290), or fXIC321S (321), 120 nM subunits, was incubated with 1 μg/mL DS. Samples were collected into nonreducing sample buffer at the indicated time points and size fractionated on 7.5% polyacrylamide gels, then stained with GelCode Blue. (F) fXIWT (○), fXIC321S,L284A (□), or fXIC321S,I290A (△), each at a concentration of 120 nM subunits, was incubated with 4 μM poly-P. At various times, fXIa generation was determined. For all panels, error bars represent 1 standard deviation.
fXI autoactivation. (A) Plasma fXI (120 nM subunits) was incubated with 1 μg/mL DS (●) or 4 μM poly-P (○). At various times, fXIa generation was determined by chromogenic substrate assay. (B) Plasma fXI (120 nM subunits) was incubated with 1 μg/mL DS. Samples were collected into nonreducing sample buffer at the indicated time points and size fractionated on 6% polyacrylamide gels, then stained with GelCode Blue. (C) 120 nM subunits of plasma fXI (○) or 1/2-fXIai (●) were incubated with 1 μg/mL DS. At various times, fXIa generation was determined by chromogenic substrate assay. The fXI preparations in this panel were treated with DIP to inactivate contaminating fXIa, accounting for the longer lag phase compared with progress curves in panel A. (D) fXIWT (○), fXIC321S (●), fXIC321S,L284A (□), or fXIC321S,I290A (△), all 120 nM subunits, was incubated with 1 μg/mL DS. At various times, fXIa generation was determined by chromogenic substrate assay. (E) fXIC321S,L284A (321/284), fXIC321S,I290A (321/290), or fXIC321S (321), 120 nM subunits, was incubated with 1 μg/mL DS. Samples were collected into nonreducing sample buffer at the indicated time points and size fractionated on 7.5% polyacrylamide gels, then stained with GelCode Blue. (F) fXIWT (○), fXIC321S,L284A (□), or fXIC321S,I290A (△), each at a concentration of 120 nM subunits, was incubated with 4 μM poly-P. At various times, fXIa generation was determined. For all panels, error bars represent 1 standard deviation.
With poly-P, sigmoidal progress curves for activation of fXIWT, fXIC321S,L284A , and fXIC321S,I290A (Figure 6F) were similar to those observed with DS (Figure 6A). Given the template mechanism involved in autoactivation,17 these findings show that fXI monomers must bind to poly-P and that binding enhances fXI activation by fXIa. Direct binding studies using SPR confirmed that fXIC321S,L284A and fXIC321S,I290A bind to poly-P with Kd of 25 to 50 nM (fXIWTKd 5–25 nM). The study shows that the capacity to undergo polyanion-induced autoactivation is intrinsic to a fXI subunit.
The effects of poly-P on fXI-dependent plasma coagulation
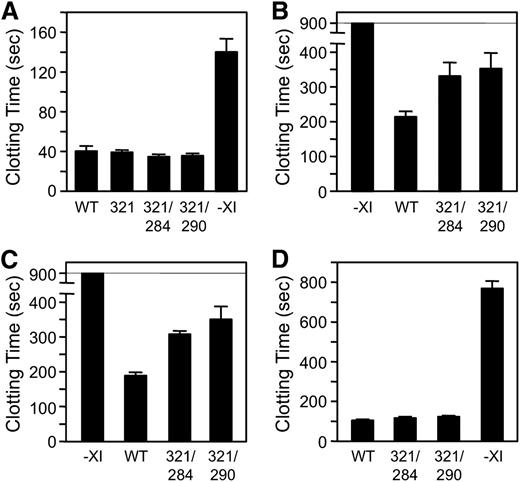
Despite the defect in fXIIa-mediated activation, fXIC321S,L284A and fXIC321S,I290A have 116% and 117%, respectively, of the specific activity of fXIWT in a PTT assay that requires fXI to be activated by fXIIa (Figure 7A). We postulated that this result, which is consistent with those shown in Figure 5D, was due to masking of the activation defect in fXI monomers by the high fXIIa concentration generated in the PTT. Accordingly, diluting the PTT reagent to reduce fXIIa generation led to longer clotting times for plasma-containing fXI monomers than dimers (Figure 7B), consistent with results shown in Figure 5A. In addition to enhancing fXI activation, poly-P induces plasma coagulation by directly promoting factor XII (fXII) activation.29 Poly-P of 70 to 85 phosphate units is a relatively weak inducer of fXII activation and, similar to results with diluted PTT reagent, induced clot formation more slowly in plasma containing fXI monomers (Figure 7C).
Plasma clotting assays. (A) fXIWT (WT), fXIC321S (321), fXIC321S,L284A (284/321), or fXIC321S,I290A was added to fXI-deficient human plasma anticoagulated with 0.32% sodium citrate to a final concentration of 30 nM. –XI indicates vehicle-treated control plasma. Plasma containing fXI (30 μL) was mixed with an equal volume of PTT reagent, followed by incubation for 5 minutes at 37°C. Thirty microliters of 25 mM CaCl2 was added and time to fibrin clot formation was determined. Proteins were tested in triplicate. (B-C) fXI-deficient plasma-containing vehicle or 30 nM fXIWT, fXIC321S,L284A, or fXIC321S,I290A supplemented with 60 μM PC–PS vesicles was incubated with (B) 30 μL 1:256 dilution of PTT-reagent or (C) 30 μL poly-P (final concentration 4 μM). Mixtures were incubated for 1 minute at 37°C before addition of CaCl2 and measuring time to clot formation, as above. (D) fXI-deficient plasma containing 8 μM corn trypsin inhibitor (to inhibit fXIIa), 60 μM PC–PS vesicles, and 16 μM poly-P (final concentration 4 μM) was mixed with an equal volume of 30 nM fXIWT, fXIC321S,L284A, or fXIC321S,I290A. β-thrombin was added to a final concentration of 1.5 nM, followed by incubation for 1 minute at 37°C. CaCl2 was added and the time to clot formation was measured. For all panels, error bars represent 1 standard deviation.
Plasma clotting assays. (A) fXIWT (WT), fXIC321S (321), fXIC321S,L284A (284/321), or fXIC321S,I290A was added to fXI-deficient human plasma anticoagulated with 0.32% sodium citrate to a final concentration of 30 nM. –XI indicates vehicle-treated control plasma. Plasma containing fXI (30 μL) was mixed with an equal volume of PTT reagent, followed by incubation for 5 minutes at 37°C. Thirty microliters of 25 mM CaCl2 was added and time to fibrin clot formation was determined. Proteins were tested in triplicate. (B-C) fXI-deficient plasma-containing vehicle or 30 nM fXIWT, fXIC321S,L284A, or fXIC321S,I290A supplemented with 60 μM PC–PS vesicles was incubated with (B) 30 μL 1:256 dilution of PTT-reagent or (C) 30 μL poly-P (final concentration 4 μM). Mixtures were incubated for 1 minute at 37°C before addition of CaCl2 and measuring time to clot formation, as above. (D) fXI-deficient plasma containing 8 μM corn trypsin inhibitor (to inhibit fXIIa), 60 μM PC–PS vesicles, and 16 μM poly-P (final concentration 4 μM) was mixed with an equal volume of 30 nM fXIWT, fXIC321S,L284A, or fXIC321S,I290A. β-thrombin was added to a final concentration of 1.5 nM, followed by incubation for 1 minute at 37°C. CaCl2 was added and the time to clot formation was measured. For all panels, error bars represent 1 standard deviation.
The effect of poly-P on thrombin-mediated fXI activation was tested in plasma containing a fXIIa inhibitor. Clotting was induced with β-thrombin,14,17 which converts fibrinogen to fibrin poorly due to disruption of anion-binding exosite I.31 As this exosite is not involved in fXI activation, β-thrombin and α-thrombin activate fXI similarly.14 In the reactions shown in Figure 7D, β-thrombin activates fXI bound to poly-P, leading to α-thrombin generation from plasma prothrombin and clot formation. β-thrombin induced clot formation more rapidly in the presence of fXIWT (∼100 seconds) than in its absence (∼750 seconds), confirming the system’s fXI dependence (Figure 7D). fXI monomers and dimers support β-thrombin–initiated clotting similarly (Figure 7D), consistent with data in Figure 5G and supporting the premise that fXI need not be a dimer for activation by thrombin in plasma in the presence of poly-P.
Discussion
The fXI gene arose from a duplication of a gene for a monomeric protein (PK).6,7 That the fXI dimer is conserved6,11,32 and that the parent homolog PK is a monomer implies that dimerization is required for specific aspects of fXI biology. In the original coagulation cascade, fXI is activated by fXIIa, and fXIa converts factor IX to factor IXaβ.1,33,-35 It is clear that fXIa does not need to be dimeric to activate factor IX, as fXIa with 1 active subunit (1/2-fXIa, fXIC321S,L284A, and fXIC321S,I290A)9,15 activates factor IX normally. Recent evidence indicates that the dimer may be required for zymogen activation. Wu et al showed that fXI monomers are activated more slowly than dimers by fXIIa and by thrombin and autoactivation in the presence of DS.9 They proposed that fXI activation involves a trans-activation mechanism in which a protease (fXIIa, thrombin, or fXIa) binds to a fXI subunit and activates the opposite subunit within the dimer. Based on this hypothesis, we postulated that fXI monomers would not reconstitute fXI−/− mice as well as dimeric fXI in a thrombosis model requiring fXI activation by fXIIa.11,26,-28 Indeed, fXI monomers support arterial thrombus formation poorly after vessel injury with FeCl3. Monomer fXIC321S,L284A demonstrated some activity in mice, while fXIC321S,I290A did not (Figure 3D). The reason for this is uncertain, but it may reflect subtle differences between the 2 proteins that are not obvious in in vitro assays. It is not surprising that a monomer would exhibit some activity, as the defect in fXIIa-mediated activation observed in vitro, while significant, is not total. Interestingly, fXI−/− mice expressed fXI dimers and monomers comparably. In the past, it was suspected that intracellular processing and secretion of fXI required dimerization. Meijers et al noted that a mutation associated with congenital fXI deficiency (Phe283Leu) caused a partial dimerization defect, with monomer retained within transfected cells.36 We reported similar results for mutation Glu350Gly.37 However, most missense mutations causing fXI deficiency result in reduced protein secretion, regardless of their impact on dimer formation.1,37,38 Subsequent work with mutations targeting the dimer interface9,10 and the data presented here show that dimerization is not a prerequisite for secretion.
Results of our in vitro analysis only partly support the published trans-activation hypothesis for fXI.9 Modeling data across a range of fXIIa concentrations suggests that fXI subunits harbor 2 productive binding sites with different affinities for fXIIa. The lower-affinity site would be contained within a subunit, while a higher-affinity site may extend onto the neighboring subunit. Supporting this, higher fXI activation rates were seen at 100 nM fXIIa than expected from the second-order rate constant calculated for activation by fXIIa at 5 to 30 nM. The behavior of the monomer at low fXIIa concentrations can be explained by absence of the higher-affinity site. As fXIIa concentration increases, the low-affinity sites common to monomers and dimers are saturated, resulting in comparable activation rates. Our results do not indicate that fXI must be a dimer for activation by thrombin. Given the absence of hemostatic abnormalities associated with factor XII deficiency, thrombin may be the more important fXI activator during hemostasis.11,,-14,35,39,40 A putative α-thrombin binding site was mapped to residues Ala45 to Arg70 in the fXI A1 domain.41 In the fXI subunit the protease domain is interposed between this site and the activation cleavage site.5 This configuration appears to be incompatible with a mechanism in which thrombin binds to a fXI subunit and activates that subunit (cis-activation). Our results, in contrast, show that any binding site for α-thrombin likely lies within the fXI subunit undergoing activation.
Regardless of the activating protease, polyanions enhance fXI activation.9,12,13,16,17 A number of physiologic counterparts to the polyanions used in vitro have been proposed. In fXII/fXI–dependent mouse thrombosis models, in particular, poly-P appears to play a role.29 It has been suggested that poly-P contributes to fXII activation in these models.29 The results presented here raise the possibility that poly-P may also support fXI activation. Phosphate polymers are ubiquitous in the biological world.42,43 Poly-P from platelet-dense granules can alter fibrin fibril thickness, accelerating factor V activation, and induce fXII activation.29,43,44 Choi et al showed that poly-P enhances fXI activation by thrombin and autoactivation.17 We noted that the defect in fXIIa-mediated activation of fXI monomers in the absence of a polyanion was also evident in the presence of poly-P. However, fXI monomers and dimers were activated similarly by thrombin and by autoactivation in the presence of poly-P. The discrepancy between these data and published work9 may partly reflect differences between poly-P (a linear polymer) and DS (a branched polymer). However, this does not completely explain the disparate results. In our study, fXI need not be a dimer to undergo autoactivation with DS or poly-P. Furthermore, results with active site–inhibited 1/2-fXIa indicate that the process does not require activation of a fXI subunit by an active subunit within that same dimer, as suggested earlier.45 The current data and published work13,16,17 are most consistent with a mechanism in which polyanions enhance fXI activation by bringing an activating protease and the substrate into proximity, and perhaps by inducing conformational changes in the protease and/or substrate. For fXI activation by thrombin or by autoactivation, in contrast to activation by fXIIa, a cis-activating mechanism appears sufficient.
The study on thrombus formation in mice demonstrated that the dimeric structure of fXI is important in vivo, at least under the conditions of the model. Based on this, it is tempting to postulate that fXI dimerization is an adaptation to enhance zymogen activation in certain circumstances. However, there are reasonable objections to this proposal. In vitro, fXI monomers were defective only when fXIIa was the activating protease. fXIIa may contribute to pathologic coagulation through fXI activation11,28,29 ; but there is no compelling evidence indicating it is required for hemostasis.1,35,39,40 PK, the parent homolog of fXI, is a monomer with a domain structure identical to that of fXI. PK is efficiently activated by fXIIa in vitro.6,-8,35 Reducing fXII expression in mice with antisense oligonucleotides results in decreased PK activation, indicating fXIIa activates PK in vivo.46 Cumulatively, these observations raise the possibility that aspects of protease function unrelated to zymogen activation drove the evolution of the fXI dimer. There is evidence that fXI needs to be dimeric to function properly in the presence of platelets. Vitamin K–dependent coagulation proteases bind to phospholipid membranes, localizing their activity to sites of vessel injury and preventing them from being removed from a wound site by blood flow.39 As a protease involved in hemostasis, it is reasonable to expect fXI activation and fXIa activity to be localized to an injury site. While fXI lacks the domain that facilitates phospholipid binding for vitamin K–dependent proteases,3,-5 it does bind to platelets.47,48 Previously, we proposed that fXI may bind to a platelet through 1 subunit, while binding to its substrate factor IX through the other.49 In this scenario, the dimer may be an adaptation to facilitate proper protease localization and function in flowing blood. Studies using flow-based models may help clarify the relevance of this mechanism in the future.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge support from awards from the National Heart, Lung, and Blood Institute (HL81326 and HL58837 [D.G.] and HL080018 [I.M.V.]). D.G. is a consultant and receives consultant’s fees from several pharmaceutical companies.
Authorship
Contribution: Y.G. performed experiments of fXI activation by fXIIa, thrombin, and autoactivation and wrote the manuscript; I.M.V. contributed to design of experiments of fXI activation in vitro and performed kinetic analyses of data; S.B.S. performed experiments with 1/2-fXIa; M.S. prepared and characterized recombinant fXI and fXI variants; A.M. was involved in assessment of fXI activation in plasma; Q.C. conducted HTI experiments; S.A.S. prepared and characterized poly-P and contributed to experimental design; J.H.M contributed to experimental design of experiments with poly-P and to writing of the manuscript; and D.G. was responsible for oversight of the project and preparation of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Gailani, Hematology/Oncology Division, Vanderbilt University, 777 Preston Research Building, 2220 Pierce Ave, Nashville, TN; e-mail: dave.gailani@vanderbilt.edu.