Recent improvement in modern analytical technologies has stimulated an explosive growth in the study of glycobiology. In turn, this has lead to a richer understanding of the crucial role of N- and O-linked carbohydrates in dictating the properties of the proteins to which they are attached and, in particular, their centrality in the control of protein synthesis, longevity, and activity. Given their importance, it is unsurprising that both gross and subtle defects in glycosylation often contribute to human disease pathology. In this review, we discuss the accumulating evidence for the significance of glycosylation in mediating the functions of the plasma glycoproteins involved in hemostasis and thrombosis. In particular, the role of naturally occurring coagulation protein glycoforms and inherited defects in carbohydrate attachment in modulating coagulation is considered. Finally, we describe the therapeutic opportunities presented by new insights into the role of attached carbohydrates in shaping coagulation protein function and the promise of carbohydrate modification in the delivery of novel therapeutic biologics with enhanced functional properties for the treatment of hemostatic disorders.
Introduction
Glycan structures are attached to more than half of all known proteins,1 and genes encoding the molecular apparatus required for glycosylation constitute 1% to 2% of the human genome.2 Despite the prevalence of glycan attachment to human proteins and lipids, the field of glycobiology has traditionally represented something of a Cinderella subject. However, recent advances in synthetic, and particularly analytic, methodologies have led to heightened awareness regarding the structural and functional significance of carbohydrate structures on proteins. Accumulating data make it clear that the glycan structures expressed on many glycoproteins play critical roles in modulating functional activity. In addition, variation in carbohydrate structures has been implicated in the pathogenesis of a number of human diseases. Moreover, it seems inevitable that evidence regarding the physiological and pathological importance of carbohydrate expression will continue to emerge in the coming years. In this context, it is perhaps unsurprising that regulation of glycan expression on novel recombinant therapeutic glycoproteins is already established as a key quality parameter within the pharmaceutical industry. In this review, we provide an overview of the critical roles played by carbohydrate determinants in regulating human hemostasis and thrombosis. In particular, using exemplar coagulation glycoproteins, we have sought to highlight some of the different molecular mechanisms through which glycan variation can influence glycoprotein biology. Although we have selected specific examples and focused on plasma coagulation glycoproteins, these concepts can nevertheless be considered a paradigm equally applicable to other human secretory glycoproteins.
Protein glycosylation
N-linked glycosylation
N-linked glycans on human glycoproteins are attached to the amide nitrogens of asparagine (Asn) side chains. N-linked glycosylation begins in the endoplasmic reticulum (ER),3,4 where a preassembled oligosaccharide core structure is transferred from a dolichol lipid donor onto specific Asn residues within nascent polypeptide chains.5 This reaction is catalyzed by the enzyme complex oligosaccharyltransferease, which targets Asn residues located in the consensus sequence Asn-X-serine (Ser)/threonine (Thr) (where X can be any amino acid except proline).6 Importantly, N-linked glycosylation within the ER is actually a cotranslational event occurring on the luminal aspect of the ER membrane. As a consequence, depending on polypeptide folding and conformation, not all Asn residues in a consensus sequence will necessarily be glycosylated. The net effect, therefore, is that polar N-linked glycans are typically found on the surfaces of glycoproteins, rather than being buried deep within the protein interior.
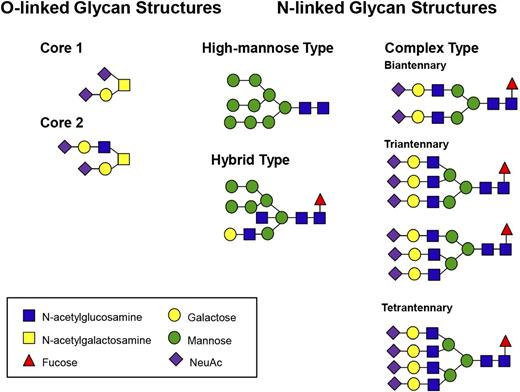
The initial 14-sugar core N-linked structure attached during protein synthesis in the ER is mannose-rich (Glc3Man9GlcNAc2). This core glycan is subsequently remodeled by a series of glycosyltransferases and glycosidases as the protein passes through the ER and onto the Golgi.7 This process commences in the ER with the removal of 2 terminal glucose moieties by the exoglycosidases glucosidase 1 and 2. Glucose cleavage enables the protein to interact with 2 homologous ER lectins, calnexin (Cnx) and calreticulin (Crt), and thereby engage in a folding cycle.8 Once properly folded, glycoproteins are subsequently transported to the Golgi, where the N-linked glycans are further modified. Unsurprisingly, given that more than a hundred different glycosyltransferases are encoded by the human genome, the final N-linked carbohydrate structures can be complex and heterogeneous in nature. Nevertheless, N-linked glycans can be classified into 1 of 3 subgroups: high-mannose, hybrid, or complex (Figure 1). This process is distinct from that of glycation, which refers to the nonenzymatic irreversible attachment of reducing sugars to proteins, and in contrast to glycosylation, it is not enzyme-controlled or dependent on predefined attachment sites.
Examples of typical N- and O-linked glycan structures expressed on human plasma glycoproteins.
Examples of typical N- and O-linked glycan structures expressed on human plasma glycoproteins.
O-linked glycosylation
O-linked glycans on human glycoproteins are attached to Ser or Thr residues. O-linked glycosylation differs from N-linked glycosylation in a number of important regards.9 First, O-linked glycosylation is a true posttranslational modification, as O-linked carbohydrate structures are only synthesized on proteins as they transit through the Golgi. Second, there is no preassembled O-linked oligosaccharide core structure. Rather, O-linked carbohydrate synthesis involves sequential addition of monosaccharide units in a stepwise manner. These reactions are catalyzed by a series of specific glycosyltransferases analogous to those required for N-linked glycans synthesis. Finally, for O-linked glycosylation to occur, Ser or Thr residues do not need to exist as part of a specific consensus sequence. Nevertheless, previous studies have shown that O-linked glycosylation of Ser or Thr is more common if Ser/Thr residues are present in clusters or are located in areas rich in proline or alanine residues.10 Final O-linked glycan structures are simpler than complex N-linked sugars (Figure 1).
Heterogeneity of glycosylation
In view of the number of distinct human glycosyltransferases and glycosidases already described, it is perhaps not surprising that glycan databases include descriptions of more than 500 different N-linked carbohydrate structures. The marked heterogeneity of N-linked glycans structures has proven one of the major obstacles to the investigation of the potential physiological and pathological significance of carbohydrate structures. A further level of complexity is added by virtue of the fact that many glycoproteins contain multiple individual N- and O-linked glycosylation sites. In addition, different types of glycan structures can be expressed on each of these specific Asn residues within the same protein. Importantly, expression levels for the individual glycosyltransferase and glycosidase enzymes vary significantly between different tissues and can also be influenced by disease state or normal aging. As a consequence, a given individual can express various glycoforms of a particular glycoprotein that differ only with respect to their carbohydrate profiles.
Many of the human proteins involved in regulating normal hemostasis circulate as soluble glycoproteins in plasma. Before their secretion, these proteins often undergo complex posttranslational modification, including significant glycosylation. As a result, complex branching carbohydrate structures can account for up to 25% of their final molecular mass. These carbohydrate structures play critical, but often underappreciated, roles in modulating many of the key biological properties of these coagulation proteins.
Role of glycans in modulating intracellular trafficking
Carbohydrate determinants regulate transit through the ER
Secretory glycoproteins, including coagulation factors, are synthesized by ER-bound ribosomes. After processing within the ER, proteins follow an intracellular pathway through the ER–Golgi intermediate compartment (ERGIC) to the Golgi before finally being secreted into the plasma. Within the lumen of the ER, significant folding and modification of newly synthesized proteins occurs. This process is regulated by a series of enzymes and molecular chaperones, including immunoglobulin-binding protein (BiP), Cxn, and Crt. Only correctly folded proteins are allowed to exit the ER. Misfolded proteins either are retained within the ER or are subject to degradation by the ER-associated protein degradation pathway.4
Previous studies have clearly defined the critical role played by carbohydrate structures in regulating glycoprotein interaction with ER-resident molecular chaperones. In particular, the 14-sugar core N-linked structure on nascent polypeptide chains is a key regulator of these interactions. Cxn and Crt are homologous lectins that bind monoglucosylated polypeptides in concert with the thiol oxidoreductase ER p57, facilitating correct folding and preventing protein aggregation. The fate of the Cxn/Crt-bound polypeptide is ultimately determined by uridine diphosphate–glucose:glycoprotein glucosyltransferase (GT), which acts as a folding sensor that detects characteristic biophysical properties of misfolded proteins. If misfolding is detected, the protein is reglucosylated by GT and reenters the Cxn/Crt cycle, where it can either continue until correctly folded or be transferred for degradation. If properly folded, GT does not reglucosylate high mannoses, and the protein is ready for transport to the Golgi apparatus.
The physiological relevance of carbohydrate determinants in regulating transit through the ER has been highlighted in a series of elegant studies examining biosynthesis of the homologous coagulation glycoproteins factor V (FV) and factor VIII (FVIII), respectively. Once activated, these glycoproteins play crucial roles in the coagulation cascade, acting as cofactors in the prothrombinase and intrinsic tenase complexes. FV and FVIII share identical domain structures (A1-A2-B-A3-C1-C2), and significant structural homology exists between their A and C domains. In contrast, the B domains of FV and FVIII exhibit limited sequence similarity.11 Nevertheless, both B domains are extensively glycosylated, containing 25 (FV) and 18 (FVIII) potential N-linked glycosylation sites, respectively. Moreover, although the amino acid sequence encoding the FVIII B domain has diverged widely between human, porcine, and murine genes, the large number of N-linked glycosylation sites has remained strikingly conserved. In spite of their homologous structures, expression studies have demonstrated that FV is secreted from mammalian cells significantly more efficiently than FVIII. Limiting steps in FVIII secretion have been identified and include extended interactions with ER chaperone molecules, which in turn limit its progress to the Golgi and onward to secretion from the cell. In particular, residues within the A1 domain of FVIII have been shown to mediate stable interaction with BiP.12 In contrast, FV does not associate with BiP.13 Furthermore, FVIII has also been shown to bind both Crt and Cxn, which also slows its secretion.14 Unsurprisingly, these interactions are mediated in large part through N-linked glycan structures expressed within the FVIII B domain. Although FV can also interact with the chaperone Crt, it does not appear to bind to Cnx.
Carbohydrate determinants regulate transit from ER to Golgi
On successful folding and packaging, new proteins travel from the ER to the Golgi for additional posttranslational modifications before secretion. This is achieved by formation of coat protein complex II (COPII) vesicles, which bud from the ER lumen and migrate to the Golgi apparatus via the ERGIC.15 Despite their different ER processing, FV and FVIII have a shared prerequisite for specialized ER-to-Golgi transport machinery. In particular, lectin mannose-BiP 1 (LMAN1; also known as ERGIC-53) and multiple coagulation factor deficiency protein 2 (MCFD2) are cargo transporters for ER-to-Golgi traffic of FV and FVIII.16,17
N-linked oligosaccharides are key to FV/FVIII interactions with the LMAN1/MCFD2 complex. LMAN1 association with FV/FVIII is enhanced by the presence of fully glucose trimmed mannose 9 structures on B domain–located carbohydrates, as demonstrated using an LMAN1 mutant with defective mannose binding ability and, consequently, severely diminished FVIII-LMAN1 interaction. LMAN1/MCFD2 gene mutations that prevent interaction with FV/FVIII (or each other) have been shown to be the cause of combined FV/FVIII deficiency, an autosomal recessive disorder associated with a mild to moderate bleeding tendency caused by reduced FV and FVIII plasma levels of 5% to 30%.18,19 Cumulatively, these data serve to emphasize the critical importance of N-linked glycan structures in regulating the intracellular trafficking of secretory glycoproteins.
Role of glycans in modulating susceptibility to proteolysis
In addition to modulating intracellular processing, carbohydrate expression on plasma glycoproteins influences a number of their biological properties. In particular, glycans have been shown to modulate susceptibility to proteolysis. The critical importance of sugar structures in this regard has been highlighted through a series of recent studies on the proteolysis of the large, multimeric sialoglycoprotein, von Willebrand Factor (VWF). Before secretion, VWF undergoes extensive posttranslational modification, including significant glycosylation. As a result, each VWF monomer contains 12 potential N-linked and 10 potential O-linked glycosylation sites with carbohydrate structures.20 The glycan structures of VWF have been characterized21 and shown to be highly complex and heterogeneous in nature. The most common N-linked structure is a monosialylated biantennary complex chain.22 In contrast, the majority of the O-linked glycans of VWF are composed of the tumor-associated T-antigen.23 Thus, both the N- and O-linked VWF glycans are commonly capped by terminal negatively charged sialic acid moieties. Unusually, covalently linked ABO(H) blood group carbohydrate determinants have also been described as terminal sugar residues on a proportion of both the N-linked (13%) and O-linked (1%) glycans of VWF.22 ,24 ,25
Plasma VWF multimer composition is a critical determinant of functional activity. High–molecular weight multimers bind both subendothelial collagen and platelet glycoprotein Ibα; with significantly higher affinities than lower–molecular weight forms.26 Interestingly, the O-linked glycans of VWF have recently been reported to modulate the critical interaction with glycoprotein Ibα.27,28 In normal plasma, the multimeric composition of circulating VWF is tightly controlled by a disintegrin and metalloproteinase with thrombospondin type repeats 13 (ADAMTS13). The physiologic importance of regulating VWF multimer composition is highlighted in type 2A VW disease and thrombotic thrombocytopenic purpura. In type 2A VWD, increased proteolysis is associated with concomitant loss of High–molecular weight multimers and a bleeding phenotype. Conversely, inherited or acquired ADAMTS13 deficiency or dysfunction results in thrombotic thrombocytopenic purpura, characterized by an excess of circulating ultralarge VWF with the subsequent development of platelet-rich thrombi in the microvasculature.29
For many years, it has been recognized that glycosylation profiles on circulating glycoproteins play critical roles in modulating susceptibility to proteolysis. In particular, loss of terminal sialic acid expression has been shown to result in increased proteolysis by various different proteases.30 ,31 For example, desialylation of VWF significantly enhances sensitivity to proteolysis by plasmin, chymotrypsin, and cathepsin B. In contrast to this accepted paradigm, however, recent data from our laboratory have shown that depletion of terminal α2-6 linked sialic acid from the N-linked glycans of VWF specifically inhibits susceptibility to ADAMTS13 proteolysis.32 Furthermore, terminal expression of ABO(H) blood group determinants on VWF glycans has also been shown to influence VWF permissiveness to ADAMTS13-mediated proteolysis (in the order O ≥ B ≥ A ≥ AB).33 ,34 Site-directed mutagenesis studies have suggested that glycan expression at Asn-1574 in the VWF A2 domain adjacent to the ADAMTS13 cleavage site may be of particular importance in this context.35 Mutation of this Asn residue with subsequent elimination of the associated glycan chain resulted in markedly enhanced susceptibility of VWF to ADAMTS13 proteolysis. In contrast, mutation of neighboring glycan Asn-1515 had no such effect. On the basis of these findings, therefore, it is clear that variations in carbohydrate expression profiles can critically regulate plasma glycoprotein susceptibility to proteolysis, and thereby influence normal physiology. Moreover, these alterations in carbohydrate structure may involve only subtle changes in terminal glycan determinant expression. In addition, glycan variation at particular N-linked sites may also be of specific importance in this regard.
Role of glycans in modulating biological activity
Previous in vitro studies have demonstrated that modification of carbohydrate determinants on plasma glycoproteins, by either exoglycosidase digestion or site-directed mutagenesis, can significantly influence key aspects of biological function. It is important to note, however, that even in normal individuals, some plasma glycoproteins naturally circulate as different glycoforms (Table 1). Although these protein isomers contain identical amino acid compositions, they differ with respect to the number and/or type of their attached glycan structures. As a consequence of altered glycosylation profiles, individual glycoforms may demonstrate clinically relevant differences in their functional properties. Within the coagulation cascade, there are several notable examples of naturally occurring, partially glycosylated plasma glycoforms that exhibit differential functional properties compared with their fully glycosylated counterparts.
Coagulation is initiated in vivo by the exposure of tissue factor (TF) on extravascular cells on vascular injury, which then interacts with activated factor VII (FVIIa) to activate factor X (FX). TF has N-linked glycosylation consensus sequences at 3 positions (Asn-11, Asn-124, and Asn-137); however, their contribution to TF procoagulant activity is subject to debate. TF possesses different glycan structures depending on whether it was derived from human placenta or generated via recombinant expression in bacterial or insect cells.36 -38 Deglycosylation of placenta-derived TF resulted in a significant (fourfold) reduction in catalytic rate (kcat) for extrinsic FXase activity, indicating an important role for N-linked glycosylation in modulating TF procoagulant function.38 In contrast, previous studies have reported that recombinant TF expressed in Escherichia coli possessed similar procoagulant activity to that expressed in mammalian cells.39 In addition, recent studies have shown that recombinant TF mutants lacking specific individual N-linked glycan consensus sequences also exhibit functional activity similar to that of wild-type TF.40
Human coagulation FX is activated in vivo by FIXa and FVIIa in the presence of cofactors FVIIIa and TF, respectively. FX possesses 2 N-linked (Asn-39 and Asn-49) and 2 O-linked (Thr-17 and Thr-29) glycosylation sites, all of which are contained within the activation peptide of the zymogen protein. Various reports have suggested that FX carbohydrate moieties can modulate FX activation.41 -43 Mutagenesis of FX N- and O-linked glycan attachment sites significantly increased FX activation by FVIIa or FIXa but exhibited a limited effect on the catalytic efficiency of either the intrinsic (FIXa/FVIIIa) or extrinsic (TF/FVIIa) FXase complexes.42 Enzymatic desialylation of FX attenuates the rate of activation by either the intrinsic or extrinsic FXase complex, implying an important role for terminal sialic acids in enhancing FXase complex formation.43 Further to their role in FX activation, N-linked (but not O-linked) glycans on the FX activation peptide have been proposed to protect FX from rapid clearance via glycan-dependent interactions with macrophages in mice, accounting for its prolonged plasma half-life in comparison with related vitamin K–dependent zymogens.44 ,45
The serine proteinase inhibitor antithrombin constitutes the major plasma inhibitor of thrombin and circulates as a single-chain glycoprotein that possesses 4 N-linked glycosylation sites at Asn-96, Asn-135, Asn-155, and Asn-192, respectively. These glycan structures exist predominantly in the form of disialylated biantennary complex chains.46 Two different plasma glycoforms of antithrombin (α- and β-antithrombin) have been described.47 Fully glycosylated α-antithrombin accounts for the majority of total plasma antithrombin. In addition, a minor glycoform (β-antithrombin) contributes 10% to 15% of total plasma antithrombin. This glycoform is identical to α-antithrombin but for the absence of any N-linked oligosaccharide expression at Asn-135.48 The loss of this specific glycan chain results in markedly enhanced protease inhibitor activity.47 ,49 As a consequence, despite representing only a small minority of plasma antithrombin, β-antithrombin has been suggested to be the principal mediator of antithrombin protease inhibitor activity in vivo.50 Kinetic and crystallographic analyses of the molecular basis underlying the enhanced activity of β-antithrombin have demonstrated that the presence of the oligosaccharide structure at Asn-135 sterically impedes a conformational change required to activate antithrombin on heparin/heparan binding.51 Thus, absence of this steric hindrance at Asn-135 in β-antithrombin enables rapid adoption of an active conformation once bound to heparin, thereby enhancing its inhibitory activity.52
Protein C (PC), similar to antithrombin, is crucial for the regulation of thrombin generation in vivo. PC circulates in zymogen form and is activated by the thrombin–thrombomodulin complex. After activation by the thrombin–thrombomodulin complex, activated PC (APC) inhibits further thrombin generation by proteolytic degradation of procoagulant-activated cofactors FVa and FVIIIa. PC possesses 4 N-linked glycosylation sequons: 1 located within its first epidermal growth factor (Asn-97) and the remaining 3 located in its protease domain (Asn-248, Asn-313, and Asn-329).
In addition, 3 different glycoforms of human PC have been described in normal human plasma: α-PC accounts for 70% of total plasma PC and is characterized by the presence of complex biantennary oligosaccharide chains at all 4 N-linked glycosylation sites, β-PC accounts for approximately 25% of total plasma PC and differs from α-PC in that it is not glycosylated at Asn-329,53 and γ-PC represents only 5% of total plasma PC and lacks oligosaccharide chains attached at both Asn-329 and Asn-248. Several lines of evidence support the hypothesis that these different glycoforms of PC have important biological differences. For example, site-directed mutagenesis studies have suggested that PC activation by the thrombin–thrombomodulin complex is modulated by the presence of N-linked oligosaccharides at Asn-313.54 APC anticoagulant activity may also be subject to modulation by its glycan structures, but reports on its importance have been conflicting. Specifically, a naturally occurring PC mutation encoding only β-PC (N329T) exhibited mildly reduced anticoagulant activity when purified from plasma, activated, and assayed for its ability to degrade FVa.
In contrast, a recombinant version of β-APC in which glycosylation at Asn-329 was eliminated exhibited approximately twofold increased anticoagulant activity compared with wild-type recombinant APC.54 ,55 In addition to its anticoagulant role, APC also possesses potent anti-inflammatory and antiapoptotic activity that is mediated at least in part by activation of protease-activated receptor 1 (PAR1).56 We have recently demonstrated that a recombinant APC mutant that mimics the glycosylation pattern of β-APC (APC-N329Q) exhibits an increased capacity to maintain endothelial cell barrier integrity and inhibit endothelial cell apoptosis compared with wild-type APC.55 Interestingly, Asn-329 is located proximal to a putative PAR-1 binding exosite on the surface of the APC protease domain,57 and recent work has indicated that elimination of the oligosaccharide chain at this position accelerates the rate of PAR1 cleavage by APC, possibly by facilitating increased PAR1 access to the binding exosite on APC (E. M. Gleeson, J. S. O'Donnell, and R. J. Preston, unpublished data). On the basis of these findings, therefore, it is clear that the different APC glycoforms present in normal human plasma exhibit important differences in their biological activities that are likely to be of physiological and pathological relevance.
Partial N-linked glycosylation resulting in the synthesis of heterogeneous glycoforms with distinct biological properties has also been reported for a number of important procoagulant plasma glycoproteins. Human FV is abundantly glycosylated, with both N- and O-linked carbohydrate structures accounting for 15% to 25% of the total molecular mass. FV is activated by limited specific proteolysis by either thrombin or FXa and then serves as a critical cofactor in the prothrombinase complex. Subsequently, FVa is inactivated by APC-catalyzed proteolysis at Arg-306 and Arg-506. Inactivation of FVa by APC plays a critical role in down-regulating thrombin formation. Two different glycoforms of FV are present in the normal human circulation. As a consequence, activation by thrombin results in the generation of 2 distinct forms of FVa (FVa1 and FVa2) that differ only with respect to their glycosylation profiles.58 Site-directed mutagenesis studies have established that unlike FVa1, the FVa2 glycoform appears to result from partial glycosylation at Asn-2181 in the C-terminal C2 domain.59 Importantly, several reports have shown that this variation in the N-linked glycan component of FVa significantly modulates its functional properties.58 -60 For example, the affinity of the human FVa2 glycoform binding to anionic phospholipids was approximately threefold higher than that of FVa1.59 Moreover, FVa1 and FVa2 also displayed differential susceptibilities to APC-mediated proteolysis. In particular, at low phospholipid concentrations, FVa1 was inactivated at a 15-fold slower rate compared with FVa2.60 These distinct biological differences serve as a further example of how the relative concentrations of naturally occurring coagulation protein glycoforms have the potential to markedly influence overall thrombin generation at sites of vascular injury.
Role of glycans in modulating clearance
N- and O-linked carbohydrate structures play major roles in determining the rate of clearance of many human glycoproteins from plasma. Terminal sialic acids are of critical importance in this regard. The removal of capping sialic acid residues leading to exposure of penultimate Gal and GalNAc moieties typically results in markedly enhanced glycoprotein clearance. In mammals, desialylation is achieved by a family of 4 sialidases (also known as neuraminidases; Neu1-Neu4) that catalytically remove α-glycosidase-linked sialic acid groups from carbohydrate structures.61 This clearance is mediated primarily via the hepatic lectin asialoglycoprotein receptor (ASGPR or Ashwell receptor). A member of the calcium-dependent (C-type) lectin receptor family abundantly expressed in the liver, ASGPR is composed of 2 homologous trans-membrane polypeptides (Asgr-1 and Asgr-2) that assemble into a hetero-oligomer on the cell surface. The C-terminal extracellular domains of Asgr-1 and Asgr-2 form a carbohydrate recognition domain that selectively binds glycoproteins expressing either β-d-galactose (βGal) or N-acetyl-d-galactosamine (GalNAc) terminal sugar determinants in a calcium-dependent manner. However, these βGal and GalNAc residues are more typically expressed on plasma glycoproteins as subterminal moieties on oligosaccharide chains capped by sialic acid. If the terminal sialic acid residue is lost, the ASGPR can bind the exposed βGal or GalNAc and mediate endocytosis.
The critical importance of sialic acid expression in determining plasma half-life has been observed for several different coagulation glycoproteins. Enzymatic removal of terminal sialic acid residues from the abundantly sialylated VWF ex vivo markedly reduces plasma half-life in rabbits (240 vs 5 minutes for normal and desialylated VWF, respectively).62 In keeping with this observation, genetic inactivation of a specific sialyltransferase (ST3Gal-IV) in a transgenic mouse also resulted in significantly reduced plasma VWF levels as a consequence of a twofold increased rate of clearance.63 The importance of the ASGPR in modulating physiological VWF clearance is further underlined by recent data demonstrating that VWF half-life is significantly increased in ASGPR-1 knockout mice.64
In addition to its role in regulating VWF plasma clearance, the ASGPR may also modulate the clearance of a number of other coagulation glycoproteins, including FVIII. As previously described, FVIII is heavily glycosylated, and the N-linked glycans of human FVIII are commonly capped by negatively charged sialic acid residues.65 Surface plasmon resonance studies have demonstrated that FVIII also binds the ASGPR with high affinity (Kd = 2 nM). This interaction is mediated through the N-linked carbohydrate structures clustered within the B domain of FVIII. Furthermore, administration of an ASGPR antagonist significantly inhibited FVIII clearance in mice, suggesting that the ASGPR may contribute to normal physiological clearance of FVIII from plasma.66
Similar to sialic acid, ABO(H) blood group determinants are also expressed as terminal sugar residues on the carbohydrate structures of both VWF and FVIII. This ABO(H) expression has direct clinical relevance, as ABO blood group is major determinant of plasma VWF levels. Group O individuals have 25% less circulating VWF compared with non-O individuals (group A, B, or AB).67 ,68 Moreover, plasma VWF levels are even lower in individuals with the rare Bombay blood group phenotype, in which H antigens are not expressed.34 The effect of ABO(H) blood group antigens on VWF levels is explained by differences in clearance rates between each blood group. As such, the VWF plasma half-life is significantly shorter in normal group O vs non-O individuals (10.0 vs 25.5 hours, respectively).67 Nevertheless, the molecular mechanism underlying the enhanced clearance of group O VWF remains unknown. However, given that the ASGPR selectively binds either GalNAc or Gal residues, it seems likely that another clearance receptor is responsible for modulating this phenomenon. A weak effect of the Secretor blood group locus on plasma VWF levels has also been reported.69 Interestingly, this blood group system is similar to ABO, in that it is characterized by the presence or absence of specific terminal carbohydrate determinants on oligosaccharide structures. To date, it remains unclear whether this Secretor influence is also modulated through an effect on VWF clearance.
In addition to the ASGPR, a variety of other lectin receptors has been characterized. These lectins typically contain a carbohydrate recognition domain that has binding specificity for particular terminal glycans moieties expressed on N- and/or O-linked carbohydrate structures. Examples of other lectins that have been implicated in modulating glycoprotein clearance include Mac-1 (αMβ2), the macrophage galactose lectin, and the scavenger receptor C-type lectin.70 The relative contribution of these individual receptors in mediating clearance of individual plasma coagulation glycoproteins has not yet been defined. However, recent data have demonstrated that macrophage-mediated endocytosis may be important in the physiological clearance of both VWF and FVIII.71 Furthermore, data from our laboratory have shown that the rate of VWF clearance by macrophages is markedly influenced by VWF glycan expression.72 Recent data have also demonstrated that galectin 1, galectin 3, and siglec 5 can also all bind to human VWF.73 ,74 In addition, other putative lectin-like receptors that may be involved in determining plasma levels of the VWF-FVIII complex have been identified through genome-wide association studies and include C-type lectin domain family 4 member M and stabilin 2.75 Thus, although the molecular mechanisms responsible for modulating the clearance of glycoproteins from plasma remain poorly understood, carbohydrate expression is of critical importance in regulating the rate of clearance.
Aberrant glycosylation can cause human disease
Although rare, almost 50 different congenital disorders of glycosylation have been identified.76 These disorders typically involve defects in N-linked glycosylation and are associated with severe multiorgan clinical phenotypes including skeletal and neurological abnormalities. Significant coagulopathies have also been observed in children with congenital disorders of glycosylation.76 -78 In particular, factor XI, PC, antithrombin, and protein S are commonly deficient. The molecular mechanism or mechanisms responsible for the reduced plasma levels of these specific coagulation glycoproteins remains unclear. Nevertheless, significant thrombotic and bleeding complications are well recognized as constituting important clinical features of these conditions.77 ,78
Aberrant glycosylation of specific proteins, including coagulation factors, has also been implicated in the etiology of human pathology. Point mutations that result in the introduction of novel N-linked glycosylation sites are of particular importance. For example, the amino acid substitution Ile359Thr within the heavy chain of FV (FV Liverpool) creates a new N-linked glycosylation consensus sequence, such that an additional glycan chain is expressed at Asn-357.79 As a result, the FVa-Ile359Thr molecule is resistant to APC-mediated proteolysis, and consequently, FV Liverpool is associated with a prothrombotic phenotype. A number of different amino acid substitutions that introduce additional N-linked glycosylation sites have also been described in patients with congenital dysfibrinogenemia. These include fibrinogens Lima (Aα Arg141Ser),80 Caracas II (Aα Ser434Asn),81 Asahi (γ Met310Thr),82 and Kaiserslauten (γ Lys380Asn). In each of these cases, the attachment of an extra N-linked glycan causes impaired functional activity and a consequent bleeding tendency. Similarly, an FIX gene mutation that results in an extra glycosylation site has been identified in a family with hemophilia B.83 Interestingly, the Arg94Ser substitution actually leads to the introduction of a new O-linked glycosylation site in the second epidermal growth factor-like domain of FIX, which in turn markedly attenuates activation by FXIa.
In contrast, mutations leading to the loss of a single specific N-linked glycosylation site have also been implicated in disease pathology. Protein S is a plasma glycoprotein that is important in regulating thrombin generation in vivo. First, protein S functions as a nonenzymatic cofactor for APC inactivation of FVa and FVIIIa. In addition, protein S may also regulate hemostasis by APC-independent inhibition mechanisms. Protein S Heerlen is found in approximately 0.5% of the population and is characterized by a Ser to Pro substitution at position 460.84 This change results in the loss of N-linked glycosylation at Asn-458 and has been associated with an increased risk for venous thromboembolism.85 The clinical phenotype relates in part to the fact that the Ser460Pro substitution results in reduced plasma protein S levels because of an enhanced clearance.86 In addition, protein S Heerlen demonstrates reduced cofactor activity for APC-inactivation of FVIIIa.85
Glycan modification: therapeutic implications and opportunities
As summarized in this review, carbohydrate structures on human coagulation proteins play essential roles in determining stability, circulatory half-life, and biological activity. As a consequence, in the production of biopharmaceuticals, glycosylation is of critical importance. In particular, for the synthesis of recombinant glycoprotein therapeutics, it is well established that glycosylation profiles can vary significantly, depending on the cell line chosen for expression. Moreover, recombinant proteins generated in vitro can also demonstrate significant heterogeneity in terms of their glycan profiles. This obviously has major implications, given that many studies of coagulation protein structure and function have used recombinant proteins that may express carbohydrate determinants that differ markedly to those expressed on the native human proteins. Unsurprisingly, these glycan variations also can have important therapeutic sequelae. For example, recombinant FVIIa (rFVIIa; NovoSeven) used for the treatment of patients with hemophilia with inhibitors contains 2 N-linked and 2 O-linked glycans and is expressed in baby hamster kidney (BHK) cells.87 Although all 4 sites are glycosylated in the purified rFVIIa molecule, approximately 10% of rFVIIa molecules from BHK cells have N-linked glycans lacking terminal sialylation. Moreover, a further 30% of the rFVIIa possesses significantly reduced N-linked sialic acid expression. This variable sialylation has important consequences in determining the plasma half-life of therapeutic rFVIIa, as hyposialylated rFVIIa is rapidly cleared from the circulation through the hepatic ASGPR.88
Patients with hemophilia A can be treated using either plasma-derived or recombinant FVIII products. Unsurprisingly, glycan expression differs significantly between plasma-derived and recombinant FVIII.89 Moreover, glycosylation variation has also been described between different commercial recombinant FVIII products that have been synthesized in different mammalian cell lines (including Chinese hamster ovary and BHK).89 For example, rFVIII from Chinese hamster ovary cells express the NeuGc epitope, which accounts for 0.5% of total sialic acid.90 In contrast, Gal-α(1,3)Gal structures have been identified on ∼3% of BHK-expressed rFVIII. Importantly, high levels of antibodies against NeuGc and Gal-α(1,3)Gal both occur naturally in most humans. Interestingly, recent studies have also demonstrated that specific glycan chains on FVIII may influence dendritic cell uptake mediated through the macrophage mannose receptor (CD206).91 Thus, removal of the mannosylated sugars at Asn-239 (A1 domain) or Asn-2118 (C1 domain) abrogated dendritic cell endocytosis of FVIII and presentation to CD4+ T-cells. Importantly, these data raise the possibility that variations in glycan expression on recombinant FVIII products may influence immunogenicity and, consequently, risk for inhibitor development in patients with hemophilia.92
As our understanding of the critical role played by glycan structures in regulating the biological activity and half-life of plasma glycoproteins continues to further develop, it seems likely that significant opportunities for the development of novel therapeutic agents will arise. In particular, glycoengineering involving targeted selective carbohydrate modification may enable the development of recombinant glycoprotein therapies with improved clinical efficacy. From the data presented in this review, the wide spectrum of coagulation factor properties that can be influenced by protein glycosylation are readily apparent. Manipulation of carbohydrate structures may be useful in prolonging plasma half-life of recombinant clotting factor concentrates; for example, through hypersialylation. Alternatively, glycoengineering may be useful in reducing the immunogenicity of recombinant therapeutic glycoproteins. Clearly, even minor glycan modification of terminal sugar moieties, or indeed the loss or introduction of a specific glycosylation site, may be enough to develop a glycoform with enhanced therapeutic properties.
Coagulation glycoproteins—carbohydrate composition and biological relevance
| Haemostatic glycoprotein . | N-linked sites . | O-linked sites . | Functional significance of glycan structures . | Physiological and pathological glycoforms . |
|---|---|---|---|---|
| Fibrinogen | 4 | 0 | Promotes fibrinogen solubility93 | Pathological: fibrinogens Lima,80 Caracas II,81 Niigata,94 Pontoise, Asahi,82 and Kaiserslautern95 |
| Prothrombin | 3 | 0 | None described | None described |
| TF | 3 | ND | Glycans modulate procoagulant activity of TF36 -38 | None described |
| Factor V | 26 | ND | Glycans modulate intracellular trafficking from ER to Golgi.13 ,14 N-linked glycans also influence FVa phospholipid binding affinity and FVa susceptibility to APC mediated proteolysis.58 -60 | Physiological: FVa1 and FVa2 |
| Pathological: factor V Liverpool (Ile359Thr)79 | ||||
| Factor VII | 2 | 2 | Influence hepatic clearance and plasma half-life of recombinant FVIIa. Loss of O-linked glycans impairs procoagulant activity of FVIIa in plasma.96 | None described |
| Factor VIII | 24 | 7 | Glycans influence FVIII folding and intracellular trafficking during biosynthesis.13 ,14 N-linked glycans also regulate FVIII uptake by dendritic cells91 ,97 and clearance by the ASGPR. | Pathological: factor VIII (Met1772Thr) and (Ile566Thr) 25 |
| Factor IX | 2 | 5 | None described | Pathological: factor IX (Arg94Ser) 83 |
| Factor X | 2 | 2 | Both N- and O-linked glycans are negative modulators of FX zymogen activation.42 N-linked glycans also regulate FX clearance.45 | None described |
| Factor XI | 5 | ND | None described | None described |
| Factor XII | 2 | 7 | None described | None described |
| Factor XIII | 3 | ND | None described | None described |
| VWF | 12 | 10 | N-linked and O-linked glycans influence VWF synthesis, secretion, and biological activity.27 ,35 ,98 Glycan expression also regulates susceptibility to ADAMTS13 proteolysis34 and is a critical determinant of VWF clearance.67 | Physiological: ABO blood group–specific glycoforms |
| PC | 4 | 0 | Modulate PC zymogen activation by thrombin thrombomodulin complex.54 Glycans also influence the anticoagulant and antiinflammatory properties of APC.55 | Physiological: α-protein C, β-protein C, γ- protein C53 |
| Protein S | 3 | ND | None described | Pathological: protein S Heerlen (Ser460Pro) 84 |
| Antithrombin | 4 | 0 | Glycans influence conformational changes in antithrombin after heparin binding, and thereby regulate serpin activity.47 ,51 | Physiological: α-antithrombin and β-antithrombin47 |
| Pathological: antithrombin (Ile7Asn),99 antithrombin (Ser82Asn) 100 |
| Haemostatic glycoprotein . | N-linked sites . | O-linked sites . | Functional significance of glycan structures . | Physiological and pathological glycoforms . |
|---|---|---|---|---|
| Fibrinogen | 4 | 0 | Promotes fibrinogen solubility93 | Pathological: fibrinogens Lima,80 Caracas II,81 Niigata,94 Pontoise, Asahi,82 and Kaiserslautern95 |
| Prothrombin | 3 | 0 | None described | None described |
| TF | 3 | ND | Glycans modulate procoagulant activity of TF36 -38 | None described |
| Factor V | 26 | ND | Glycans modulate intracellular trafficking from ER to Golgi.13 ,14 N-linked glycans also influence FVa phospholipid binding affinity and FVa susceptibility to APC mediated proteolysis.58 -60 | Physiological: FVa1 and FVa2 |
| Pathological: factor V Liverpool (Ile359Thr)79 | ||||
| Factor VII | 2 | 2 | Influence hepatic clearance and plasma half-life of recombinant FVIIa. Loss of O-linked glycans impairs procoagulant activity of FVIIa in plasma.96 | None described |
| Factor VIII | 24 | 7 | Glycans influence FVIII folding and intracellular trafficking during biosynthesis.13 ,14 N-linked glycans also regulate FVIII uptake by dendritic cells91 ,97 and clearance by the ASGPR. | Pathological: factor VIII (Met1772Thr) and (Ile566Thr) 25 |
| Factor IX | 2 | 5 | None described | Pathological: factor IX (Arg94Ser) 83 |
| Factor X | 2 | 2 | Both N- and O-linked glycans are negative modulators of FX zymogen activation.42 N-linked glycans also regulate FX clearance.45 | None described |
| Factor XI | 5 | ND | None described | None described |
| Factor XII | 2 | 7 | None described | None described |
| Factor XIII | 3 | ND | None described | None described |
| VWF | 12 | 10 | N-linked and O-linked glycans influence VWF synthesis, secretion, and biological activity.27 ,35 ,98 Glycan expression also regulates susceptibility to ADAMTS13 proteolysis34 and is a critical determinant of VWF clearance.67 | Physiological: ABO blood group–specific glycoforms |
| PC | 4 | 0 | Modulate PC zymogen activation by thrombin thrombomodulin complex.54 Glycans also influence the anticoagulant and antiinflammatory properties of APC.55 | Physiological: α-protein C, β-protein C, γ- protein C53 |
| Protein S | 3 | ND | None described | Pathological: protein S Heerlen (Ser460Pro) 84 |
| Antithrombin | 4 | 0 | Glycans influence conformational changes in antithrombin after heparin binding, and thereby regulate serpin activity.47 ,51 | Physiological: α-antithrombin and β-antithrombin47 |
| Pathological: antithrombin (Ile7Asn),99 antithrombin (Ser82Asn) 100 |
ND, not determined.
Acknowledgments
This work was supported by a Science Foundation Ireland Principal Investigator Award (11/PI/1066) (J.S.O.) and a Science Foundation Ireland Starting Investigator Research Grant (09/SIRG/I1590) (R.J.S.P.).
Authorship
Contribution: R.J.S.P., O.R., E.M.G., and J.S.O. drafted the first version of different sections of the manuscript and critically reviewed the final manuscript.
Conflict-of-interest disclosure: J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Leo Pharma, and Octapharma; has served on the advisory boards of Baxter, Bayer, Octapharma, and Pfizer; and has received research grant funding awards from Baxter, Bayer, and Novo Nordisk. R.J.S.P. has received research funding from Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: James O’Donnell, Haemostasis Research Group, Institute of Molecular Medicine, St. James’s Hospital, Trinity College Dublin, Dublin 8, Ireland; e-mail: jodonne@tcd.ie.