Key Points
The Mnk inhibitor cercosporamide suppresses human leukemic progenitors and exhibits antileukemic effects in a xenograft mouse model.
Cercosporamide enhances the antileukemic effects of cytarabine in vitro and in vivo.
Abstract
Mnk kinases regulate the phosphorylation and activation of the eukaryotic initiation factor 4E (eIF4E), a protein that plays key roles in the initiation of messenger RNA translation and whose activity is critical for various cellular functions. eIF4E is deregulated in acute myeloid leukemia (AML), and its aberrant activity contributes to leukemogenesis. We determined whether cercosporamide, an antifungal agent that was recently shown to act as a unique Mnk inhibitor, exhibits antileukemic properties. Treatment of AML cells with cercosporamide resulted in a dose-dependent suppression of eIF4E phosphorylation. Such suppression of Mnk kinase activity and eIF4E phosphorylation by cercosporamide resulted in dose-dependent suppressive effects on primitive leukemic progenitors (CFU-L) from AML patients and enhanced the antileukemic properties of cytarabine (Ara-C) or mammalian target of rapamycin (mTOR) complex 1 inhibition. Similarly, the combination of cercosporamide with cytarabine resulted in enhanced antileukemic responses in a xenograft mouse model in vivo. Altogether, this work demonstrates that the unique Mnk inhibitor cercosporamide suppresses phosphorylation of eIF4E and exhibits antileukemic effects, in support of future clinical-translational efforts involving combinations of Mnk inhibitors with cytarabine and/or mTOR inhibitors for the treatment of AML.
Introduction
The need for novel therapies for acute myeloid leukemia (AML) remains urgent and of high clinical importance. Multiple signaling pathways that promote leukemic cell survival and proliferation are constitutively activated in AML cells, providing potential therapeutic targets. Among them, the mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) pathways play central roles in leukemogenesis.1-3 MAPK pathways have previously been shown to be involved in the regulation of gene transcription, cell proliferation, and survival.4 There is extensive evidence that these pathways regulate normal and malignant hematopoiesis and transduce signals generated by engagement of growth factor and cytokine receptors.1
A family of kinases that are key effectors for MAPK pathways includes the Mnk1 and Mnk2 kinases, which regulate phosphorylation of the eukaryotic initiation factor 4E (eIF4E) in response to a variety of signals.5-13 eIF4E is a key component of the messenger RNA (mRNA) cap-binding complex.14 The phosphorylation of this protein by Mnk1/2 has important functional consequences for mRNA translation and the regulation of malignant cell proliferation.5,6 Inhibition of eIF4E may be an important approach for the development of novel treatments for patients with various malignancies, as this protein appears to be critical for the growth and survival of cancer cells15,16 as well as malignant transformation.17,18 On the other hand, Mnk activity does not appear to be required for normal development.19
In prior studies, we demonstrated that Mnk kinases may act as negative feedback regulators in response to antileukemic agents, including arsenic trioxide (As2O3)11 and chemotherapy (cytarabine).20 These studies demonstrated that pharmacologic inhibition or small interfering RNA targeting of Mnk kinases suppresses leukemic progenitor growth and enhances the antileukemic properties of other antileukemia agents.11,20 However, efforts to therapeutically target Mnk pathways for the treatment of leukemias have been limited by the lack of Mnk inhibitor compounds with the potential for clinical development.
Cercosporamide was recently identified during a chemical screen for Mnk1 inhibitors.21 It was demonstrated that this known, orally bioavailable antifungal agent is a potent and selective Mnk inhibitor.21 Cercosporamide was found to suppress the growth of melanoma lung metastases and colon carcinoma xenograft tumors,21 but its potential activity against AML cells and other leukemias is unknown. In the present study, we examined the effects of cercosporamide on different AML cell lines and primary leukemic progenitors from AML patients. Our data show that cercosporamide is a potent inhibitor of phosphorylation of eIF4E at Ser209 in AML cells and results in potent inhibitory effects on primitive leukemic progenitors (CFU-L) from AML patients. In addition, we found that combinations of low-dose cytarabine with cercosporamide result in enhanced antileukemic responses, raising the potential for combinations of cercosporamide with other agents for the treatment of AML.
Materials and methods
Cells and reagents
The U937, MM6, and K562 human leukemia cell lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and gentamycin. MV4-11 acute myelogenous leukemia cells were purchased from the American Type Culture Collection (Manassas, VA) and cultured in Iscove’s modified Dulbecco’s medium with l-glutamine, 25 mM HEPES with 10% fetal bovine serum adjusted to contain 1.5 g/L sodium bicarbonate. Cytarabine was purchased from Sigma-Aldrich (St. Louis, MO). Antibodies against the phosphorylated form of eIF4E on Ser209 and eIF4E were obtained from Cell Signaling Technology (Danvers, MA). The antibody against glyceraldehyde-3-phosphate dehydrogenase was purchased from Millipore Corporation (Billerica, MA). The mTOR inhibitor rapamycin was purchased from Calbiochem/EMD (San Diego, CA). Cercosporamide was from Eli Lilly and Company (Indianapolis, IN).
Cell lysis and immunoblotting
Animal studies
All animal work was approved by the Eli Lilly and Company Institutional Animal Care and Use Committee and performed in an Association for Assessment of Laboratory Animal Care–certified facility. MV4-11 cells were implanted at a density of 5 × 106 cells per mouse as described elsewhere.21 Tumors were measured by caliper and tumor volume calculated as described previously.21 Once tumors reached a group mean of 100 mm3, animals were randomized to the following treatment groups: Ara-C (20 mg/kg daily dosed intraperitoneally), cercosporamide (10 mg/kg twice daily, 20 mg/kg daily dosed orally by gavage), Ara-C plus cercosporamide combinations (as above), or the relative vehicle controls (captisol for cercosporamide and water for Ara-C). Cercosporamide was formulated as described previously.21 Tumor lysates were taken 2 hours after dosing, and western blot analyses were performed with antibodies against eIF4E, peIF4E on serine 209, and β-actin, as described previously.21
Cell proliferation/viability assays
Hematopoietic progenitor cell assays
Results
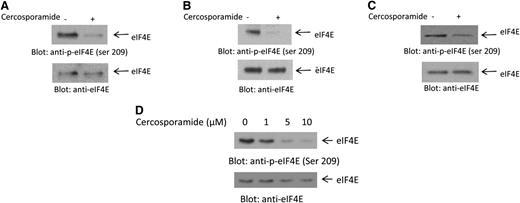
In initial studies, we examined the effects of cercosporamide on phosphorylation of eIF4E in AML cells. In experiments using the U937 acute myelomonocytic leukemia cell line, we found that cercosporamide potently inhibited phosphorylation of eIF4E on serine 209 (Figure 1A). Similar results were obtained when the effects of cercosporamide were assessed on the AML line MM6 (Figure 1B) and the acute erythroleukemia cell line K562 (Figure 1C). The inhibitory effects of cercosporamide on eIF4E were dose dependent, with partial suppression of phosphorylation seen at concentration of cercosporamide of 1 μM and maximizing at 5 to 10 μM (Figure 1D).
Cercosporamide suppresses phosphorylation of eIF4E. (A) U937 cells were incubated with cercosporamide (10 µM) for 24 hours. Cell lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209. The same blot was stripped and reprobed with an antibody against eIF4E, as indicated. (B) MM6 cells were incubated with cercosporamide for 24 hours. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209. The same blot was stripped and reprobed with an antibody against eIF4E, as indicated. (C) K562 cells were incubated with cercosporamide for 24 hours. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209. The same blot was stripped and reprobed with an antibody against eIF4E, as indicated. (D) Serum-starved U937 cells were treated for 1 hour with increasing doses of cercosporamide, as indicated. Lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209. The same blot was striped and reprobed with an antibody against eIF4E, as indicated. ser, serine.
Cercosporamide suppresses phosphorylation of eIF4E. (A) U937 cells were incubated with cercosporamide (10 µM) for 24 hours. Cell lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209. The same blot was stripped and reprobed with an antibody against eIF4E, as indicated. (B) MM6 cells were incubated with cercosporamide for 24 hours. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209. The same blot was stripped and reprobed with an antibody against eIF4E, as indicated. (C) K562 cells were incubated with cercosporamide for 24 hours. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209. The same blot was stripped and reprobed with an antibody against eIF4E, as indicated. (D) Serum-starved U937 cells were treated for 1 hour with increasing doses of cercosporamide, as indicated. Lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209. The same blot was striped and reprobed with an antibody against eIF4E, as indicated. ser, serine.
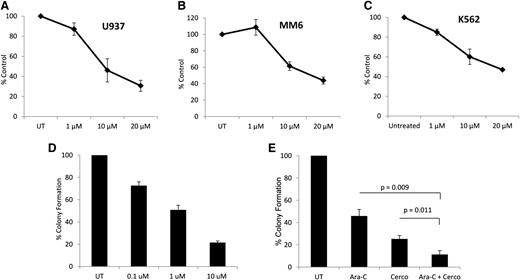
To determine whether cercosporamide exhibits negative regulatory effects on cell proliferation and viability of leukemia cells, MTT assays were conducted. When U937 cells were incubated in the presence or absence of the increasing doses of cercosporamide, we found a dose-dependent suppression of cell growth (Figure 2A). Similar experiments with comparable results were seen when the effects of cercosporamide on MM6 (Figure 2B) and K562 (Figure 2C) cells were examined. Altogether, these studies demonstrated that cercosporamide blocks phosphorylation of eIF4E in human AML lines and that such an inhibitory effect correlates with decreased cell viability/suppression of leukemic cell proliferation.
Antileukemic effects of cercosporamide. (A) U937 cells were incubated for 5 days in the presence or absence of the indicated doses of cercosporamide. Cell proliferation was assessed by an MTT assay. Data are expressed as means ± standard error (SE) of 5 independent experiments. (B) MM6 cells were incubated for 5 days in the presence or absence of the indicated doses of cercosporamide. Cell proliferation was assessed by an MTT assay. Data are expressed as means ± SE of 3 independent experiments. (C) K562 cells were incubated for 5 days in the presence or absence of the indicated doses of cercosporamide. Cell proliferation was assessed by an MTT assay. Data are expressed as means ± SE of 4 independent experiments. (D) U937 cells were plated in methylcellulose culture assay system with increasing concentrations of cercosporamide, as indicated. Data are expressed as percentage control of leukemic colonies for untreated cells and represent means ± SE of 5 experiments. (E) U937 cells were plated in methylcellulose culture assay system with cytarabine (1 ng/mL) and/or cercosporamide (10 µM), as indicated, and CFU-L leukemic colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± SE of the values from 5 independent experiments are shown. Paired t test analysis for the combinations of cytarabine plus cercosporamide showed P = .011 compared with cercosporamide alone and P = .009 compared with cytarabine alone. UT, untreated.
Antileukemic effects of cercosporamide. (A) U937 cells were incubated for 5 days in the presence or absence of the indicated doses of cercosporamide. Cell proliferation was assessed by an MTT assay. Data are expressed as means ± standard error (SE) of 5 independent experiments. (B) MM6 cells were incubated for 5 days in the presence or absence of the indicated doses of cercosporamide. Cell proliferation was assessed by an MTT assay. Data are expressed as means ± SE of 3 independent experiments. (C) K562 cells were incubated for 5 days in the presence or absence of the indicated doses of cercosporamide. Cell proliferation was assessed by an MTT assay. Data are expressed as means ± SE of 4 independent experiments. (D) U937 cells were plated in methylcellulose culture assay system with increasing concentrations of cercosporamide, as indicated. Data are expressed as percentage control of leukemic colonies for untreated cells and represent means ± SE of 5 experiments. (E) U937 cells were plated in methylcellulose culture assay system with cytarabine (1 ng/mL) and/or cercosporamide (10 µM), as indicated, and CFU-L leukemic colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± SE of the values from 5 independent experiments are shown. Paired t test analysis for the combinations of cytarabine plus cercosporamide showed P = .011 compared with cercosporamide alone and P = .009 compared with cytarabine alone. UT, untreated.
In subsequent studies, the effects of Mnk inhibition on CFU-L colony formation were examined. When U937 cells were treated with increasing concentrations of cercosporamide in a methylcellulose culture system, there were dose-dependent suppressive effects on leukemic progenitor colony formation (CFU-L) (Figure 2D). Also, when the effects of Mnk inhibition on the antileukemic effects of cytarabine were examined, we found that the combination of cercosporamide and cytarabine led to more potent inhibitory effects on CFU-L growth than with either alone (Figure 2E).
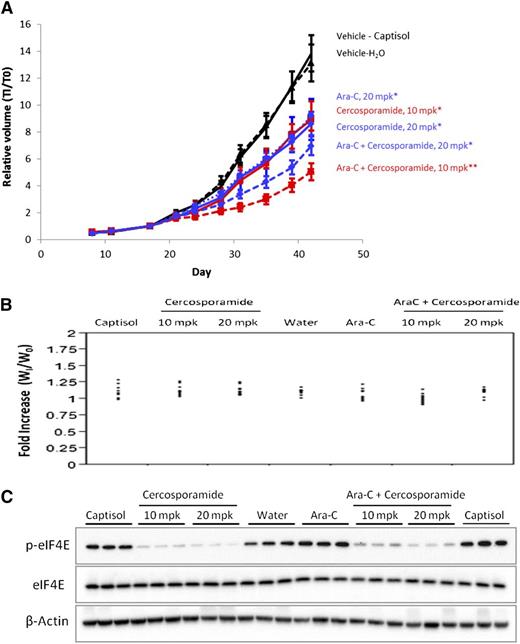
We next sought to determine whether Mnk inhibition might similarly enhance the effects of cytarabine (Ara-C) in vivo using MV-411 AML xenograft tumors. MV4-11 cells were used to generate the xenograft tumors, as in immunoblotting studies we found that these cells do not express the Jak3 protein (data not shown), whose kinase activity is also inhibited by cercosporamide.21 MV4-11 cells were injected subcutaneously into nude mice. Once palpable tumors reached a group mean size of ∼100 mm3, mice were randomized to the following treatment groups: vehicle (captisol for cercosporamide or H2O for Ara-C), cercosporamide (10 mg/kg orally by gavage twice daily or 20 mg/kg once daily) alone, Ara-C alone (20 mg/kg intraperitoneally), or cercosporamide plus cytarabine (10 mg/kg twice-daily cercosporamide plus 20 mg/kg Ara-C or 20 mg/kg daily cercosporamide plus 20 mg/kg Ara-C). Treatment with cercosporamide or Ara-C alone significantly suppressed xenograft growth when compared with the respective vehicle (P < .011 for 10 mg/kg twice-daily cercosporamide; P < .006 for cercosporamide 20 mg/kg daily; P < .0374 for Ara-C). The combination of cercosporamide 10 mg/kg twice daily plus Ara-C was significantly more effective than either agent alone (P < .0009 vs cercosporamide; P = .005 vs Ara-C; P < .0001 vs either vehicle) (Figure 3A). Cercosporamide (20 mg/kg once daily) in combination with Ara-C showed similar effects, with significant inhibition of tumor growth vs captisol (P < .0001) or water (P = .0003), but did not show statistical significance vs cercosporamide alone (20 mg/kg) or Ara-C alone. In each treatment group, mean body weights increased over the course of the study (Figure 3B). Further, tumor volume data were evaluated in relation to body weight for each mouse during treatment and revealed no significant effects of body weight on tumor volume (data not shown). Finally, eIF4E serine 209 phosphorylation was reduced in all xenografted tumors treated with cercosporamide (Figure 3C).
Combining cercosporamide and Ara-C suppresses growth of MV4-11 AML xenograft tumors. (A) Tumor-bearing mice were randomized to treatment groups and dosed daily with 20 mg/kg Ara-C, or with cercosporamide (20 mg/kg daily or 10 mg/kg twice daily), or with Ara-C in combination with either cercosporamide treatment. The vehicle-treated mice (captisol for cercosporamide and water for Ara-C) are shown. Relative tumor volumes measured by caliper are plotted at each time point divided by initial tumor volume for each animal (T1/T0) ± standard error of the mean. All treatments showed statistically significant activity when compared with the appropriate vehicle-treated controls* (cercosporamide 10 mg/kg twice daily, P < .011; cercosporamide 20 mg/kg daily, P < .006; Ara-C, P < .0374; cercosporamide 10 mg/kg twice daily plus Ara-C vs either vehicle, P < .0001). Cercosporamide 10 mg/kg twice daily plus Ara-C was also significantly more effective than cercosporamide (P < .0009**) or Ara-C alone (P = .0005**). Data are representative of 4 separate xenograft studies. (B) Body weight distribution across all treatment groups was calculated by normalizing body weight for each animal at the end of study (day 42) (WI) with that immediately prior to treatment (W0). (C) Western blot analyses for p-eIF4E were run on tumor lysates harvested at the end of study. Cell lysates for the indicated treatment conditions were analyzed by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E (upper panel). Equal amounts of lysates from the same experiment shown in the upper panel were analyzed separately by SDS-PAGE and immunoblotted with an antibody against eIF4E (middle panel). The same blot shown in the middle panel was immunoblotted with an anti-actin antibody, as indicated (lower panel). Each lane represents an individual tumor. mpk, mg/kg.
Combining cercosporamide and Ara-C suppresses growth of MV4-11 AML xenograft tumors. (A) Tumor-bearing mice were randomized to treatment groups and dosed daily with 20 mg/kg Ara-C, or with cercosporamide (20 mg/kg daily or 10 mg/kg twice daily), or with Ara-C in combination with either cercosporamide treatment. The vehicle-treated mice (captisol for cercosporamide and water for Ara-C) are shown. Relative tumor volumes measured by caliper are plotted at each time point divided by initial tumor volume for each animal (T1/T0) ± standard error of the mean. All treatments showed statistically significant activity when compared with the appropriate vehicle-treated controls* (cercosporamide 10 mg/kg twice daily, P < .011; cercosporamide 20 mg/kg daily, P < .006; Ara-C, P < .0374; cercosporamide 10 mg/kg twice daily plus Ara-C vs either vehicle, P < .0001). Cercosporamide 10 mg/kg twice daily plus Ara-C was also significantly more effective than cercosporamide (P < .0009**) or Ara-C alone (P = .0005**). Data are representative of 4 separate xenograft studies. (B) Body weight distribution across all treatment groups was calculated by normalizing body weight for each animal at the end of study (day 42) (WI) with that immediately prior to treatment (W0). (C) Western blot analyses for p-eIF4E were run on tumor lysates harvested at the end of study. Cell lysates for the indicated treatment conditions were analyzed by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E (upper panel). Equal amounts of lysates from the same experiment shown in the upper panel were analyzed separately by SDS-PAGE and immunoblotted with an antibody against eIF4E (middle panel). The same blot shown in the middle panel was immunoblotted with an anti-actin antibody, as indicated (lower panel). Each lane represents an individual tumor. mpk, mg/kg.
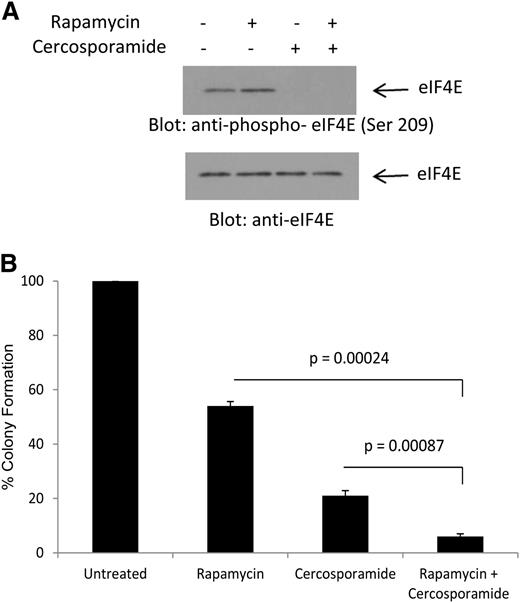
We have previously shown that treatment of AML cells with rapamycin results in phosphorylation of eIF4E on Ser 209, consistent with Mnk activation via a feedback loop.20 To determine whether cercosporamide can modulate the effects of antileukemic effects of rapamycin by blocking this negative feedback loop, its effects on rapamycin-dependent phosphorylation of eIF4E were first examined. As shown in Figure 4A, treatment with rapamycin enhanced phosphorylation of eIF4E on Ser209 (Figure 4A). Such phosphorylation was completely abrogated by concomitant treatment of the cells with cercosporamide (Figure 4A). In addition, the combination of rapamycin with cercosporamide resulted in enhanced suppressive effects on CFU-L colony formation, as compared with each agent alone (Figure 4B). Thus, cercosporamide enhances the antileukemic effects of mTOR complex 1 (mTORC1) inhibition by blocking Mnk/eIF4E phosphorylation.
Combined inhibition of mTOR and Mnk activity results in enhanced antileukemic effects. (A) U937 cells were incubated for 2 hours with rapamycin, in the presence or absence of cercosporamide, as indicated. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209 or with an antibody against total eIF4E, as indicated. (B) U937 cells were plated in methylcellulose culture assay system with rapamycin (20 nM) and/or cercosporamide (10 µM), as indicated, and CFU-L leukemic colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± SE of the values from 4 independent experiments are shown. Paired t test analysis for the combination of rapamycin plus cercosporamide showed P = .00087 compared with cercosporamide alone and P = .00024 compared with rapamycin alone.
Combined inhibition of mTOR and Mnk activity results in enhanced antileukemic effects. (A) U937 cells were incubated for 2 hours with rapamycin, in the presence or absence of cercosporamide, as indicated. Cell lysates were resolved by SDS-PAGE and immunoblotted with an antibody against the phosphorylated form of eIF4E on serine 209 or with an antibody against total eIF4E, as indicated. (B) U937 cells were plated in methylcellulose culture assay system with rapamycin (20 nM) and/or cercosporamide (10 µM), as indicated, and CFU-L leukemic colony formation was assessed. Data are expressed as a percentage control of CFU-L for untreated cells. Means ± SE of the values from 4 independent experiments are shown. Paired t test analysis for the combination of rapamycin plus cercosporamide showed P = .00087 compared with cercosporamide alone and P = .00024 compared with rapamycin alone.
In subsequent studies, we examined the effects of cercosporamide on leukemic precursors from different AML patients. The effects of cercosporamide against leukemic CFU-L were assessed in clonogenic assays in methylcellulose. There was dose-dependent suppression of primary CFU-L colony formation (Figure 5A). Importantly, combinations of cercosporamide with rapamycin (Figure 5B) or with low doses of cytarabine (Figure 5C) resulted in enhanced antileukemic activity when compared with either of these agents alone.
Antileukemic effects of cercosporamide on primary leukemic progenitors from AML patients. (A) Dose-dependent suppression of primitive leukemic precursors from AML patients by cercosporamide. Effects were assessed in clonogenic assays in methylcellulose. Data are expressed as percentage control of leukemic colonies for untreated cells and represent means ± SE of 3 experiments, using cells from 3 different patients. (B) Effects of the combination of rapamycin (20 nM) and cercosporamide (1 µM) on primitive leukemic precursors from AML patients. Leukemic CFU-L colony formation was assessed in clonogenic assays in methylcellulose. Data are expressed as percentage control of leukemic colonies for untreated cells and represent means ± SE of 4 experiments, using cells from 4 different patients. Paired t test analysis of the combination of rapamycin and cercosporamide showed P = .0188 compared with rapamycin alone and P = .0125 compared with cercosporamide alone. (C) Effects of the combination of Ara-C (1 ng/ml) and cercosporamide (1 µM) on primitive leukemic precursors from AML patients. Leukemic CFU-L colony formation was assessed in clonogenic assays in methylcellulose. Data are expressed as percentage control of leukemic colonies for untreated cells and represent means ± SE of 4 experiments. Paired t test analysis of the combination of Ara-C and cercosporamide showed P = .0013 compared with Ara-C alone and P = .0193 compared with cercosporamide alone.
Antileukemic effects of cercosporamide on primary leukemic progenitors from AML patients. (A) Dose-dependent suppression of primitive leukemic precursors from AML patients by cercosporamide. Effects were assessed in clonogenic assays in methylcellulose. Data are expressed as percentage control of leukemic colonies for untreated cells and represent means ± SE of 3 experiments, using cells from 3 different patients. (B) Effects of the combination of rapamycin (20 nM) and cercosporamide (1 µM) on primitive leukemic precursors from AML patients. Leukemic CFU-L colony formation was assessed in clonogenic assays in methylcellulose. Data are expressed as percentage control of leukemic colonies for untreated cells and represent means ± SE of 4 experiments, using cells from 4 different patients. Paired t test analysis of the combination of rapamycin and cercosporamide showed P = .0188 compared with rapamycin alone and P = .0125 compared with cercosporamide alone. (C) Effects of the combination of Ara-C (1 ng/ml) and cercosporamide (1 µM) on primitive leukemic precursors from AML patients. Leukemic CFU-L colony formation was assessed in clonogenic assays in methylcellulose. Data are expressed as percentage control of leukemic colonies for untreated cells and represent means ± SE of 4 experiments. Paired t test analysis of the combination of Ara-C and cercosporamide showed P = .0013 compared with Ara-C alone and P = .0193 compared with cercosporamide alone.
Discussion
eIF4E is a key eukaryotic initiation translation factor in mammalian cells, and its function is critical for mRNA translation of genes whose protein products mediate mitogenic responses, such as c-Myc and cyclin D1.5,26 Deregulation of eIF4E promotes leukemogenesis, making it an attractive target for the treatment of leukemias.27-29 This has resulted in efforts to inhibit its function and/or expression for the treatment of AML.29 One approach to directly target eIF4E in the past has been the use of antisense compounds against eIF4E.30 LY2275796 is an antisense oligonucleotide against eIF4E that was developed and studied in solid tumors and found to decrease tumor growth in vitro and in vivo,30 prompting clinical trials in patients with advanced cancers. In a phase 1 trial, of the 30 patients who received at least 1 dose of the eIF4E antisense oligonucleotide, 7 patients had stable disease of at least 6 weeks duration and 2 patients had disease control for over 3 months.31 Other efforts have included the use of the ribavirin, an antiviral drug that may mimic the m7G cap to block eIF4E activity.27 A clinical trial of ribavirin was conducted in patients with AML, and among the 11 evaluable patients, there was 1 complete remission and 2 partial remissions.32 Notably, response was found to be associated with relocalization of nuclear eIF4E to the cytoplasm and a decrease in the levels of eIF4E expression.32
The clinical-translational efforts to target eIF4E by inhibiting its expression30 or directly targeting its function32 have been encouraging and underscore the importance of the Mnk pathway in tumorigenesis. As Mnk kinases are required for phosphorylation of eIF4E on Ser209,5-13 efforts to target and block their kinase activities may provide an important approach for the treatment of malignancies. Such approaches may be particularly attractive because, although necessary for malignant transformation,17,18 Mnk-kinase–mediated phosphorylation of eIF4E does not appear to be a requirement for normal cellular development and growth.19
In the current study, we examined the effects of cercosporamide, a relatively selective and orally bioavailable Mnk1 inhibitor,21 which was previously shown to have activity against experimental melanoma lung metastases and subcutaneous colon carcinoma xenograft tumors.21 Our data demonstrate that cercosporamide inhibits phosphorylation of eIF4E on Ser209 in AML cells and exhibits direct suppressive effects on leukemic progenitor cell growth, enhancing the antileukemic properties of mTORC1 inhibition by rapamycin and promoting the suppressive effects of cytarabine in vitro. In addition, it strongly enhances the antileukemic effects of cytarabine in MV4-11 xenografts in vivo. These data suggest that the antileukemic effects of cercosporamide are attributable to Mnk inhibition. Indeed, the kinase selectivity profiles of cercosporamide were previously tested in a panel of 76 kinases.21 The only kinase inhibited with potency similar to that on Mnk (ie, an 50% inhibition/inhibitory concentration <100 nmol/L) was Jak3.21 The AML xenograft chosen for these studies, MV411, does not express Jak3 protein (data not shown), further substantiating the notion that the antileukemic effects of cercosporamide reflect Mnk inhibitory activity. Nevertheless, to definitively establish the activity and mode of action, further preclinical studies in the future utilizing a bone marrow–residing model of AML involving the use of immunocompetent animals would be useful.
Altogether, our findings underscore the relevance of targeting Mnk pathways for the treatment of AML20 and raise the possibility of developing cercosporamide as an antileukemic agent. Also, because the mechanism of action of cercosporamide is distinct from that of ribavirin,32 these studies raise the potential of future combinations of cercosporamide with ribavirin to target Mnk pathways in AML. In addition, efforts to combine cercosporamide or other Mnk targeting agents with dual mTORC1/mTOR complex 2 catalytic inhibitors, which were recently shown to exhibit potent antileukemic properties against AML precursors,33 may be warranted. Such efforts may be particularly relevant, as our data suggest that in response to mTORC1 inhibition in AML cells, there is engagement of a feedback loop involving Mnk/eIF4E (Altman et al20 and the current study). It is also known from prior work that in malignant cells there is an S6K-PI3′K-Ras–dependent activation of Mek/Erk,34 which is upstream of Mnk/eIF4E.6 Taken together, our work suggests that targeting Mnk/eIF4E may provide a selective and potent approach to block effectors of MAPK cascades during mTOR inhibition and enhance antileukemic responses in AML and possibly other leukemias.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yan Wang and Dr Kenn Henry (Lilly Research Labs, Eli Lilly and Company) for purifying cercosporamide.
This work was supported by grants from the National Institutes of Health, National Cancer Institute (CA155566, CA121192, and CA77816) and by a Merit review grant from the Department of Veterans Affairs.
Authorship
Contribution: J.K.A. performed and designed the research, analyzed the data, and wrote the manuscript; A. Szilard, B.W.K., P.W.I., B.K., H.G., A. Sassano, and E.V. performed the research; J.R.G. designed the research, analyzed the data, and edited the manuscript; L.C.P. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: B.W.K., P.W.I., and J.R.G. are employees and stockholders of Eli Lilly and Company. The remaining authors declare no competing financial interests.
Correspondence: Leonidas C. Platanias, Robert H. Lurie Comprehensive Cancer Center, 303 East Superior St, Lurie 3-107, Chicago, IL 60611; e-mail: l-platanias@northwestern.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal