In this issue of Blood, Kanakry and colleagues report that increased pretreatment levels of plasma Epstein Barr virus DNA (EBV-DNA), as determined by quantitative real-time polymerase chain reaction (QRT-PCR), are associated with inferior outcomes among patients with previously untreated, advanced-stage Hodgkin lymphoma.1
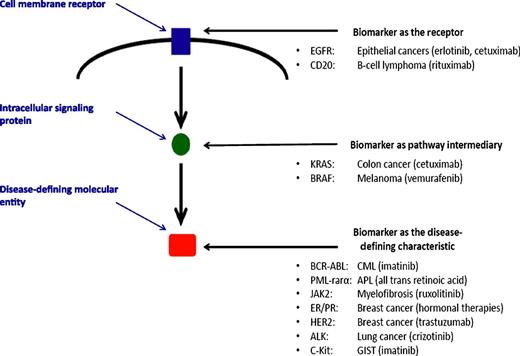
Biomarkers may be “prognostic” and separate patients into favorable or unfavorable groups or be “predictive” and direct those expressing or not expressing the biomarker to different therapies. The figure shows that a current list of validated predictive cancer biomarkers is limited to molecular entities that define a disease and to proteins that are intimately involved in the mechanism of action of a targeted therapy. Kanakry et al suggest a role for plasma EBV-DNA levels as a prognostic biomarker for patients with Hodgkin lymphoma. Establishing a role of EBV in the continued pathogenesis of Hodgkin lymphoma and developing therapies against this process would create the potential for plasma EBV-DNA to be a predictive biomarker. APL, acute promyelocytic leukemia; CML, chronic myelocytic leukemia; EGFR, epidermal growth factor receptor; ER/PR, estrogen receptor/progesterone receptor; GIST, gastrointestinal stromal tumor.
Biomarkers may be “prognostic” and separate patients into favorable or unfavorable groups or be “predictive” and direct those expressing or not expressing the biomarker to different therapies. The figure shows that a current list of validated predictive cancer biomarkers is limited to molecular entities that define a disease and to proteins that are intimately involved in the mechanism of action of a targeted therapy. Kanakry et al suggest a role for plasma EBV-DNA levels as a prognostic biomarker for patients with Hodgkin lymphoma. Establishing a role of EBV in the continued pathogenesis of Hodgkin lymphoma and developing therapies against this process would create the potential for plasma EBV-DNA to be a predictive biomarker. APL, acute promyelocytic leukemia; CML, chronic myelocytic leukemia; EGFR, epidermal growth factor receptor; ER/PR, estrogen receptor/progesterone receptor; GIST, gastrointestinal stromal tumor.
The authors evaluated 274 patients who were included in the 794-patient Eastern Cooperative Oncology Group–led North American Intergroup study comparing doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) with Stanford V.2 Using a plasma cutoff level of 60 copies/100 μL, failure-free survival was inferior among those with higher EBV-DNA levels (HR = 2.0; 95% CI 1.2, 3.5; P = .01).
Scientific advancements associated with Hodgkin lymphoma have provided extraordinary examples of principles that influence modern cancer treatments. Hodgkin lymphoma was an initial setting for demonstrating the importance of radiation treatment, careful anatomical staging, non–cross-resistant combination chemotherapy, autologous stem cell transplantation, and understandings of detrimental late-treatment effects.3 Ironically, Hodgkin lymphoma has not served as a prominent example of the principles associated with “personalized medicine,” in which advances in molecular oncology lead to identification of cancer cell–specific therapeutic targets or markers that direct patient-specific treatment.4 The mainstay of modern treatment remains nonspecific cytotoxic chemotherapy, and the major prognostic categorization schema, the International Prognostic Factors Project described by Hasenclever and colleagues,5 is based on mathematical modeling of nonspecific clinical features and laboratory results. It is thus not surprising that current management controversies, such as use of ABVD or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, prednisone, and procarbazine, continue to emphasize principles of dose intensity, risk-benefit tradeoffs, and patient preferences,3 or that major research strategies include response-adapted treatment that incorporates positron emission tomography in an attempt to more selectively affect risk-benefit proportions.
Central to strategies of personalized medicine is use of a biomarker, defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”6 Molecular abnormalities that define a disease process (eg, bcr-abl for chronic myelocytic leukemia; pml-rarα for acute promyelocytic leukemia) epitomize opportunities associated with biomarkers because these are not only a diagnostic criterion of the disease, but also are targets for therapeutic intervention (eg, imatinib; all-trans-retinoic acid) and serve as quantitative measures of the disease process (eg, QRT-PCR assessment of BCR-ABL1 transcript levels), which can be used to monitor therapeutic response in individuals and as surrogate end points in clinical trials. Such biomarkers are exquisitely rare.
Instead, many biomarkers provide information about the clinical course of a disease and abilities to distinguish patients destined to do well from those who will have poorer outcomes. Biomarkers with such “prognostic” features can be helpful in communicating with individual patients and are an important aid used to stratify groups within clinical trials and to inform future research directions.7 However, other than contributing to risk-benefit discussions (eg, avoidance of therapy in patients with a favorable prognosis), underappreciated limitations exist with use of these prognostic biomarkers when selecting from a choice of therapies for individual patients. Results of randomized trials commonly conclude that the superior therapy is the treatment of choice for patients in both favorable and unfavorable prognostic groups. To select from a choice of therapies according to evolving paradigms of personalized medicine requires demonstration that a biomarker is “predictive” and that an interaction exists in which patients expressing or not expressing the biomarker are best treated with different therapies.7
The list of cancer-related biomarkers that have predictive properties is surprisingly short.4,8 Those currently in use have a common feature: all either represent a molecular entity that defines the disease or are intimately involved in the mechanism of action of the targeted therapy as either a cellular membrane or intracellular signaling protein that may serve as the therapeutic agent’s binding site and affects the downstream molecular machinery that ultimately determines cancer cell survival (see figure). Thus far, none of the many prognostic biomarkers associated with secondary biologic events, including those identified in Hodgkin lymphoma,9 have demonstrated predictive capacities; specific association with the fundamental molecular basis of the cancer or with the mechanism of action of the anticancer therapy appear to be an essential property of predictive markers.
There is long-standing recognition of a relation between Hodgkin lymphoma and EBV. Hypothesized aspects of the relation have included primary causation and a contributing role to pathogenesis.10 Various associations with prognosis have been observed.9 Kanakry’s observations provide a strong stimulus to strive to better understand these relations and also suggest a pathway for clinical evaluation. A prognostic role of pretreatment plasma EBV-DNA levels is conceptually supported by Kanakry’s finding in a smaller subset that persistent levels following treatment are also prognostic. Observations in their post hoc training sample now require prospective testing in a validation sample, including with use of their cutoff level of 60 copies/100 μL. Processes of “technology transfer” are needed to demonstrate that results obtained from a research laboratory setting can be reproduced across clinical service laboratories. If validated, exciting opportunities will exist to test plasma EBV-DNA for clinical utility as a prognostic and predictive biomarker. If validated as prognostic, plasma EBV-DNA levels may facilitate conduct of clinical trials through use of the biomarker to stratify or enrich populations based on risk; as part of clinical care, patients might be better informed about treatment choices within a risk-benefit, patient-preference paradigm.
Perhaps the most important role of plasma EBV-DNA levels will come with better understandings of the role of EBV in the pathogenesis of Hodgkin lymphoma and whether this varies across patients. If EBV is confirmed to have a central role in the continued pathogenesis of disease (at least in some patients), therapeutic strategies to either directly target the virus or to target critical steps in the molecular pathway through which the virus propagates lymphomagenesis will require reliable identification of these patients. Our current understandings of biomarkers suggest that such intimate association with the underlying biology of the disease is needed if predictive capacities are to exist and allow for selecting specific therapies for specific populations.
Conflict-of-interest disclosure: Dr Meyer has received consulting fees from Celgene regarding his role on two Independent Response Assessment Committees of clinical trials and from Lilly regarding his role on an Independent Data Safety Monitoring Committee for a clinical trial. He is Director of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG), which has received research funding to support the conduct of cancer clinical trials from Amgen Canada, Ariad Pharmaceuticals, Astex Therapeutics, AstraZeneca, Boston Biomedical Inc, Bristol-Myers Squibb, Celgene, Geron Corp, GlaxoSmithKline, Janssen-Ortho, Lilly, Merck Frosst Canada, Novartis, Oncolytics Biotech, Oncothyreon, Orthobiotech, Pfizer, Roche, Sanofi-Aventis, Schering Canada. The NCIC CTG participated in the Eastern Cooperative Oncology Group (ECOG) 2496 clinical trial but did not contribute to the correlative analysis evaluating plasma EBV-DNA.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal