Key Points
ADAMTS13 derived peptides presented on HLA-DR; implications for acquired TTP.
CUB2 domain peptide binds to risk-allele HLA-DRB1*11.
Abstract
Autoantibodies directed against ADAMTS13 prohibit the processing of von Willebrand factor multimers, initiating a rare and life-threatening disorder called acquired thrombotic thrombocytopenic purpura (TTP). Recently, HLA-DRB1*11 has been identified as a risk factor for the development of acquired TTP. Here, we identified ADAMTS13-derived peptides presented on MHC class II alleles from 17 healthy donors. Dendritic cells from a panel of both HLA-DRB1*11–positive and -negative donors were pulsed with ADAMTS13, and the HLA-DR–presented peptide repertoire was analyzed by mass spectrometry. Interestingly, at low antigen concentrations, HLA-DRB1*11- or DRB1*03-positive donors presented a limited number of CUB2-derived peptides. Pulsing of dendritic cells using higher concentrations of ADAMTS13 resulted in the presentation of larger numbers of ADAMTS13-derived peptides by both HLA-DRB1*11–positive and -negative donors. Although the presented peptides were derived from several ADAMTS13 domains, inspection of the peptide profiles revealed that CUB2 domain–derived peptides were presented with a higher efficiency when compared with other peptides. Remarkably, dendritic cells from DRB1*11 donors pulsed with higher concentrations of ADAMTS13-present derivatives of a single CUB2-derived peptide. We hypothesize that functional presentation of CUB2-derived peptides on HLA-DRB1*11 contributes to the onset of acquired TTP by stimulating low-affinity, self-reactive CD4+ T cells.
Introduction
Acquired thrombotic thrombocytopenic purpura (TTP) is a rare and life-threatening disorder characterized by the production of autoantibodies directed toward ADAMTS13, a plasma metalloprotease responsible for the cleavage of ultra-large von Willebrand factor (UL-VWF) multimers.1-3 Deficiency of ADAMTS13 causes the accumulation of UL-VWF strings on the surface of endothelial cells of the vessel wall and in the circulation.4 Prolonged exposure of UL-VWF strings on the surface of endothelial cells promotes platelet adhesion, leading to low platelet counts and microvascular thrombosis.5,6 The majority of the inhibitory anti-ADAMTS13 antibodies that develop in patients affected by acquired TTP targets a single epitope composed of Arg568, Phe592, Arg660, Tyr661, and Tyr665 on the outer surface of the spacer domain. This exposed region is crucial for binding of ADAMTS13 to unfolded VWF A2 domain.7-9 Antibodies targeting the CUB 1-2 and TSP2-8 regions of ADAMTS13 have also been detected in the plasma of several patients with acquired TTP.10,11 The pathogenic role of anti–TSP2-8 and anti–CUB1-2 is still unclear. The carboxy-terminal ADAMTS13 TSP2-8 and CUB 1-2 regions not only modulate processing of VWF under flow12-14 but are also necessary for the binding of ADAMTS13 to endothelial cells.15
A number of studies have shown that anti-ADAMTS13 antibodies detected in the plasma of TTP patients are composed of subclasses IgG1 and IgG4.8,10,16,17 Isotype switching from IgM to IgG is a characteristic feature of the humoral immune response, which depends on help from CD4+ T cells.10,16 Anti-ADAMTS13 antibodies have undergone affinity maturation through somatic hypermutation in germinal centers.18,19 Both isotype switching and affinity maturation are dependent on help from CD4+ T lymphocytes. It has been postulated that development of anti-ADAMTS13 antibodies might require activation of low-affinity CD4+ T cells that have not been efficiently eliminated from the repertoire in the thymus and can therefore recognize self-antigens.10 This has been shown previously for other autoimmune disorders like multiple sclerosis. Analyses of the autoimmune TCR-peptide-MHC II complexes in patients with multiple sclerosis revealed that the overall stability of the complex is significantly reduced, allowing autoreactive T cells to escape negative selection.20,21 Peripheral activation of autoreactive T cells occurs at higher antigen concentrations. Under these condition the TCR-peptide-MHC II complex is stabilized. Autoreactive T cells are activated and able to differentiate in memory cells that have reduced requirements for activation when encountering the presented self-antigen.22 Autoimmunity has also been linked to defects in regulatory T cells.23,24 In a recent study, reduced levels of CD4+CD25+ T cells were found in patients with recurrent TTP.25 The observed alterations in subsets of regulatory T cells may contribute to the pathogenesis of acquired TTP.
Activation of ADAMTS13-specific CD4+ T cells requires uptake of ADAMTS13 by antigen presenting cells (APCs) and subsequent presentation of ADAMTS13 peptides on human leukocyte antigen (HLA) molecules on the surface of APCs. Genetic and epidemiological findings have strongly implicated polymorphic sites within MHC class II molecules in the pathogenesis of autoimmune diseases.26-28 Recently HLA-DRB1*11 has been identified as a risk factor for the development of acquired TTP. It has been observed that the HLA-DRB1*11 is more frequent in patients with acquired TTP when compared with a control population.29-31 These findings indicate that presentation of ADAMTS13-derived peptides on MHC II molecules by APC is necessary to activate ADAMTS13-specific CD4+ T cells. We have recently shown that ADAMTS13 is efficiently internalized by immature dendritic cells (iDCs) through the macrophage mannose receptor.32 In this study, the repertoire of naturally MHC class II–presented ADAMTS13-derived peptides was explored using ADAMTS13-pulsed DC. Our findings show that CUB2 domain–derived peptides are presented in a DRB1*11-dependent manner. We hypothesize that functional presentation of these CUB2 domain–derived peptides contributes to the onset of acquired TTP by stimulating low-affinity, self-reactive CD4+ T cells that have escaped negative selection in the thymus. Together, these findings provide further insight into the initiation of the autoimmune reactivity against ADAMTS13 in patients affected by TTP.
Materials and methods
Generation of immature dendritic cells
Human monocytes were isolated from peripheral blood mononuclear cells (PBMCs) as described previously.32 In brief, fresh blood was drawn from healthy HLA class II–typed volunteers in accordance with Dutch regulations and after approval from the Sanquin Ethical Advisory Board in accordance with the Declaration of Helsinki. Monocytes were isolated with anti-CD14+ magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and differentiated into immature DCs by culturing them in the presence of 800 U/mL IL-4 and 1000 U/mL GM-CSF (CellGenix, Freiburg, Germany) for 5 days.
Materials
The following reagents were used in this study: wild-type, full-length, recombinant ADAMTS13 was produced in stable HEK 293 cells and purified as described previously7 ; anti–CD14-PE (Sanquin Reagents, Amsterdam, the Netherlands); anti–CD80-FITC; anti–CD83-APC; and anti–CD86-APC (BD Biosciences, San Jose, CA). Lipopolysaccharide (LPS) was obtained from Sigma-Aldrich (St. Louis, MO). The hybridoma producing the HLA-DR–specific monoclonal antibody (L243) was purchased from ATCC (Wesel, Germany). The antibody was purified from hybridoma supernatant via protein A-sepharose and coupled at a final concentration of 5 mg/mL to CNBr Sepharose 4B (Amersham Biosciences, Buckinghamshire, UK).
Endocytosis of ADAMTS13
After 5 days of differentiation, immature DCs at a concentration of 5 × 106 cells/mL were incubated with 100 nM and 500 nM of recombinant ADAMTS13 in Cellgro medium supplemented with IL-4 and granulocyte-macrophage colony-stimulating factor. After 5 hours of incubation, cells were maturated for 24 hours by adding 1 μg/mL of LPS in the presence of 1% human serum. Subsequently, adherent cells were detached with phosphate-buffered saline (PBS) containing 0.25% trisodiumcitrate and then washed in PBS before analysis.
Flow cytometric analysis
Immature and mature DCs (mDCs) were analyzed for their surface expression of MHC class II molecules as well as other surface molecules. Cells were incubated with 50 μL of staining buffer (PBS, 0.5% human serum albumin) and 1 μg/mL of the appropriate primary monoclonal antibodies or isotype control for 30 minutes at 4°C. Cells were then washed twice in PBS and analyzed by flow cytometry (LSRII flow cytometer, BD Biosciences). Histograms were processed using Flowjo software version 7.5.5 (Tree Star Inc., Ashland, OR).
Affinity purification of HLA-DR–restricted peptides
MHC class II–peptide complexes from ADAMTS13-treated mature DCs were purified as described previously.33 Briefly, cell pellets were resuspended in lysis buffer (50 mM Tris-HCl pH 7.0 and 4% Igepal CA-630 [Sigma]) end-over-end at 4°C for 30 minutes. Cell lysates were cleared by centrifugation for 15 minutes at 4°C at 14.000 rpm. Subsequently, HLA-DR–peptide complexes were purified from the soluble fraction by adding L243-coupled CNBr Sepharose 4B (Amersham Biosciences). Immunoaffinity chromatography was performed in the presence of complete protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany) overnight at 4°C. After overnight incubation, L243 Sepharose was washed 3 times with 10 mM Tris-HCl pH 7.0 supplemented with complete protease inhibitor cocktail (1 tablet per 50 mL of buffer) and 5 times with 10 mM Tris-HCl pH 7.0 without complete protease inhibitor cocktail. Peptides were eluted from HLA-DR by adding 10% acetic acid, which was incubated with the L243 Sepharose for 15 minutes at 70°C. PBS-pulsed immature DCs were used as a control. In parallel experiments, cell lysates were incubated with an isotype control antibody coupled to CNBr Sepharose 4B (clone CLB-T4/1, mouse IgG2a; Sanquin Reagents, Amsterdam, the Netherlands).
Mass spectrometry analysis of purified peptides
Peptide identification was performed essentially by mass spectrometry as described previously.33 In brief, eluted peptides were purified from the acetic acid eluate using a C18 ziptip (Millipore, Billerica, MA) and separated using a reverse-phase C18 column (50 μm × 20 cm, 5 μm particles [Nanoseparations, Nieuwkoop, the Netherlands]) at a flow rate of 100 nL/min with a gradient from 0% to 35% (vol/vol) acetonitrile with 0.1 M HAc. Once separated, the peptides were sprayed directly into the LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific Inc., Bremen, Germany) using a nanoelectrospray source with a spray voltage of 1.9 kV. A collision-induced dissociation was performed for the 5 most intense precursor ions selected from each full scan in the Orbitrap (300 to 2000 m/z, resolving power 30 000). On a monthly basis, the LTQ Orbitrap was calibrated as recommended by the manufacturer to ensure a high mass accuracy.
Characterization of peptides
Peptides were identified by screening each SEQUEST output file against the UniprotKB nonredundant protein 25.H_sapiens.fasta database using Proteome Discoverer version 1.2 software (Thermo Scientific). During the search, we allowed a mass deviation of 20 ppm and a fragment mass tolerance of 0.8 Da. Peptide evaluation was done according to strict quality requirements based on the sequences-variables cross-correlation (Xcorr) and ranking of identified peptides. Only peptides of rank 1 and with the following Xcorr score were considered: all peptides with a charge state of 2 have a Xcorr score of 2.0; for peptides with charge state of 3, the minimal Xcorr score is 2.2; for charge states of 4, the Xcorr score is 2.5; and for peptides with a charge state of 5, 6, or 7, the Xcorr score is 2.75, 3.0, and 3.2, respectively. All peptides that did not meet these criteria were excluded.
Results
Identification of HLA-DR ADAMTS13-derived peptides on dendritic cells
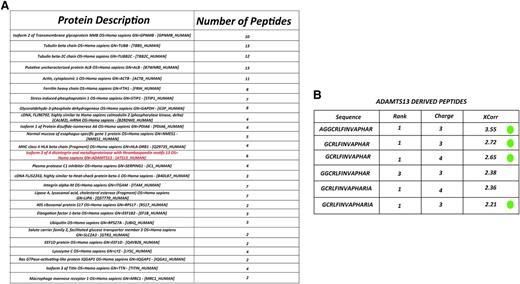
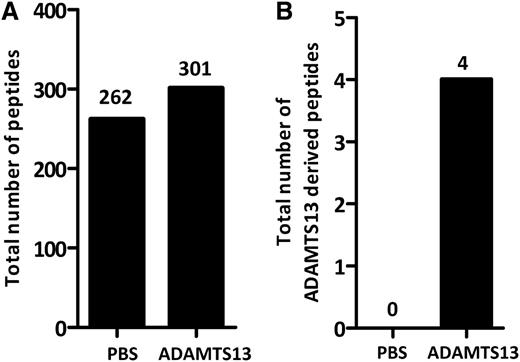
To study whether ADAMTS13 is processed and presented on MHC class II molecules, immature monocyte–derived dendritic cells from healthy-typed donors were pulsed with 100 nM of ADAMTS13. After overnight maturation, cells were lysed and MHC II–peptide complexes were purified by affinity chromatography using HLA-DR–specific monoclonal antibody L243. Maturation of DCs was monitored by flow cytometry. Cells were analyzed for the presence of CD14, CD80, CD83, and CD86. An increase of maturation markers was observed in cells treated with LPS (data not shown). Peptides bound to purified MHC II were eluted and analyzed by mass spectrometry and analyzed using Proteome Discoverer version 1.2 software. In agreement with previous findings, the majority of the identified peptides were derived from proteins that belong to compartments of the MHC II pathway such as plasma membrane, cytosol, endosomes, and lysosomes, as well as other intracellular compartments such as mitochondria and the nucleus.33,34 A representative list of endogenous proteins derived from donor 4 (DRB1*1101/1301) that were processed for presentation on MHC class II is provided in Figure 1A. Interestingly, peptides derived from the macrophage mannose receptor (CD206) are also presented on MHC class II (Figure 1A). Previously, we have shown that CD206 is involved in internalization of ADAMTS13.32 Six different peptides derived from ADAMTS13 are presented on MHC class II of this particular DRB1*1101/DRB1*1301-positive donor. Inspection of the amino acid sequence of the 6 peptides reveals that all peptides are derived from the same core peptide sequence, GCRLFINVAPHAR, that corresponds to amino acid sequence 1325-1338 (numbering according to www.uniprot.org) in the CUB2 domain (Figure 1B). Peptides 1, 2, 3, and 6 fulfill our inclusion criteria that are based on rank and charge cross-correlation criteria as documented in “Materials and methods.” Peptides 4 and 5 did not fulfill these criteria. The results demonstrate that the CUB2 domain–derived peptide GCRLFINVAPHAR is presented by ADAMTS13-pulsed DCs generated from this DRB1*1101/DRB1*1301-positive donor. In parallel, we also determined whether ADAMTS13-derived peptides could be recovered from DCs pulsed with PBS. Similar numbers of peptides that meet the required rank and cross-correlation criteria were presented on MHC class II of PBS- and ADAMTS13-pulsed DCs (Figure 2A). In contrast to ADAMTS13-pulsed DCs, no ADAMTS13-derived peptides were found on DCs pulsed with PBS (Figure 2B). This demonstrates that under these experimental conditions, ADAMTS13 is efficiently presented by mDCs.
Identification of MHC II-bound peptides. (A) Representation of several endogenous proteins processed and presented on MHC II molecules isolated from ADAMTS13-treated mDCs of donor 4 (DRB1*1101/DRB1*1301). (B) List of ADAMTS13-derived peptides presented on MHC II of donor 4. The first column shows the amino acid sequence of the peptides derived from ADAMTS13. Following are the rank, charge, and Xcorr value as provided by Sequest. Peptides meeting the required rank and cross-correlation criteria as documented in “Material and methods” are indicated by a green dot.
Identification of MHC II-bound peptides. (A) Representation of several endogenous proteins processed and presented on MHC II molecules isolated from ADAMTS13-treated mDCs of donor 4 (DRB1*1101/DRB1*1301). (B) List of ADAMTS13-derived peptides presented on MHC II of donor 4. The first column shows the amino acid sequence of the peptides derived from ADAMTS13. Following are the rank, charge, and Xcorr value as provided by Sequest. Peptides meeting the required rank and cross-correlation criteria as documented in “Material and methods” are indicated by a green dot.
Peptide counts of MHC II–bound peptides in ADAMTS13-treated and PBS-treated DCs. Cells were treated with either 100 nM ADAMTS13 or PBS. All samples were immunoprecipitated using anti–MHC II antibody L243. Eluted peptides were analyzed by mass spectrometry and identified using Proteome Discoverer version 1.2. (A-B) Total amount of peptides (A) or ADAMTS13-derived peptides (B) was calculated in both samples. Peptides that did not fulfill our requirements based on rank and charge cross-correlation were excluded.
Peptide counts of MHC II–bound peptides in ADAMTS13-treated and PBS-treated DCs. Cells were treated with either 100 nM ADAMTS13 or PBS. All samples were immunoprecipitated using anti–MHC II antibody L243. Eluted peptides were analyzed by mass spectrometry and identified using Proteome Discoverer version 1.2. (A-B) Total amount of peptides (A) or ADAMTS13-derived peptides (B) was calculated in both samples. Peptides that did not fulfill our requirements based on rank and charge cross-correlation were excluded.
Analysis of ADAMTS13-presented peptides on MHC class II molecules
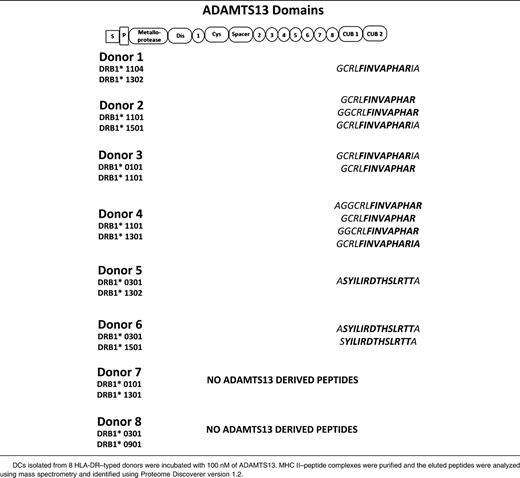
To study the repertoire of naturally presented ADAMTS13 peptides, we analyzed peptide-repertoires of DCs derived from 8 HLA-DR–typed donors. Four of the donors were heterozygous for DRB1*11, which was recently identified as a risk factor for the development of acquired TTP (Table 1).29-31 DCs derived from 4 DRB1*11-negative donors are also included (Table 1). The total number of HLA-DR–presented peptides meeting our criteria ranged between 100 and 360 unique presented peptides (supplemental Figure 1A). All 4 DRB1*11-positive donors presented ADAMTS13-derived peptides (supplemental Figure 1B, Table 1). Strikingly, all HLA-DRB1*11–positive donors presented variants of the same CUB domain peptide: GCRLFINVAPHARIA (Table 1). The remaining 4 donors did not express the HLA-DRB1*11 allele and presented different peptides DCs generated from monocytes of donors 5 and 6, who both expressed HLA-DRB1*0301, presented a different CUB2 domain–derived peptide: ASYILIRDTHSLRTTA (residues 1356-1369). Although similar amounts of peptides were eluted from the MHC class II molecules isolated from the mDCs of donors 7 and 8, no ADAMTS13-derived peptides were detected in these samples (supplemental Figure 1A-B). Taken together, these data suggest that mDCs preferentially present antigenic peptides derived from the CUB2 domain of ADAMTS13. Presentation of the peptides containing the core sequence GCRLFINVAPHARIA appears to be linked to DRB1*11, whereas peptides containing ASYLIRDTHSLRTTA seem to be preferentially presented by HLA-DRB1*0301.
Pulsing of DCs with higher concentrations of ADAMTS13 increases diversity and quantity of ADAMTS13-derived peptides
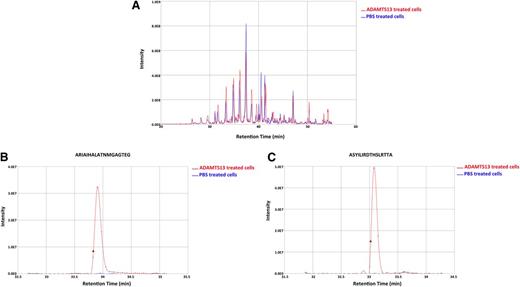
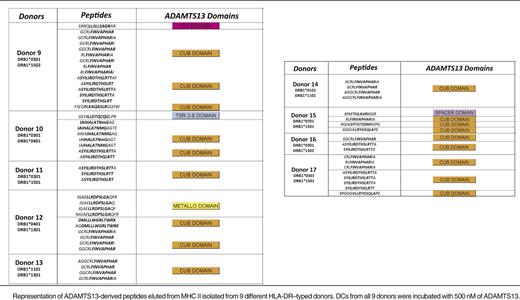
To investigate whether mDCs are able to present a higher number of ADAMTS13 peptides, iDCs were incubated with 500 nM instead of 100 nM of ADAMTS13. After uptake, cells were maturated and lysed and MHC class II molecules were purified as described before. The eluted peptides were identified and analyzed also as described previously. To ensure specificity, we checked whether PBS-treated mDCs derived of a DRB1*0301/0401-positive donor presented ADAMTS13-derived peptides (Figure 3). The chromatogram of total ions did not reveal major differences for the ADAMTS13- and PBS-pulsed DCs (Figure 3A). The total number of peptides presented under the 2 experimental conditions is similar (683 for PBS-treated DCs, 594 for ADAMTS13-pulsed DCs). No ADAMTS13-derived peptides were presented in the PBS-treated DCs, whereas 8 ADAMTS13-derived peptides were identified in ADAMTS13-treated DCs (Table 2; see donor 10). To gain insight into this relative abundance, we interrogated the reconstructed ion chromatogram from donor 10 for peptides AIAHALATNMGAGTEG and ASYILIRDTHSLRTTA (Figure 3B-C). The specific mass of these peptides was only detected in the chromatogram of ADAMTS13 and not in PBS-treated DCs (Figure 3B-C). These results confirm that these ADAMTS13-derived peptides are not recovered from PBS-treated DCs. To verify that the ADAMTS13-derived peptides were specifically binding to MHC II, immunoprecipitations were performed on ADAMTS13-treated mDCs obtained from donor 15 using L243-Sepharose and were compared with control immunoprecipitations using an isotype-control antibody coupled to CNBr Sepharose 4B. No ADAMTS13-derived peptides were obtained in the sample immunoprecipitated with the isotype-control antibody (Figure 4). Having established specificity, we incubated DCs derived from 9 different donors with 500 nM of ADAMTS13. Incubation of DCs with a higher concentration of ADAMTS13 resulted in presentation of a larger number of ADAMTS13-derived peptides (Table 2). All 9 donors, regardless of their HLA-DRB1 allele, presented ADAMTS13-derived peptides. Although the majority of the presented peptides were derived from the CUB domain regions of ADAMTS13, peptides derived from other regions of ADAMTS13 were also identified. Donor 9 presented a single peptide belonging to the disintegrin domain, donor 10 presented a peptide derived from the thrombospondin 2-8 repeats, donor 12 presented a peptide derived from the metalloproteinase domain, and donor 15 presented a peptide belonging to the spacer domain of ADAMTS13. Donors 11, 13, 14, 16, and 17 presented only CUB domain peptides. Our data show that endocytosis of higher amounts of ADAMTS13 increases the diversity of ADAMTS13-derived peptides. In addition, at higher concentrations of ADAMTS13, presentation of ADAMTS13-derived peptides is independent of the presence of HLA-DRB1*11; also, other MHC II alleles can present ADAMTS13-derived peptides under these conditions. Interestingly, the diversity of ADAMTS13-derived peptides presented by iDCs of donors HLA-DRB1*11 was not affected by pulsing of iDCs with higher concentration of ADAMTS13. Under these conditions, the only peptides that were presented were derivatives of the GCRFINVAPHAR core sequence that was also presented at lower concentrations of ADAMTS13. To address this in a quantitative manner, iDCs from DRB1*1101-positive donors 3 and 4 were incubated with either 100 nM or 500 nM of ADAMTS13 for 5 hours at 37°C. After maturation of the cells, the MHC II–peptide complex was purified and the eluted peptides were analyzed by mass spectrometry. As expected, ADAMTS13-derived peptides are presented in both samples (Figure 5A). Interestingly, incubating iDCs with a higher concentration of ADAMTS13 did not lead to presentation of different peptides. In all samples, we detected peptide variants derived from the CUB2 domain peptide: GCRLFINVAPHARIA. We quantified the relative abundance of two of these peptides using SIEVE 1.2 software. Figure 5B-C shows that the amount of ADAMTS13 CUB2 domain–derived peptide GCRLFINVAPHARIA is higher in the samples incubated with 500 nM of ADAMTS13 when compared with samples incubated with 100 nM of ADAMTS13.
Relative abundance of ADAMTS13 CUB2–derived peptides in ADAMTS13 and PBS-treated DCs. DCs isolated from donor 10 were treated with 500 nM of ADAMTS13 or PBS. The MHC II–eluted peptides isolated from the cells were analyzed by mass spectrometry. SIEVE was used to compare intensities of individual peptides eluted from MHC II isolated from ADAMTS13- or PBS-treated DCs. (A) Total ion current chromatogram of ADAMTS13- and PBS-treated cells. (B-C) Reconstructed ion chromatogram of 2 identified ADAMTS13 peptides are shown for both ADAMTS13 and PBS samples. Red and blue chromatograms represent peptides obtained from ADAMTS13 and PBS samples, respectively.
Relative abundance of ADAMTS13 CUB2–derived peptides in ADAMTS13 and PBS-treated DCs. DCs isolated from donor 10 were treated with 500 nM of ADAMTS13 or PBS. The MHC II–eluted peptides isolated from the cells were analyzed by mass spectrometry. SIEVE was used to compare intensities of individual peptides eluted from MHC II isolated from ADAMTS13- or PBS-treated DCs. (A) Total ion current chromatogram of ADAMTS13- and PBS-treated cells. (B-C) Reconstructed ion chromatogram of 2 identified ADAMTS13 peptides are shown for both ADAMTS13 and PBS samples. Red and blue chromatograms represent peptides obtained from ADAMTS13 and PBS samples, respectively.
Endocytosis of higher concentrations of ADAMTS13 leads to presentation of different ADAMTS13-derived peptides
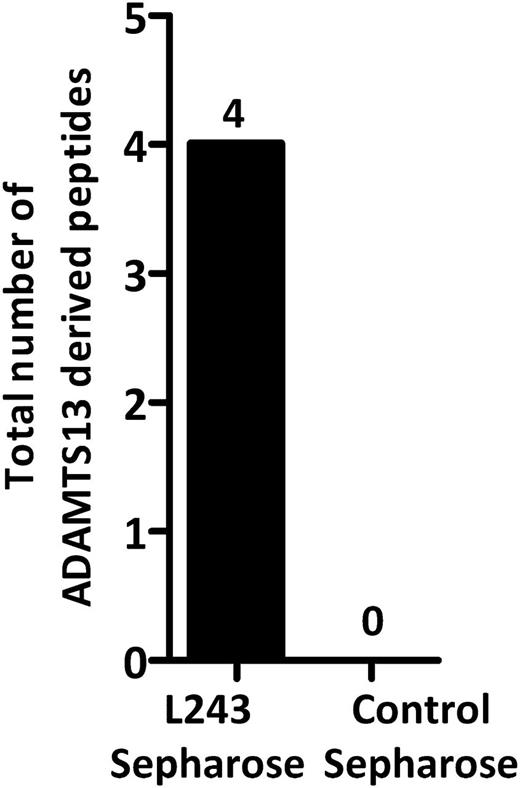
Analysis of MHC II ligands identified in ADAMTS13-treated mDCs. ADAMTS13-treated cells isolated from donor 15 were immunoprecipitated using either an anti–MHC II antibody or an isotype control antibody. Eluted peptides were analyzed by mass spectrometry and identified by Proteome Discoverer version 1.2. The total number of ADAMTS13-derived peptides was determined by counting the specific peptides that met our exclusion criteria.
Analysis of MHC II ligands identified in ADAMTS13-treated mDCs. ADAMTS13-treated cells isolated from donor 15 were immunoprecipitated using either an anti–MHC II antibody or an isotype control antibody. Eluted peptides were analyzed by mass spectrometry and identified by Proteome Discoverer version 1.2. The total number of ADAMTS13-derived peptides was determined by counting the specific peptides that met our exclusion criteria.
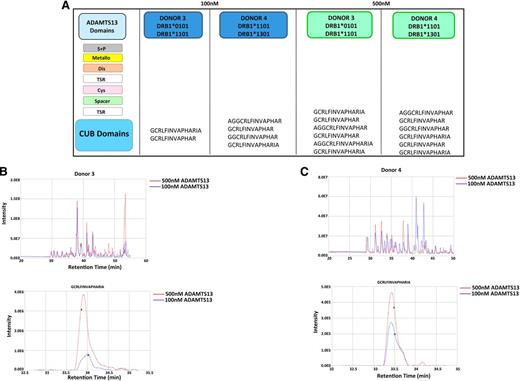
HLA-DRB1*11 donors only present ADAMTS13 CUB domain peptides. DCs from donors 3 and 4 were incubated with 100 nM or 500 nM of ADAMTS13. MHC II–peptide complexes were purified and the eluted peptides were identified using mass spectrometry and Proteome Discoverer version 1.2. (A) Representation of peptides obtained for all 4 samples. (B-C) Total ion current chromatogram of peptides recovered from cells pulsed with 100 nM (blue) and 500 nM (red) ADAMTS13 for donors 3 and 4. Reconstructed ion chromatogram of GCRLFINVAPRIA peptide obtained from cells pulsed with 100 nM (blue) and 500 nM (red) ADAMTS13 for donors 3 and 4.
HLA-DRB1*11 donors only present ADAMTS13 CUB domain peptides. DCs from donors 3 and 4 were incubated with 100 nM or 500 nM of ADAMTS13. MHC II–peptide complexes were purified and the eluted peptides were identified using mass spectrometry and Proteome Discoverer version 1.2. (A) Representation of peptides obtained for all 4 samples. (B-C) Total ion current chromatogram of peptides recovered from cells pulsed with 100 nM (blue) and 500 nM (red) ADAMTS13 for donors 3 and 4. Reconstructed ion chromatogram of GCRLFINVAPRIA peptide obtained from cells pulsed with 100 nM (blue) and 500 nM (red) ADAMTS13 for donors 3 and 4.
These data demonstrate that even though increasing the concentration of ADAMTS13 leads to presentation of a higher absolute amount of ADAMTS13-derived peptides, HLA-DRB1*11–positive donors present only variants of the CUB2 domain peptide: GCRLFINVAPHARIA.
Discussion
It is well established that inhibitory antibodies that develop in patients with acquired TTP target an exposed surface in the spacer domain, which is crucial for binding of ADAMTS13 to unfolded VWF.7-10,18,19,35,36 However, our knowledge with respect to the mechanism underlying the development of anti-ADAMTS13 antibodies in previously healthy individuals is still limited. Polymorphisms in MHC class II genes are associated with many autoimmune disorders. Three recent studies showed that HLA-DRB1*11 is more frequently observed in patients with acquired TTP compared with a healthy control population.29-31 The findings reported in our study show that the CUB2 domain–derived peptide GCRLFINVAPHAR is presented on APCs derived from HLA-DRB1*11–positive individuals. At first glance, it may seem surprising that CUB2 domain–derived peptides are preferentially presented on MHC class II, whereas the spacer domain of ADAMTS13 is the major target of autoantibodies in patients with acquired TTP.8,11,35,36 T cells recognize linear peptides that are loaded on MHC class I or II after proteolytic degradation of antigens by APCs. B cells recognize surface-exposed conformational or linear epitopes. On the basis of these properties, T-cell epitopes do not necessarily overlap with B-cell epitopes. We anticipate that efficient loading of the CUB2 domain peptide GCRLFINVAPHAR on HLA-DRB1*11 underlies the preferential presentation of this peptide on HLA-DRB1*11–positive APCs.
Crystal structures of MHC class II–peptide complexes have shown that epitopes bind to MHC class II in an extended conformation that is dictated by hydrogen bonding between conserved MHC class II residues and the peptide backbone. The side-chains of peptide residues at specific positions—designated anchor residues P1, P4, P6, and P9—interact with distinctive pockets in the peptide-binding groove of MHC class II and further stabilize the MHC class II-peptide complex.27 A binding motif for HLA-DR11 has been established based on the identification of amino acid sequences of natural peptides binding to HLA-DR11.37 The DR11 consensus sequence contains 4 anchor residues at P1, P4, P6, and P9. Inspection of the sequence of the GCRLFINVAPHAR peptide identifies F1327 as the most likely P1 residue. The putative P4 residue V1330 follows the predicted binding motif. Interestingly, P1329 (P6) and R1332 (P9) have different properties because P6 has been predicted to be composed primarily of R/K and P9 of A/G/S/P.37 Several MHC II–binding prediction algorithms have been developed, including NetMHCIIpan Sever 1.2.38 In agreement with our experimental findings, NetMHCIIpan predicts a high affinity of FINVAPHAR-containing peptides for DRB1*1101 and DRB1*1104 (supplemental Figure 2). When low concentrations of ADAMTS13 are used, another CUB2-derived peptide, ASYILIRDTHSLRTTA, is presented by DCs derived from 2 DRB1*11-negative donors (see Table 1). On the basis of its NetMHCIIpan score, this peptide is most likely presented on DRB1*0301 (supplemental Figure 2).
Pulsing of DCs with higher concentrations of ADAMTS13 resulted in increased diversity of peptides presented on DCs of DRB1*11-negative individuals (Table 2). Under these conditions, ADAMTS13-derived peptides can apparently compete more efficiently for binding to MHC class II. The relatively high affinity of nearly all of these different ADAMTS13-derived peptides for MHC class II, as predicted by NetMHCIIpan, is consistent with this hypothesis (supplemental Table 1). Interestingly, FINVAPHARIA-derived peptides are also presented under these conditions. Apparently, this peptide can be presented by multiple MHC class II alleles, although higher concentrations of ADAMTS13 are required for its presentation on DCs derived from non-DRB1*11–positive donors. The NetMHCIIpan server predicts that FINVAPHARIA-derived peptides can indeed be presented by multiple alleles (supplemental Table 2). It is interesting that even at higher concentrations of ADAMTS13, DCs derived from DRB1*11-positive donors present only the GCRLFINVAPHAR peptide (Table 2). In none of the DRB1*11-positive donors were other ADAMTS13-derived peptides identified. Exclusive presentation of the GCRLFINVAPHAR peptide might be caused by the high-affinity binding of this peptide to multiple MHC class II alleles. The affinity of GCRLFINVAPHAR peptide for HLA-DRB1*1101, 0101, and 1301, as predicted by NetMHCIIpan, supports this hypothesis (supplemental Table 2). HLA-DRB1*0401 has been associated with a protective effect in acquired TTP.30,31 The precise mechanism by which protective HLA alleles mediate their effect has not yet been fully elucidated. The protective effect of HLA-DQB1*601 in experimental autoimmune encephalomyelitis is mediated via the antiinflammatory effects of high levels of interferon-γ, which is produced by HLA-DQB1*601–restricted CD4+ T cell responses.39 In view of this finding, it is possible that HLA-DRB1*04–restricted CD4+ T cell responses contribute to the observed protective effect of HLA-DRB1*04 in acquired TTP.
Altogether, our results show that GCRLFINVAPHAR- related peptides are efficiently presented on DCs derived from HLA-DRB1*11–positive donors. The increased frequency of DRB1*11 in patients with acquired TTP together with the results of this study suggests that preferential presentation of GCRLFINVAPHAR by DRB1*11-positive individuals contributes to the onset of acquired TTP. Our results clearly demonstrate that this peptide is more efficiently presented when compared to other ADAMTS13-derived peptides. Therefore, it is likely that CD4+ T-cell responses directed against this peptide can be generated. Presentation of self-antigens by medullary thymic epithelial cells results in the elimination of self-reactive CD4+T cells, which bind with high affinity to cognate MHC class II-peptide complexes.40 This mechanism ensures that autoreactive T cells are deleted from the repertoire. Autoimmunity arises from the escape of autoreactive CD4+ T cells from the negative selection in the thymus. These CD4+ T cells can bind with low or intermediate affinity to self-peptides presented on MHC class II.20 We speculate that the efficient presentation of FINVAPHARIA-derived peptides on DRB1*11 provokes the proliferation of low-affinity CD4+ T cells that have escaped negative selection in the thymus. The same ADAMTS13 CUB2 domain–derived peptide can also be presented by non-HLA-DRB1*11–positive donors, although higher concentrations of ADAMTS13 are needed to allow sufficient presentation of this peptide. Higher densities of self-peptide MHC complexes in the periphery may allow self-reactive CD4+ T cells to overcome their low affinity for the antigen and recognize relatively unstable ligands. This compensatory mechanism has been shown in other autoimmune disorders. In patients affected by multiple sclerosis the immunodominant epitope binds very weakly to HLA-DR4 but still allows T-cell activation at high antigen concentrations.22 It should be emphasized that TTP is a rare autoimmune disorder; therefore we anticipate that the threshold for the activation of self-reactive T cells by the GCRLFINVAPHAR- and/or other ADAMTS13-derived peptides is high. Currently, we do not know which clinical conditions promote loss of tolerance to ADAMTS13 in patients with acquired TTP. Viral and bacterial infections have been implicated in the etiology of TTP.10 As yet, no single pathogen has been linked with the onset of TTP rendering molecular mimicry of a specific viral or bacterial peptide with the FINVAPHARIA peptide identified in this study unlikely. However, cross-reactive CD4+ T cells arising during bacterial or viral challenges may be able to recognize complexes of the MHC class II and FINVAPHARIA peptides. Altogether, our study provides evidence for preferential presentation of a CUB2 domain–derived peptide on DRB1*1101-positive APCs. Further studies are needed to establish whether presentation of peptides harboring the FINVAPHARIA core sequence contributes to the onset of acquired TTP.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Aleksandra Wroblewska and Eszter Herzcenik for helpful discussions; Wouter Pos for input during the initial phase of the project; and Jacqueline Klein-Gebbinck for her help with mass spectrometry.
This study was supported by PPOC-08-021 (N.S.).
Authorship
Contribution: N.S., P.H.K., and S.D.v.H. performed experiments; N.S., R.F., and J.V. conceived the study; A.t.B. and A.B.M. provided expertise; N.S. and J.V. wrote the manuscript; all authors provided input for the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.D.v.H. is the Department of Medicine, Division of Infectious Diseases, Boston Children’s Hospital, Boston, MA.
Correspondence: Jan Voorberg, Department of Plasma Proteins, Sanquin Research, Plesmanlaan 125, 1066 CX Amsterdam, the Netherlands; e-mail: j.voorberg@sanquin.nl.