Key Points
Novel, more potent codon-optimized human FVIII variant (codop-hFVIII-V3).
Codop-hFVIII-V3 is safe and efficacious in mice and nonhuman primates, thus improving the prospects of gene therapy for hemophilia A.
Abstract
Recombinant adeno-associated virus (rAAV) vectors encoding human factor VIII (hFVIII) were systematically evaluated for hemophilia A (HA) gene therapy. A 5.7-kb rAAV-expression cassette (rAAV-HLP-codop-hFVIII-N6) containing a codon-optimized hFVIII cDNA in which a 226 amino acid (aa) B-domain spacer replaced the entire B domain and a hybrid liver-specific promoter (HLP) mediated 10-fold higher hFVIII levels in mice compared with non–codon-optimized variants. A further twofold improvement in potency was achieved by replacing the 226-aa N6 spacer with a novel 17-aa peptide (V3) in which 6 glycosylation triplets from the B domain were juxtaposed. The resulting 5.2-kb rAAV-HLP-codop-hFVIII-V3 cassette was more efficiently packaged within AAV virions and mediated supraphysiologic hFVIII expression (732 ± 162% of normal) in HA knock-out mice following administration of 2 × 1012 vector genomes/kg, a vector dose shown to be safe in subjects with hemophilia B. Stable hFVIII expression at 15 ± 4% of normal was observed at this dose in a nonhuman primate. hFVIII expression above 100% was observed in 3 macaques that received a higher dose of either this vector or the N6 variant. These animals developed neutralizing anti-FVIII antibodies that were abrogated with transient immunosuppression. Therefore, rAAV-HLP-codop-hFVIII-V3 substantially improves the prospects of effective HA gene therapy.
Introduction
Hemophilia A (HA), the most common inherited bleeding disorder, caused by a deficiency of factor VIII (FVIII) is well suited for a gene replacement approach, primarily because a modest increase in the level of FVIII (>1% of normal) can ameliorate the severe bleeding phenotype.1 Several gene transfer strategies for FVIII replacement have been evaluated.2 However, adeno-associated viral (AAV) vectors currently show the greatest promise because of their excellent safety profile and ability to direct long-term transgene expression from postmitotic tissues such as the liver.3-5 Indeed, our ongoing gene therapy clinical trial for hemophilia B, a related bleeding diathesis, has demonstrated that a single peripheral vein administration of AAV vector leads to stable (>30 months) expression of human factor IX (FIX) at levels between 1% to 6% of normal. This is sufficient for conversion of the hemophilia phenotype from severe to moderate or mild.5 Two-thirds of the participants in this study have discontinued prophylaxis and remain free of spontaneous hemorrhage. The other participants have increased the interval between FIX prophylaxes.
The use of AAV vectors for HA gene therapy, however, poses new challenges because of the distinct molecular and biochemical properties of human FVIII (hFVIII). Compared with other proteins of similar size, expression of hFVIII is highly inefficient.6 Bioengineering of the FVIII molecule has resulted in improvement of the FVIII expression. For instance, deletion of the hFVIII B domain, which is not required for co-factor activity, resulted in a 17-fold increase in mRNA levels over full-length wild-type FVIII and a 30% increase in secreted protein.6-8 This has led to the development of recombinant B domain–deleted (BDD) FVIII, which is now widely used clinically (Refacto; Pfizer). Pipe and colleagues have shown that the inclusion of the proximal 226 amino acid (aa) portion of the B domain (FVIII-N6) that is rich in asparagine-linked oligosaccharides significantly increases expression over that achieved with BDD-FVIII.6,9 This is in part due to improved secretion of hFVIII, which is thought to be facilitated by interactions of 6 N-linked glycosylation triplets with the mannose-binding lectin, LMAN1, within this region. Additionally, FVIII-N6 has a reduced tendency to evoke an unfolded protein response when compared with the full-length and BDD-hFVIII variant, thus making it a useful variant for further evaluation in the context of gene transfer.10
Another obstacle to AAV-mediated gene transfer for HA gene therapy is the size of the FVIII coding sequence, which at 7.0 kb, far exceeds the normal packaging capacity of AAV vectors. Packaging of large expression cassettes into AAV vectors has been reported, but this is a highly inconsistent process resulting in low yields of vector particles with reduced infectivity.11,12 AAV vectors encoding the canine BDD-FVIII variant that is ∼4.4 kb in size, show promising results, but further evaluation of this approach using human BDD-FVIII is required.13 Other innovative approaches to overcome the size constraint involve the need for simultaneous transduction with 2 AAV vectors for functional FVIII activity, which has limited their transition to the clinic.14-17
We and others have shown that expression of FVIII can be improved in the context of lentiviral vectors by reorganization of the wild-type cDNA of hFVIII according to the codon usage of highly expressed human genes as described before for human factor IX (hFIX).3,18,19 However, these codon-optimized (codop) FVIII variants remain hitherto untested in the setting of recombinant AAV (rAAV).
In this report, we describe a novel 5.2-kb AAV expression cassette (rAAV-HLP-codop-hFVIII-V3) in which HLP is a hybrid liver-specific promoter and the N6 226-aa B-domain spacer has been replaced with a 17-aa peptide that contains the 6 N-linked glycosylation signals required for efficient and safe expression of hFVIII. We show that rAAV-HLP-codop-hFVIII-V3 is efficiently packaged into rAAV vectors and more potent, mediating higher levels of FVIII expression in mice and non-human primates than is possible with other FVIII variants containing wild-type or codop DNA sequences, thus improving the prospects of safe and effective gene transfer for HA.
Methods
Construction of rAAV-hFVIII variants
The BDD deleted and N6 (kindly provided by Professor Steven Pipe) hFVIII variants containing the wild-type DNA sequences were cloned downstream of our previously described liver-specific LP1 promoter.3,6 rAAV-LP1-BDD-hFVIII contained the BDD hFVIII complementary DNA (cDNA) as described previously.20 In rAAV-LP1-hFVIII-N6, the first 226 aa residues of the B domain containing 6 asparagine-linked glycosylation sites (hFVIII-N6) replaced the SQ sequence in BDD-hFVIII.6,9 The DNA sequences in hFVIII-N6 were further modified using the codon-optimization algorithm previously used to optimize expression of the hFIX cDNA.3,21 The resulting 5012-bp codop-hFVIII-N6 cDNA was 87% identical to that previously used by our group in the lentiviral gene transfer studies.18 The codop-hFVIII-N6 cDNA was synthesized and cloned downstream of the LP1 promoter as described previously.3,21 The smaller HLP enhancer/promoter was constructed by synthesizing a 251-bp fragment containing a 34-bp core enhancer from the human apolipoprotein hepatic control region upstream of a modified 217-bp SERINA1 (α-1-antitrypsin) gene promoter in which the distal X and the proximal A+B regulatory domains were brought together (see supplemental Figure 1 for full sequence). rAAV-HLP-codop-hFVIII-N6 was generated by cloning the codop-hFVIII-N6 cDNA downstream of the HLP promoter but upstream of a 60-bp synthetic polyadenylation signal,22 thus reducing the overall size of this cassette to 5.7 kb.
Three smaller rAAV-codop-hFVIII variants were also developed to reduce the overall size of the expression cassette. The smallest of these new variants consisted of rAAV-HLP-codop-BDD-hFVIII, which was identical to rAAV-HLP-codop-hFVIII-N6 except that the 226-aa B-domain spacer was replaced with a 14-aa peptide (SFSQNPPVLKRHQR) consisting of the previously described SQ sequence including the furin cleavage site (RHQR).20 rAAV-HLP-codop-BDD-hFVIII was further modified to develop rAAV-HLP-codop-hFVIII variants 1 (V1) and 3 (V3) which contained following peptides bearing the 6 N-linked glycosylation triplets respectively:
Peptide 1: SFSQFNATTIQNVSSNNSLSDNTSSNDSKNVSSPPVLKRHQR, and
Peptide 3: SFSQNATNVSNNSNTSNDSNVSPPVLKRHQR.
Asparagine (N) in the glycosylation triplet (N-X-T/S) is underlined for clarity. Consequently, both the codop-hFVIII V1 and V3 constructs also have a furin cleavage site.
Vector production and purification
rAAV-hFVIII vector particles were made by the 293T transient transfection method described before using an adenoviral helper plasmid (HGT1) and chimeric AAV2 Rep-5Cap and AAV2 Rep-8Cap packaging plasmid called pLT-RCO3 and pAAV8-2 to generate AAV5 and 8 pseudotyped vector particles, respectively.23,24 Serotypes 5 and 8 capsid pseudotyped hFVIII vectors were purified by the previously described ion exchange chromatography method.23 Vector particles were purified, titered, and characterized as described elsewhere.25 The size of the genome packaged was determined using denaturing agarose gel electrophoresis as previously described.3,25 Gel-based titers were used for dosing of animals as described in Fagone et al.25
Animal studies
All animal work was performed in accordance with institutional guidelines under protocols approved by the Institutional and/or National Committees for the Care and Use of Animals in the United States and Europe. The potency of the rAAV-hFVIII gene transfer efficiency was initially assessed in 6- to 10-week-old male wild-type immunocompetent C57Bl/6 mice (Harlan, UK) by assessing hFVIII antigen (hFVIII:Ag) levels in plasma at varying time points following tail vein injection of vector. In addition, hFVIII:Ag, FVIII activity (hFVIII:C), and anti-FVIII immunoglobulin G (IgG) were assessed in 8- to 10-week-old immunocompetent male FVIII-deficient mice (F8−/− mice) following tail vein injection of rAAV-hFVIII variants. These animals, bred in-house on a mixed C57BL/29S4/SvJae background, carry a deletion in murine FVIII-exon 16.26 Thrombin-antithrombin levels in murine plasma were determined using the Enzygnost TAT kit (Dade-Behring, Milton Keynes, UK).
For nonhuman primate studies, male rhesus monkeys were purchased from either Charles River Laboratories (Sparks, NV) or Covance Research Products (Alice, TX) and transduced by administering the vector into the saphenous vein as per our previously described procedures.4 Blood samples were collected following venipuncture at various time points for analysis of coagulation profile (prothrombin and activated partial thromboplastin time), hFVIII:Ag, anti-FVIII antibody levels as well as complete blood count (CBC), serum chemistry, and cytokine profiles (R&D Systems, Oxford, UK). Samples for CBC, serum chemistry (blood urea nitrogen and liver function test), and serum interleukin-6 (IL-6) levels were obtained every 48 hours for 2 weeks after vector infusion to assess toxicity. The CBC, serum chemistry, and coagulation profile were performed by the St. Jude Animal Core Laboratory Facility. Monkeys that developed anti-hFVIII antibodies received eradication therapy with rituximab (250 mg/m2, intravenously at 4 weekly intervals, total of 4 bolus infusions) and cyclophosphamide (300 mg/m2 slow intravenous infusions every 15 days, total of 8 doses over 4 months).
Assessment of hFVIII levels in murine plasma
An immunocapture assay was used to determine hFVIII:Ag levels in plasma samples from wild-type C57Bl/6 mice. In brief, 96-well plates were coated overnight at 4°C with a combination of mouse monoclonal antibodies against human FVIII light (ESH2 from Axis-Shield, Dundee; N77110M from AMS Biotechnology, Abingdon, UK). After blocking at room temperature for 1 hour (phosphate-buffered saline [PBS]/6% bovine serum albumin [BSA]/0.05% Tween 20), a hFVIII enzyme-linked immunosorbent assay (ELISA) kit was used for subsequent steps (F8C-EIA, Quadratech Diagnostic Ltd, Epsom, UK). Standards and samples (50 µL/well) diluted 1:10 in diluent buffer were loaded in duplicates. Standards were made by serial dilutions of murine plasma spiked with recombinant human FVIII, starting concentration 4 IU/mL (11th FVIII British Standard: 95/608 6.9 IU/mL, National Institute for Biological Standards and Control, South Mimms, UK). Pooled plasma from wild-type C57Bl/6 mice was used for the dilutions of the standards and experimental samples where necessary. A total of 100 µL/well of the polyclonal horseradish peroxidase–labeled detecting antibody was used as a secondary antibody. hFVIII:Ag levels were determined spectrophotometric (492 nm) using o-phenylenediamine dihydrochloride peroxidase substrate (Sigma, Poole, UK). This ELISA format resulted higher sensitivity and consistency with hFVIII as low as 0.75% being detectable as opposed to a lower limit of detection of 3% when plates were coated with antibody supplied with the ELISA kit.
hFVIII:Ag levels in F8−/− mice were quantitated by P.J.L.’s laboratory as described previously using monoclonal antibodies (833 and D4H1) directed against the light chain of hFVIII.27 Standards consisted of normal pooled human plasma diluted in F8−/− murine plasma. hFVIII:C levels in F8−/− plasma were assessed by a 2-stage clotting assay (Diagnostica Stago, France) per the manufacturer’s instructions. Correction of the bleeding phenotype was determined using a modified tail clip assay. In brief, the distal part of the tail (at 3-mm diameter) of anesthetized F8−/− mice was cut and immediately immersed into 50 mL of saline solution at 37°C. The amount of blood loss was determined by measuring the absorbance of hemoglobin (A492 nm) after lysis of the collected red blood cells in the saline solution in which the tail was placed. Samples containing known quantities of red cells were used as a reference. The time to arrest of bleeding was measured from the moment of transection using a stopwatch.
Quantitating hFVIII:Ag in rhesus plasma
The amount of hFVIII:Ag expressed in rhesus monkeys following rAAV-mediated gene transfer of the hFVIII variants was determined using an ELISA. In brief, monkey (RQ7155) was immunized with recombinant hFVIII protein and TiterMax (an adjuvant). This animal developed high titers of anti-hFVIII antibodies that neutralized hFVIII activity but did not significantly impair the function of rhesus FVIII as determined by a normal coagulation profile in this animal. The rhesus–anti-hFVIII antibody was isolated by purification over a hFVIII column that was generated by coupling 2 mg of recombinant FVIII (Kogenate, Bayer Healthcare) to National Health Service–activated fast flow Sepharose (GE Healthcare, Chalfont, UK). Rhesus anti-hFVIII antibody was eluted with 0.1 M glycine pH 2.7, and peak fractions collected and dialyzed against PBS. A 1:50 dilution of this rhesus anti-hFVIII antibody (50 µL/well) was used to coat ELISA plates overnight at 4°C. The plates were blocked with 200 µL/well of 6% BSA (Sigma) in phosphate buffered saline pH 7.4 + 0.1% Tween 20 at 37°C for a 1-hour incubation. Standards were made by serial dilutions of rhesus plasma spiked with recombinant human FVIII (11th FVIII British Standard: 95/608 6.9IU/mL, National Institute for Biological Standards and Control, South Mimms). Rhesus samples and standards were diluted 1:5 in phosphate buffered saline pH 7.4 + 0.1% Tween 20 containing 2% BSA before loading 50 µL in duplicate. Following a 2-hour incubation at 37°C, the plates were washed and incubated for a further hour with 100 µL of horseradish peroxidase conjugated goat anti-hFVIII polyclonal secondary antibody at a final concentration of 1 µg/mL (Affinity Biologicals Inc., Ontario, Canada). After a final wash step, the amount of FVIII in rhesus plasma was determined spectrophotometrically.8,11 Using this assay, we could consistently detect between 3% and 150% hFVIII in rhesus plasma.
Western blotting
Heat-inactivated mouse plasma containing protease inhibitors was loaded was loaded on a 10% sodium dodecyl sulfonate polyacrylamide gel electrophoresis gel and transferred to a nitrocellulose membrane after electrophoresis. The blots were probed either with a 1:5000 dilution of sheep anti-hFVIII horseradish peroxidase antibody (SAF8C-HRP, Quadratech Diagnostic Ltd, Epsom, UK) or a 1:10 000 dilution of our rhesus anti-hFVIII polyclonal antibody derived from RQ7155, which was biotinylated with the BiotinTag Micro Biotinylation Kit (Sigma). The signal was developed with the ECL chemiluminescence kit (GE HealthCare, Amersham UK). Dilutions of recombinant hFVIII in murine plasma served as a positive control.
Detection of anti-hFVIII antibodies
Anti-hFVIII IgG levels in murine plasma were determined using an ELISA assay. In brief, the microtiter wells were coated with recombinant full-length hFVIII (0.1 µg/well, Kogenate, Bayer Healthcare) and then incubated with various dilutions of murine plasma samples. Negative control consisted of FVIII-deficient plasma from nontreated mice, whereas positive control consisted of murine FVIII-deficient plasma spiked with mouse monoclonal antibody D4H1. The concentration of the bound murine IgG antibodies was determined using a peroxidase-labeled goat anti-mouse IgG antibody (Sigma) using the spectrometric method described previously.
The presence of antibody against hFVIII in macaque plasma was determined using a modification of our previously described ELISA.3 In brief, plates were coated overnight with recombinant hFVIII (0.1 µg/well, Kogenate, Bayer Healthcare). Then 50 μL of dilutions of rhesus plasma (1:50, 1:500, 1:5000, 1:10 000) were applied to these wells in duplicate. Antibodies against hFVIII were detected with a 1:40 000 dilution of horseradish peroxidase conjugated rabbit anti-rhesus IgG (Sigma). Dilutions of plasma from RQ7155, a monkey immunized with recombinant hFVIII protein, were included as positive controls. All anti-hFVIII antibody titers (in mice and monkeys) were expressed as the end-point titer, defined as the reciprocal of the interpolated dilution with an absorbance value equal to 5 times the mean absorbance background value.
Positive samples were subjected to a Bethesda assay with the Nijmegen modification. Citrated rhesus test plasma was first incubated at 60°C for an hour to inactivate rhesus FVIII without significantly moderating the activity of rhesus anti-hFVIII antibody activity. Doubling dilutions of the test plasma in Imidazole buffer were then incubated with an equal volume of normal pooled human plasma or normal rhesus plasma. Following incubation at 37°C for 2 hours, the residual FVIII activity was determined using a 1-step activated partial thromboplastin time. One Bethesda unit was defined as the reciprocal of the dilution of test plasma at which 50% of human FVIII activity was inhibited. The sensitivity of the assay was 1 Bethesda unit/mL.
Molecular studies
To determine the configuration of the rAAV-codop-hFVIII genome, high-molecular-weight genomic DNA (15 µg) was extracted from murine or macaque liver tissue samples using our previously described method.3 Undigested DNA or that digested with Not I (single cutter) or Kpn I (double cutter) was electrophoresed through a 0.8% agarose gel, transferred to a nylon membrane (Hybond-N+; GE Health Systems), and hybridized with a γ-32P-labeled 480-bp fragment from the 3′ end of codop-hFVIII cDNA at 42°C. The intensity of the hybridization was determined using the STORM phosphorimager and ImageQuant software (Molecular Dynamics, Sunnyvale, CA). A quantitative real-time polymerase chain reaction assay was used to evaluate rAAV-hFVIII transgene copy number in genomic DNA extracted from murine or monkey liver tissues samples using the following primer set: forward hFVIII primer, 5′-TCATCATGTACAGCCTGGATGGCA-3′; reverse hFVIII primer, 5′- AGATGTTGTGCTTGATGCCAGAGC-3′ that amplifies a 113-bp region in the C1 domain of FVIII that was conserved in all the FVIII variants.
Integrity of the murine DNA was assessed using the following GAPDH primers: forward: 5′-TGGAGAGCCCGCTCAGACCC-3′ and reverse: 5′-GGATTGGGTGTCCCTGCG CC-3′. The lower limit of sensitivity for this assay was determined to be 10 viral copies/µg genomic DNA.
Results
Construction and evaluation of rAAV-hFVIII expression cassettes
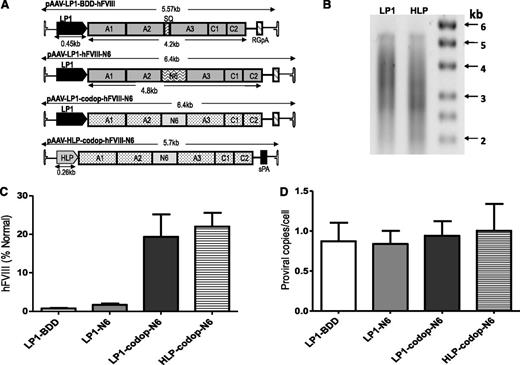
Initial studies in wild-type C57Bl/6 mice were designed to compare the effects of codon optimization of the FVIII N6 coding sequence and the use of a smaller liver-specific promoter using 4 single-stranded rAAV vectors containing either wild-type or codop hFVIII sequences under the control of our previously described LP1 promoter3 or a new, smaller HLP (Figure 1A). All 4 rAAV-hFVIII expression cassettes were packaged with serotype 5 capsid using a conventional HEK293T transient transfection method, with production efficiency (∼1 × 104 AAV-hFVIII particles/293T cell) being comparable to that reported previously for self-complementary AAV-hFIX vectors.3 Assessment of viral DNA extracted from rAAV5-LP1-codop-hFVIII-N6 and rAAV5-HLP-codop-hFVIII-N6 vector particles showed bands of approximately 5.6 kb followed by a smear on alkaline agarose gels, suggesting the packaging of heterogeneously sized genomes as previously reported for expression cassettes that exceed the ∼5.0-kb packaging capacity of rAAV (Figure 1B).12,28,29
Comparison of AAV hFVIII variants. (A) Schematics of 4 rAAV vector genomes encoding hFVIII variants under the control of the LP1 or the smaller HLP regulatory element and upstream of either rabbit globin or synthetic polyadenylation sites. Shown are the A1, A2, A3, C1, and C2 domains of hFVIII either with the SQ B-domain sequences (top schematic) or with the proximal 226-aa region of the B domain (N6). The hFVIII cDNA sequence in the lower 2 constructs are codop. (B) Alkaline gel analysis of viral genomes extracted from 5 × 1010 rAAV5-LP1-codop-hFVIII-N6 (lane 1) and rAAV5-HLP-codop-hFVIII-N6 (lane 2) viral particles. (C) Mean hFVIII:Ag levels ± SD in murine plasma at 6 weeks after a single tail vein administration of rAAV-hFVIII constructs pseudotyped with serotype 5 capsid in wild-type C57Bl/6 mice (N = 3, dose = 2 × 1013 vg/kg). (D) Mean (± SD) proviral copy number in murine liver transduced with rAAV5-hFVIII variants.
Comparison of AAV hFVIII variants. (A) Schematics of 4 rAAV vector genomes encoding hFVIII variants under the control of the LP1 or the smaller HLP regulatory element and upstream of either rabbit globin or synthetic polyadenylation sites. Shown are the A1, A2, A3, C1, and C2 domains of hFVIII either with the SQ B-domain sequences (top schematic) or with the proximal 226-aa region of the B domain (N6). The hFVIII cDNA sequence in the lower 2 constructs are codop. (B) Alkaline gel analysis of viral genomes extracted from 5 × 1010 rAAV5-LP1-codop-hFVIII-N6 (lane 1) and rAAV5-HLP-codop-hFVIII-N6 (lane 2) viral particles. (C) Mean hFVIII:Ag levels ± SD in murine plasma at 6 weeks after a single tail vein administration of rAAV-hFVIII constructs pseudotyped with serotype 5 capsid in wild-type C57Bl/6 mice (N = 3, dose = 2 × 1013 vg/kg). (D) Mean (± SD) proviral copy number in murine liver transduced with rAAV5-hFVIII variants.
Each of the 4 rAAV5-hFVIII vectors were administered by tail vein injection into 4- to 10-week-old male wild-type C57Bl/6 mice (N = 3/group) at a dose of 2 × 1013 vector genomes (vg)/kg. At 6 weeks after gene transfer, mice transduced with rAAV5-LP1-BDD-hFVIII expressed detectable hFVIII:Ag levels of 1 ± 0.3% (Figure 1C). Consistent with previous reports, hFVIII:Ag expression in the rAAV-LP1-hFVIII-N6 cohort of mice was approximately twofold higher (2 ± 0.6%) than in animals transduced with the rAAV5-LP1-BDD-hFVIII.6,9 Significantly higher levels (P = .0003, 1-way analysis of variance [ANOVA]) of hFVIII:Ag were observed in mice transduced with rAAV5-LP1-codop-hFVIII-N6 and rAAV5-HLP-codop-hFVIII-N6 at 19 ± 6% and 22 ± 6%, respectively. Importantly, hFVIII transgene copy number in the liver of mice transduced with the 4 different vectors was comparable at ∼0.9 to 1 copy/cell (Figure 1D), as was the mRNA level (data not shown), indicating higher levels of hFVIII protein in the codop-hFVIII-N6 cohorts of mice was likely due to improved translation or protein processing.3,18
These studies also confirmed that the potency of the LP1 and HLP promoters was similar. As with the LP1 promoter, direct intramuscular or intrasplenic injection of 5 × 1010 self-complementary AAV vector particles encoding our previously described codop hFIX gene under control of the HLP promoter resulted in efficient transduction but no detectable transcription as assessed by reverse transcriptase-polymerase chain reaction analysis of skeletal muscle or spleen (supplemental Figure 2). This suggests that the HLP regulatory element is not efficient at directing transgene expression in nonhepatic murine tissues.
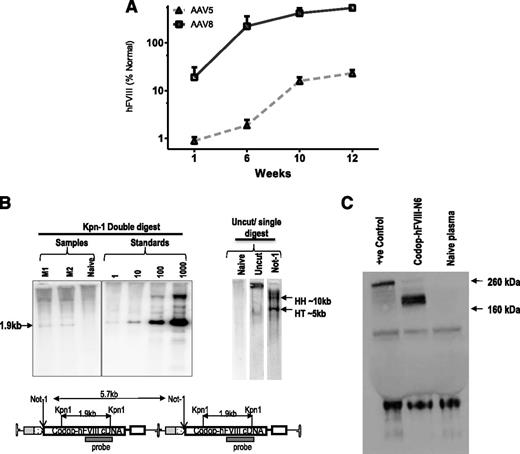
Tail vein administration of 2 × 1013 vg/kg resulted in 10-fold higher levels of transgene expression in male wild-type C57Bl/6 mice transduced with rAAV8-HLP-codop-hFVIII-N6 (hFVIII:Ag, 540 ± 218%) at 12 weeks after gene transfer when compared with cohorts that received serotype 5 pseudotyped vector (hFVIII:Ag, 23 ± 4%) containing the same expression cassette (N = 3/group, Figure 2A). This is consistent with previous reports of improved transduction of mouse liver by serotype 8 vectors.3,30 Additionally, despite the heterogeneous nature of the proviral DNA packaged into AAV virions, Southern blot analysis of genomic DNA from the liver of mice transduced with 2 × 1013 vg/kg rAAV8-HLP-codop-hFVIII-N6 only detected the full-length 5.7-kb proviral DNA in transduced murine hepatocytes (Figure 2B). Western blot analysis with an hFVIII-specific antibody showed only a single-chain ∼210-kd protein species in plasma of mice transduced with rAAV-HLP-codop-hFVIII-N6, which is the expected size for full-length codop-hFVIII-N6 (Figure 2C).
Evaluation of transduction with rAAV-HLP-codop-hFVIII-N6 in immunocompetent mice. (A) Kinetics of hFVIII:Ag expression following a single tail vein administration of 2 × 1013 vg/mouse of rAAV-HLP-codop-hFVIII-N6 pseudotyped with serotype 5 or 8 capsid in wild-type C57Bl/6 mice (N = 3/group). Shown are mean FVIII:Ag levels ± SD. (B, upper left) Southern blot of liver genomic DNA derived from mice (M1 and M2) transduced with rAAV8-HLP-codop-hFVIII-N6 was digested with Kpn I, a double cutter. Standards consist of rAAV-HLP-codop-hFVIII-N6 plasmid DNA spiked into naive mouse liver genomic DNA at the stipulated genome copies per cell concentration and then digested with Kpn I. (Upper right) Uncut DNA or DNA digested with a single cutter (Not I). HH, head-to-head and HT head-to-tail expected band size from proviral DNA in concatemeric configurations, respectively. (Bottom) A schematic showing the relative positions of Kpn I and Not I endonucleases in rAAV-HLP-codop-hFVIII-N6 when in a head to tail concatemeric configuration. (C) Western blot showing a single ∼210-kd band in the plasma of mice transduced with rAAV8-HLP-codop-hFVIII-N6, which is not present in naive mouse plasma. Positive control (+ve control) consisting of full-length recombinant hFVIII (Helixate) diluted in mouse plasma.
Evaluation of transduction with rAAV-HLP-codop-hFVIII-N6 in immunocompetent mice. (A) Kinetics of hFVIII:Ag expression following a single tail vein administration of 2 × 1013 vg/mouse of rAAV-HLP-codop-hFVIII-N6 pseudotyped with serotype 5 or 8 capsid in wild-type C57Bl/6 mice (N = 3/group). Shown are mean FVIII:Ag levels ± SD. (B, upper left) Southern blot of liver genomic DNA derived from mice (M1 and M2) transduced with rAAV8-HLP-codop-hFVIII-N6 was digested with Kpn I, a double cutter. Standards consist of rAAV-HLP-codop-hFVIII-N6 plasmid DNA spiked into naive mouse liver genomic DNA at the stipulated genome copies per cell concentration and then digested with Kpn I. (Upper right) Uncut DNA or DNA digested with a single cutter (Not I). HH, head-to-head and HT head-to-tail expected band size from proviral DNA in concatemeric configurations, respectively. (Bottom) A schematic showing the relative positions of Kpn I and Not I endonucleases in rAAV-HLP-codop-hFVIII-N6 when in a head to tail concatemeric configuration. (C) Western blot showing a single ∼210-kd band in the plasma of mice transduced with rAAV8-HLP-codop-hFVIII-N6, which is not present in naive mouse plasma. Positive control (+ve control) consisting of full-length recombinant hFVIII (Helixate) diluted in mouse plasma.
Codop-hFVIII-V3 is a potent hFVIII variant that is efficiently packaged into rAAV
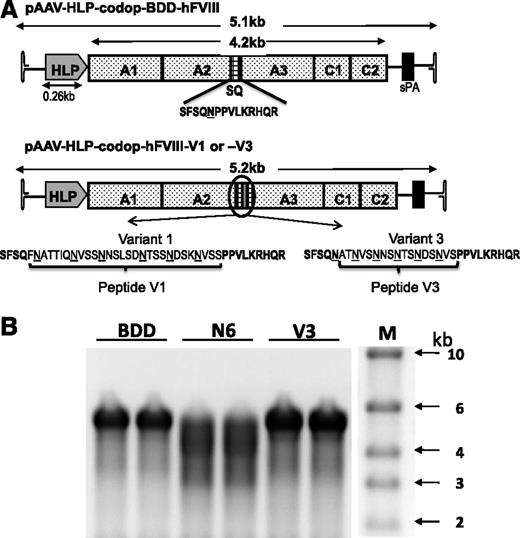
Smaller codop rAAV expression cassettes were next generated with the aim of retaining the potency of rAAV-HLP-codop-hFVIII-N6, while improving the packaging efficiency of vector genomes within AAV capsids (Figure 3A). In rAAV-HLP-codop-hFVIII-V3, the 226-aa B-domain N6 encoding spacer was replaced with a novel sequence (51 bp) encoding a 17-aa peptide, which brought into juxtaposition the 6 N-linked glycosylation triplets within the B domain of rAAV-HLP-codop-hFVIII-N6. The V3 peptide was flanked by 14-aa SQ residues from the N- and C-terminal ends of the B domain, as described before for BDD-hFVIII.20,31 rAAV-HLP-codop-hFVIII-V1 was identical to rAAV-HLP-codop-hFVIII-V3 except that the N-linked glycosylation triplets were flanked by 2 additional amino acids that are normally adjacent to these moieties in the native hFVIII B-domain, thus extending the size of the peptide from 17 to 29 aa. rAAV-HLP-codop-BDD-hFVIII (∼5.1 kb in size) served as a control vector and was identical to rAAV-HLP-codop-hFVIII-V3 except that it only contained the SQ B-domain sequences.
Comparison of smaller rAAV-HLP-codop-hFVIII constructs. (A) Schematic of smaller codon-optimized hFVIII expression cassettes. Top schematic consists of B domain–deleted variant, rAAV-HLP-codop-BDD-hFVIII, which is ∼5.1 kb in size. It comprises A1-A2-A3-C1-C2 FVIII domains and contains the SQ sequences between the A2 and A3 domains. rAAV-HLP-codop-hFVIII-V1 and rAAV-HLP-codop-hFVIII-V3 are identical to rAAV-HLP-codop-BDD-hFVIII except that peptides V1 or V3 have been placed within the SQ sequence. Both peptides contain the same 6 N-linked glycosylation triplets. (B) Analysis of viral DNA extracted from 5 × 1010 particles of each of the codon-optimized hFVIII vector preparation following separation on an alkaline agarose gel and run in duplicate showing bands of ∼5 kb, the expected size for the rAAV-HLP-codop-BDD-hFVIII (BDD) and rAAV-HLP-codop-hFVIII-V3 (V). In comparison, a rather diffuse signal was observed for the genomes extracted from AAV8-HLP-codop-hFVIII-N6 (N6), suggesting the packaging of a more heterogeneous proviral species. (Right) Size standards.
Comparison of smaller rAAV-HLP-codop-hFVIII constructs. (A) Schematic of smaller codon-optimized hFVIII expression cassettes. Top schematic consists of B domain–deleted variant, rAAV-HLP-codop-BDD-hFVIII, which is ∼5.1 kb in size. It comprises A1-A2-A3-C1-C2 FVIII domains and contains the SQ sequences between the A2 and A3 domains. rAAV-HLP-codop-hFVIII-V1 and rAAV-HLP-codop-hFVIII-V3 are identical to rAAV-HLP-codop-BDD-hFVIII except that peptides V1 or V3 have been placed within the SQ sequence. Both peptides contain the same 6 N-linked glycosylation triplets. (B) Analysis of viral DNA extracted from 5 × 1010 particles of each of the codon-optimized hFVIII vector preparation following separation on an alkaline agarose gel and run in duplicate showing bands of ∼5 kb, the expected size for the rAAV-HLP-codop-BDD-hFVIII (BDD) and rAAV-HLP-codop-hFVIII-V3 (V). In comparison, a rather diffuse signal was observed for the genomes extracted from AAV8-HLP-codop-hFVIII-N6 (N6), suggesting the packaging of a more heterogeneous proviral species. (Right) Size standards.
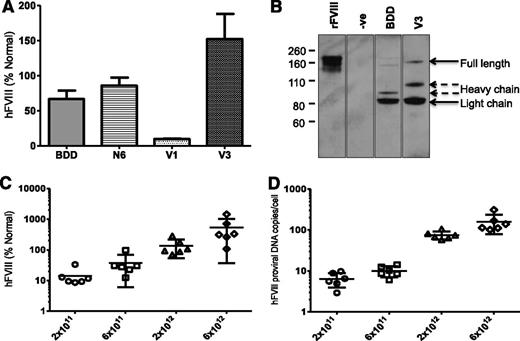
The yield of the smaller codop hFVIII vectors, pseudotyped with serotype 8 capsid, was comparable (range = 0.9-2.5 × 104 vg/cell) to that of rAAV8-HLP-codop-FVIII-N6. In contrast with rAAV8-HLP-codop-hFVIII-N6, analysis of viral DNA extracted from 2.5 × 1010 particles of rAAV8-HLP-codop-hFVIII-V3 showed a single band of ∼5 kb on an alkaline agarose gel, the expected size for the rAAV8-HLP-codop-BDD-hFVIII and codop-hFVIII-V3 (Figure 3B). The rAAV8-HLP-codop-hFVIII-V1 genome was also uniformly packaged as full-length ∼5 kb proviral DNA (data not shown). Despite its superior packaging into AAV vectors compared with codop-N6 vector, tail vein administration of 2 × 1012 vg/kg of rAAV8-HLP-codop-BDD-hFVIII in wild-type C57Bl/6 mice resulted in hFVIII:Ag levels of 56 ± 27% at 6 weeks after gene transfer (Figure 4A), which was lower than that observed in cohorts of mice transduced with the same dose of rAAV8-HLP-codop-hFVIII-N6 (hFVIII:Ag, 83 ± 24%) consistent with previous reports.6 However, mice transduced with rAAV8-HLP-codop-FVIII-V3 consistently expressed hFVIII:Ag at levels that were at least 2-fold higher (177 ± 34%). This difference in hFVIII:Ag levels between rAAV-HLP-codop-hFVIII-V3, rAAV-HLP-BDD-hFVIII, and rAAV-HLP-hFVIII-N6 was highly significant (P = .003, 1-way ANOVA). Heavy and light chain peptides with a small quantity of a 170 kDa nonprocessed, primary translation product were readily visible by western blot analysis of plasma (Figure 4B) from mice transduced with rAAV8-HLP-codop-hFVIII-V3 and rAAV8-HLP-BDD-hFVIII. Surprisingly, mice transduced with rAAV8-HLP-codop-hFVIII-V1, which contained the same 6 N-linked glycosylation moieties as rAAV8-HLP-codop-hFVIII-V3, had significantly lower levels of hFVIII:Ag at 9 ± 2% (P < .0001). Consistent with this, hFVIII was undetectable by western blot analysis in the plasma (Figure 4B) from mice transduced with rAAV8-HLP-codop-hFVIII-V1 (data not shown).
Evaluation of AAV FVIII variants in vivo. (A) Mean hFVIII:Ag levels ± SD in murine plasma derived from male wild-type C57Bl/6 mice (N = 6) at 6 weeks after tail vein injection of 2 × 1012 vg/kg of rAAV8-HLP-codop-hFVIII variants. (B) Sodium dodecyl sulfonate polyacrylamide gel electrophoresis/western blotting of murine plasma following gene transfer with rAAV-HLP-codop-BDD-hFVIII (BDD) and rAAV8-HLP-codop-hFVIII-V3 (V3) using a polyclonal rhesus anti-hFVIII antibody showing the heavy (∼90 kDa) and light (∼80 kDa) chains as well as a 170-kDa nonprocessed, primary translation product. Negative control (−ve) is naive mouse plasma and positive control consists of recombinant BDD hFVIII (ReFacto, first lane) diluted in murine plasma containing protease inhibitors. (C) Relationship between rAAV8-HLP-codop-hFVIII-V3 dose and hFVIII:Ag levels (mean ± SD) in murine plasma and (D) transgene copy number (mean ± SD) at 12 weeks following gene transfer.
Evaluation of AAV FVIII variants in vivo. (A) Mean hFVIII:Ag levels ± SD in murine plasma derived from male wild-type C57Bl/6 mice (N = 6) at 6 weeks after tail vein injection of 2 × 1012 vg/kg of rAAV8-HLP-codop-hFVIII variants. (B) Sodium dodecyl sulfonate polyacrylamide gel electrophoresis/western blotting of murine plasma following gene transfer with rAAV-HLP-codop-BDD-hFVIII (BDD) and rAAV8-HLP-codop-hFVIII-V3 (V3) using a polyclonal rhesus anti-hFVIII antibody showing the heavy (∼90 kDa) and light (∼80 kDa) chains as well as a 170-kDa nonprocessed, primary translation product. Negative control (−ve) is naive mouse plasma and positive control consists of recombinant BDD hFVIII (ReFacto, first lane) diluted in murine plasma containing protease inhibitors. (C) Relationship between rAAV8-HLP-codop-hFVIII-V3 dose and hFVIII:Ag levels (mean ± SD) in murine plasma and (D) transgene copy number (mean ± SD) at 12 weeks following gene transfer.
Further evaluation of rAAV8-HLP-codop-hFVIII-V3 over an extended dose range (2 × 1011 to 6 × 1012 vg/kg) showed a dose-dependent increase in hFVIII:Ag in murine plasma (Figure 4C; Table 1) that even at the highest dose was not associated with organ toxicity or a hypercoagulable state as determined by assessment of tissue histology and plasma D-dimer assessment. A linear relationship was noted between vector dose and transgene copy number in the liver (Figure 4D), the organ that is preferentially transduced following peripheral vein delivery of AAV vectors.4
rAAV-HLP-codop-hFVIII-V3 corrects the bleeding diathesis in HA knock-out mice
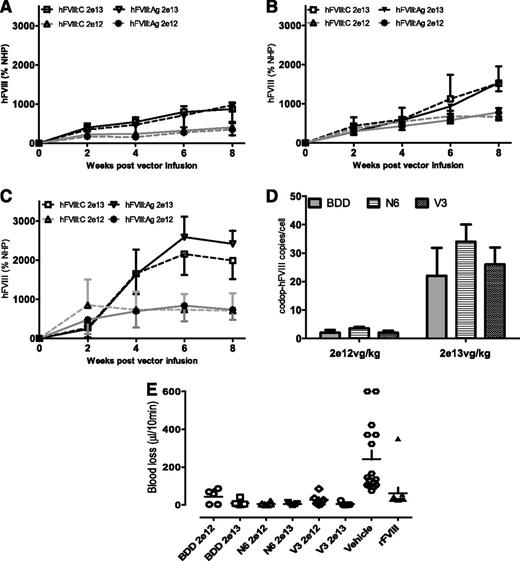
To directly compare the biologic potency of the rAAV8-codop-hFVIIII expression cassettes, serotype 8 pseudotyped vector was administered into the tail vein of male F8−/− mice at a dose of 2 × 1012 (low-dose cohort, n = 6) or 2 × 1013 (high-dose cohort, n = 6) vg/kg. For a given construct, peak hFVIII:C levels were roughly twofold higher in animals transduced with the high dose of vector when compared with the low dose (Figure 5) at 8 weeks after gene transfer, whereas the transgene copy number difference between the 2 dose levels was roughly 10-fold (Figure 5A-D). Perhaps the lower level of hFVIII/copy of transgene in the high-dose animals is due to repeat induced silencing.32 Regardless, significantly higher levels of hFVIII:C were observed in mice transduced with rAAV8-HLP-codop-hFVIII-V3 weeks after gene transfer when compared with cohorts that received codop-hFVIII-N6 or codop-BDD-hFVIII variants (P < .05 Student t test). The average ratio of hFVIII:C to hFVIII:Ag was approximately 1.0 for each of the time points assessed and at both dose levels, suggesting that the transgenic hFVIII molecules were fully active. The amount of blood lost following a tail clip assay was comparable for all groups of rAAV8-codop-hFVIII–transduced mice and significantly lower (P < .001 1-way ANOVA test) than that observed in F8−/− mice treated with vehicle (PBS) but comparable to levels observed following treatment with recombinant hFVIII. rAAV-mediated expression of hFVIII therefore restored hemostasis to levels observed with recombinant hFVIII (Figure 5E). Importantly, neutralizing anti-hFVIII antibodies were not detected at any time point in any of the 6 cohorts of F8−/− mice assessed.
Expression of hFVIII expression in F8−/− mice. (A-D) Kinetics of hFVIII expression shown as activity (hFVIII:C; mean ± SD) and antigen (hFVIII:Ag; mean ± SD) levels in male F8−/− mice following a single tail vein administration of low (2 × 1012 vg/kg) and high dose (2 × 1013 vg/kg) of (A) rAAV-HLP-codop-BDD-hFVIII (BDD), (B) rAAV-HLP-codop-hFVIII-N6 (N6), or (C) rAAV-HLP-codop-hFVIII-V3 (V3). Standards consisted of normal human pool plasma (NHP) diluted in murine plasma. (D) Transgene copy number in the liver at 8 weeks following gene transfer of rAAV-HLP-codop-BDD-hFVIII, rAAV-HLP-codop-hFVIII-N6, and rAAV-HLP-codop-hFVIII-V3. (E) Blood loss in F8−/− mice following gene transfer with rAAV-HLP-codop-BDD-hFVIII, rAAV-HLP-codop-hFVIII-N6, and rAAV-HLP-codop-hFVIII-V3 (V3) compared with that in F8−/− mice treated with vehicle alone or recombinant FVIII.
Expression of hFVIII expression in F8−/− mice. (A-D) Kinetics of hFVIII expression shown as activity (hFVIII:C; mean ± SD) and antigen (hFVIII:Ag; mean ± SD) levels in male F8−/− mice following a single tail vein administration of low (2 × 1012 vg/kg) and high dose (2 × 1013 vg/kg) of (A) rAAV-HLP-codop-BDD-hFVIII (BDD), (B) rAAV-HLP-codop-hFVIII-N6 (N6), or (C) rAAV-HLP-codop-hFVIII-V3 (V3). Standards consisted of normal human pool plasma (NHP) diluted in murine plasma. (D) Transgene copy number in the liver at 8 weeks following gene transfer of rAAV-HLP-codop-BDD-hFVIII, rAAV-HLP-codop-hFVIII-N6, and rAAV-HLP-codop-hFVIII-V3. (E) Blood loss in F8−/− mice following gene transfer with rAAV-HLP-codop-BDD-hFVIII, rAAV-HLP-codop-hFVIII-N6, and rAAV-HLP-codop-hFVIII-V3 (V3) compared with that in F8−/− mice treated with vehicle alone or recombinant FVIII.
Therapeutic expression of hFVIII in non-human primates following peripheral vein administration of rAAV8-HLP-codop-hFVIII vectors
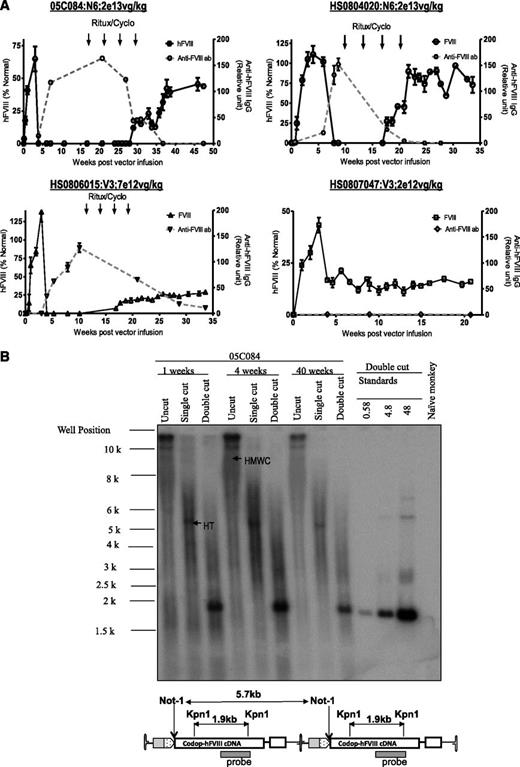
The non-human primate model provides an opportunity to assess scaleup of efficacy in a context relevant to humans.33 Peripheral vein administration of rAAV8-HLP-codop-hFVIII-N6 or rAAV8-HLP-codop-hFVIII-V3 into male adolescent rhesus monkeys resulted in detectable levels of hFVIII:Ag in monkey plasma within 72 hours of vector administration, reaching peak levels by 3 weeks (Figure 6A). Peripheral vein administration of 2 × 1013 vg/kg of rAAV8-HLP-codop-hFVIII-N6 resulted in peak hFVIII:Ag levels of 65 ± 10% and 105 ± 16% in macaques 05C084 and HS0804020, respectively. rAAV8-HLP-codop-hFVIII-V3 was administered at lower doses of vector, but despite this, 7 × 1012 vg/kg and 2 × 1012 vg/kg resulted in peak hFVIII:Ag levels of 138 ± 23% and 43 ± 10% in macaques HS0806015 and HS0807047, respectively. Except for the low-dose monkey (HS0807047), all 3 animals developed neutralizing hFVIII antibodies within 6 weeks of gene transfer. The peak anti-hFVIII IgG titer for macaques 05C084, HS0804020, and HS0806015 was 163 ± 19, 151 ± 21, and 127 ± 15 relative units. The respective peak inhibitory titers for these animals were 15, 12, and 3 Bethesda units/mL. They were treated with rituximab and cyclophosphamide as previously described,4 resulting in complete eradication of the neutralizing anti-hFVIII antibody in 2 animals and a partial reduction in antibody titer in monkey HS0806015. hFVIII:Ag was once again detectable in these animals following immune-modulatory treatment rituximab and cyclophosphamide (Figure 6A). Southern blot analysis of genomic DNA extracted from liver biopsy samples of monkey 05C084 obtained at 1 and 4 weeks showed that the approximate proviral genome copy number was between 25 and 40 copies per diploid genome. DNA species corresponding to high molecular weight concatamers, most of which were present in a head-to-tail configuration (Figure 6B), were detected in uncut genomic DNA and following digestion with Not I, which cuts once within the cassette.
Evaluation of AAV FVIII variants in non-human primates. (A) hFVIII:Ag levels (solid black line; mean ± SD, left y-axis) and anti-hFVIII antibody titers (dashed gray line; relative units, right y-axis) as assessed by immunocapture assays over time in 4 2- to 4-year-old male monkeys following a single peripheral vein tail vein administration of either rAAV8-HLP-codop-hFVIII-N6 (N6) or rAAV8-HLP-codop-hFVIII-V3 (V3). Animals that developed neutralizing anti-hFVIII antibodies were treated with 4 monthly cycles of rituximab and cyclophosphamide shown by arrows. (B) Southern blot analysis showing molecular configuration of rAAV8-HLP-codop-hFVIII-N6 genome in macaque liver. Approximately 15 µg of DNA from each time point (1, 4, and 40 weeks after gene transfer) was electrophoresed uncut (uncut) or following digestion with Not I (single cutter) or Kpn I (double cutter). Shown are bands representing high-molecular-weight concatemers (HMWC) in the head-tail (HT) and head-to-head (HH) formation. (Bottom) A schematic of rAAV-HLP-codop-hFVIII-N6 in the HT configuration together with the cleavage sites for Kpn I and Not I.
Evaluation of AAV FVIII variants in non-human primates. (A) hFVIII:Ag levels (solid black line; mean ± SD, left y-axis) and anti-hFVIII antibody titers (dashed gray line; relative units, right y-axis) as assessed by immunocapture assays over time in 4 2- to 4-year-old male monkeys following a single peripheral vein tail vein administration of either rAAV8-HLP-codop-hFVIII-N6 (N6) or rAAV8-HLP-codop-hFVIII-V3 (V3). Animals that developed neutralizing anti-hFVIII antibodies were treated with 4 monthly cycles of rituximab and cyclophosphamide shown by arrows. (B) Southern blot analysis showing molecular configuration of rAAV8-HLP-codop-hFVIII-N6 genome in macaque liver. Approximately 15 µg of DNA from each time point (1, 4, and 40 weeks after gene transfer) was electrophoresed uncut (uncut) or following digestion with Not I (single cutter) or Kpn I (double cutter). Shown are bands representing high-molecular-weight concatemers (HMWC) in the head-tail (HT) and head-to-head (HH) formation. (Bottom) A schematic of rAAV-HLP-codop-hFVIII-N6 in the HT configuration together with the cleavage sites for Kpn I and Not I.
Discussion
Three phase 1/2 clinical trials of FVIII gene transfer have failed to achieve persistent phenotypic correction in patients with severe HA.2 These trials used either ex vivo fibroblast transfection,34 intravenous delivery of a retroviral vector,35 or a helper-dependent adenoviral vector for FVIII gene delivery. On the other hand, in an ongoing clinical trial, we have demonstrated stable expression of hFIX at 1% to 6% of normal with correction of the bleeding diathesis in patients with severe hemophilia B following a single administration of an AAV vector, without long-term toxicity.5 This demonstration of the safety and efficacy of rAAV vectors supports the further development of approaches using this vector system for gene therapy of HA. However, progress has been hampered by low levels of FVIII transgene expression and the large size of the FVIII cDNA.16,17,36
In this study, we show that a single administration of rAAV that contains a codop hFVIII construct that includes the proximal 226 aa of the hFVIII B domain resulted in therapeutic expression of hFVIII in murine and non-human primate models. For a given dose of vector, expression mediated by rAAV-HLP-codop-hFVIII-N6 was almost 10-fold higher than observed with rAAV containing wild-type hFVIII sequences for the BDD or N6 variants (Table 1). Therefore, as with other human coagulation factors including FVII and FIX, codon optimization of hFVIII improves transgene expression.18,37 Because of its relatively large size, a significant proportion of the 5.7-kb rAAV-HLP-codop-hFVIII-N6 proviral DNA was packaged into rAAV as partial transgene fragments. Despite this, Southern blot analysis of murine and nonhuman primate liver showed that the rAAV-HLP-codop-hFVIII-N6 transgene was maintained predominantly as a full-length 5.7-kb expression cassette in vivo, thus raising the possibility that a degree of intracellular reassembly may occur following transduction resulting in reconstitution of the full-length 5.7-kb rAAV-HLP-codop-hFVIII-N6 proviral DNA from partial fragments of this cassette, as described previously.12,28,29
These initial studies highlighted the need for smaller rAAV-hFVIII expression cassettes to improve the proviral packaging efficiency without compromising the higher potency of codop hFVIII-N6. An important consideration in this development was that even though the B domains from different species are not highly conserved, the 6 N-linked glycosylation consensus sequences (N-X-T/S) dispersed across the proximal 226 aa of this region are maintained, suggesting that they play an important biological role.38 Therefore, to reduce the size of rAAV-HLP-codop-hFVIII-N6, the 226-aa B domain spacer was replaced with a 17-aa V3 peptide in which the 6 N-linked glycosylation triplets were placed adjacent to each other.
We found that rAAV-HLP-codop-hFVIII-V3 mediated higher hFVIII:Ag expression in 2 different murine models (wild-type C57Bl/6 and F8−/− mice) when compared with rAAV-HLP-codop-BDD-hFVIII and rAAV-HLP-codop-hFVIII-N6 variants. Additionally, this construct was efficiently packaged within rAAV virions. Importantly, the manner in which the N-glycosylation triplets from the B domain are brought together appeared to be important, because minor changes to the 17-aa peptide, which involved retention of extra amino acids flanking the N-glycosylation triplet in the native B domain, greatly reduced the efficiency of hFVIII:Ag expression. Further studies are required to assess the glycosylation status of the 6 N-linked triplets and to fully understand how this novel hFVIII configuration that includes the V3 peptide influences expression of hFVIII following gene transfer.
Irrespective of the mechanism of enhancement, the higher potency of AAV8-HLP-codop-hFVIII-V3 vector raises the possibility that a vector dose between 2 × 1011 vg/kg and 2 × 1012 vg/kg will likely be sufficient for therapeutic hFVIII expression in humans. This is the same rAAV8 vector dose range that proved to be efficacious in our ongoing hemophilia B clinical trial.5
Juxtaposition of novel amino acid sequences as has been done in rAAV-HLP-codop-hFVIII-V3 could lead to neo-antigenicity, thereby increasing the risk of provoking a neutralizing antibody response to the transgenic protein. This was also a concern when recombinant BDD-FVIII (ReFacto) was first introduced for use in man. ReFacto contains the “SQ” link of 14 aa (SFSQNPPVLKRHQR) between the A2 and A3 domains, generated by fusion of Ser743 in the N-terminus with Gln1638 in the C-terminus of the B-domain, creating a neo-antigenic site. However, despite extensive clinical use of ReFacto, an increase in frequency of neutralizing hFVIII antibodies in patients treated with this product has not been observed.39-41 Additionally, antibodies to epitopes in the B domain that are occasionally seen in patients with severe HA treated with hFVIII protein concentrates are devoid of inhibitory activity because they bind to nonfunctional FVIII epitopes.42 Neutralizing anti-hFVIII antibodies (inhibitors) were not observed in immunocompetent murine models but did occur in some monkeys. Epitope mapping studies have yet to be performed, but, as with anti-hFIX inhibitors, we showed that in the context of sustained AAV-mediated hFVIII expression following gene transfer, it is possible to induce peripheral tolerance to transgenic hFVIII with a short course of a rituximab-containing immunosuppressive regimen. This resulted in abrogation of the neutralizing anti-hFVIII antibody and sustained long-term expression of hFVIII without the need for repeated or persistent treatment with immunosuppressive therapy.3,4,32 Importantly, in the context of gene therapy for HA, initial eligible patients will have received numerous exposures to FVIII protein concentrates, an inclusion criterion that likely screens out many of those who are likely to develop inhibitors.
In summary, the inclusion of a novel 17-aa peptide has improved the potency and packaging efficiency of codop hFVIII B domain–deleted expression cassettes in rAAV virion, thus improving the prospects of effective rAAV-mediated gene transfer for HA. We are in the process of developing clinical grade rAAV-HLP-codop-hFVIII-V3 for use in human subjects in the context of a clinical trial.
Summary of hFVIII expression in murine models
| Vector . | Capsid serotype . | Dose (vg/kg) . | Mouse model . | hFVIII:Ag levels (%; mean ± SD) . |
|---|---|---|---|---|
| rAAV-LP1-BDD-hFVIII | 5 | 2 × 1013 | Wild-type C57Bl/6 | 1 ± 0.3 |
| rAAV-LP1-hFVIII-N6 | 2 ± 0.6 | |||
| rAAV-LP1-codop-hFVIII-N6 | 19 ± 6 | |||
| rAAV-HLP-codop-hFVIII-N6 | 22 ± 6 | |||
| rAAV-HLP-codop-hFVIII-N6 | 8 | 2 × 1013 | Wild-type C57Bl/6 | 540 ± 218* |
| rAAV-HLP-codop-BDD-hFVIII | 8 | 2 × 1012 | Wild-type C57Bl/6 | 56 ± 27 |
| rAAV-HLP-codop-hFVIII-N6 | 83 ± 24 | |||
| rAAV-HLP-codop-hFVIII-V1 | 9 ± 2 | |||
| rAAV-HLP-codop-hFVIII-V3 | 177 ± 34 | |||
| rAAV-HLP-codop-BDD-hFVIII | 8 | 2 × 1012 | FVIII−/− | 243 ± 112 |
| rAAV-HLP-codop-hFVIII-N6 | 542 ± 124 | |||
| rAAV-HLP-codop-hFVIII-V3 | 732 ± 162 | |||
| rAAV-HLP-codop-BDD-hFVIII | 8 | 2 × 1013 | FVIII−/− | 671 ± 209 |
| rAAV-HLP-codop-hFVIII-N6 | 1330 ± 427 | |||
| rAAV-HLP-codop-hFVIII-V3 | 2578 ± 527 |
| Vector . | Capsid serotype . | Dose (vg/kg) . | Mouse model . | hFVIII:Ag levels (%; mean ± SD) . |
|---|---|---|---|---|
| rAAV-LP1-BDD-hFVIII | 5 | 2 × 1013 | Wild-type C57Bl/6 | 1 ± 0.3 |
| rAAV-LP1-hFVIII-N6 | 2 ± 0.6 | |||
| rAAV-LP1-codop-hFVIII-N6 | 19 ± 6 | |||
| rAAV-HLP-codop-hFVIII-N6 | 22 ± 6 | |||
| rAAV-HLP-codop-hFVIII-N6 | 8 | 2 × 1013 | Wild-type C57Bl/6 | 540 ± 218* |
| rAAV-HLP-codop-BDD-hFVIII | 8 | 2 × 1012 | Wild-type C57Bl/6 | 56 ± 27 |
| rAAV-HLP-codop-hFVIII-N6 | 83 ± 24 | |||
| rAAV-HLP-codop-hFVIII-V1 | 9 ± 2 | |||
| rAAV-HLP-codop-hFVIII-V3 | 177 ± 34 | |||
| rAAV-HLP-codop-BDD-hFVIII | 8 | 2 × 1012 | FVIII−/− | 243 ± 112 |
| rAAV-HLP-codop-hFVIII-N6 | 542 ± 124 | |||
| rAAV-HLP-codop-hFVIII-V3 | 732 ± 162 | |||
| rAAV-HLP-codop-BDD-hFVIII | 8 | 2 × 1013 | FVIII−/− | 671 ± 209 |
| rAAV-HLP-codop-hFVIII-N6 | 1330 ± 427 | |||
| rAAV-HLP-codop-hFVIII-V3 | 2578 ± 527 |
These values are from the 12-wk time point. The rest of the data in the table are from 6 wk after gene transfer to allow comparisons between different experiments. The shaded rows are a summary of studies in FVIII−/− mice, which were performed by the laboratory of P.J.L. using a different set of assays and standards.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Ghasem Rastegarlari and Sushmita Roy Nawathe for experimental support, and Dr Stephen Pipe for providing plasmid containing the cDNA for BDD-FVIII (wild-type sequence) and FVIII-N6 (wild-type sequence).
This work was supported by Medical Research Council, National Institute for Health Research Programme (grant RP-PG-0310-1001), French National Agency for Research (grant ANR-CEXC-08-018-0), the ASSISI Foundation of Memphis, the American Lebanese Syrian Associated Charities, Howard Hughes Medical Institute, and the National Heart, Lung, and Blood Institute (grant HL094396). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research Programme, or the Department of Health in the United Kingdom.
Authorship
Contribution: J.M., S.R., D.R., N.P., C.R., D.L., F.M., S.-Z.Z., P.F., and J.T.G. contributed to the design of the FVIII expression cassettes, performed the cloning, manufactured the vectors, and conducted the preclinical evaluation of the FVIII constructs in normal mice; P.J.L., S.W., and O.D.C. performed the evaluation of the FVIII variants in FVIII−/− mice; C.Y.C.N., J.Z., C.L.M., and M.C. performed the evaluation of the FVIII variants in nonhuman primates; E.G.D.T., J.H.M., A.W.N., and K.A.H. helped with the design of the research strategies; and A.M.D. and A.C.N. designed research and wrote the manuscript.
Conflict-of-interest disclosure: J.M., E.G.D.T., A.M.D., and A.C.N. are listed as inventors in a patent filing for rAAV-HLP-codop-hFVIII-V3. The remaining authors declare no competing financial interests.
Correspondence: Amit C. Nathwani, UCL Cancer Institute, Paul O'Gorman Building, University College London, 72 Huntley St, London WC1E 6BT; e-mail: a.nathwani@ucl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal