Key Points
CD19-deficient donors augmented Scl-cGVHD.
Donor regulatory B cells suppressed Scl-cGVHD.
Abstract
Chronic graft-versus-host disease (cGVHD) is an increasingly frequent cause of morbidity and mortality of allogeneic hematopoietic stem-cell transplantation. Sclerodermatous cGVHD (Scl-cGVHD) is characterized by fibrosis and autoimmune features resembling those of systemic sclerosis (SSc). Transplantation of B10.D2 bone marrow and splenocytes into irradiated BALB/c mice is an established model of human Scl-cGVHD. To examine the role of B cells in Scl-cGVHD, CD19-deficient (CD19−/−) mice were used as donors or recipients. CD19−/− donors induced more severe Scl-cGVHD than wild-type donors, but use of CD19−/− recipients resulted in no significant differences compared with wild-type recipients. Moreover, CD19 deficiency on donor B cells resulted in the expansion of splenic interleukin (IL) -6–producing monocytes/macrophages, cytotoxic CD8+ T cells, and Th1 cells during the early stage of disease and increased the infiltration of T cells, TGF-β–producing monocytes/macrophages, and Th2 cells into the skin in the later stage of Scl-cGVHD. IL-10–producing regulatory B cells (B10 cells) were not reconstituted by CD19−/− donor cells, and early adoptive transfer of B10 cells attenuated the augmented manifestations of CD19−/− donor-induced Scl-cGVHD. Therefore, donor-derived B10 cells have a suppressive role in Scl-cGVHD development, warranting future investigation of regulatory B-cell–based therapy for treatment of Scl-cGVHD and SSc.
Introduction
Graft-versus-host disease (GVHD) is the most common cause of morbidity and mortality in allogeneic hematopoietic stem-cell transplantation.1,2 Chronic GVHD (cGVHD) is characterized by fibrosis with scleroderma-like changes and autoimmune features, and thus it resembles autoimmune diseases, including systemic sclerosis (SSc).3 Transplantation of B10.D2 (major histocompatibility complex molecules; H-2d) bone marrow (BM) and splenocytes across minor histocompatibility loci into sublethally irradiated BALB/c (H-2d) recipients is a well-established animal model for human sclerodermatous cGVHD (Scl-cGVHD) and SSc.4
Alloreactive reactions between donor-derived immune cells and host cell populations are critical in the pathogenesis of GVHD. Donor T cells have classically been considered the main effector cells, although recent studies have suggested that B cells also play fundamental roles in GVHD.5-8 Circulating autoantibodies are frequently detected in cGVHD patients.9 Their direct pathogenicity remains unclear, although a recent study has demonstrated that pathogenic antibody production from germinal centers plays a causal role in development of cGVHD.10 Furthermore, donor B cells prolong the survival of pathogenic CD4+ T cells.7 Clinically, studies have demonstrated the efficacy of B-cell–targeting therapy using rituximab for cGVHD.11,12 Collectively, B cells play pathogenic roles in the development of cGVHD through diverse mechanisms.13 To the contrary, recent studies have clarified the protective role of regulatory B cells (Bregs) in various inflammatory and autoimmune diseases. Bregs are required for prolonged allograft survival associated with interleukin-10 (IL-10).14 Another study indicated an increase in Bregs in kidney transplant recipients after alemtuzumab treatment.15 In acute GVHD, host B cells have a protective effect since B-cell–deficient mice experience more severe symptoms than wild-type mice.16 However, the role of Bregs in cGVHD remains unclear.
CD19 is a B-cell–specific, cell-surface protein that serves as a positive response regulator.17 CD19-deficient (CD19−/−) mice exhibit severely decreased numbers of marginal zone (MZ) B cells and B-1 cells. Specifically, CD1dhiCD5+ B cells, a fraction containing potent IL-10–producing B cells (B10 cells), are nearly absent in CD19−/− mice.18,19 Furthermore, B10 cells can promote tolerogenic immune responses through IL-10–dependent mechanisms.19-25 Contact hypersensitivity, experimental autoimmune encephalomyelitis, lupus, and inflammatory bowel diseases are augmented in CD19−/− mice due to the lack of B10 cells.18,19,24,26,27 In this study, we investigated the role of Bregs in Scl-cGVHD using CD19−/− mice.
Methods
Mice
BALB/c and B10.D2 mice were purchased from Japan SLC (Shizuoka, Japan). CD19−/− mice were generated as described28 and backcrossed onto the BALB/c or B10.D2 background for ≥12 generations. Mice were housed in a specific pathogen-free barrier facility. The Committee on Animal Experimentation of Kanazawa University Graduate School of Medical Science approved all studies and procedures.
BM transplantation
Male wild-type or CD19−/− B10.D2 mice and female wild-type or CD19−/− BALB/c mice (8 to 12 weeks old) were used as donors and recipients, respectively. BM was T-cell–depleted (TCD) with anti-CD90.2 microbeads (Miltenyi Biotec, Auburn, CA). Splenic T and B cells were isolated with anti-CD90.2 and anti-B220 microbeads (Miltenyi Biotec), respectively. Recipients were irradiated with 800 cGy (MBR-1520R; Hitachi, Tokyo, Japan) and were injected with 10 × 106 TCD BM cells, 5 × 106 splenic T cells, and 5 × 106 splenic B cells in 0.5 mL of phosphate-buffered saline to generate Scl-cGVHD. Control groups received donor cells from BALB/c (syngeneic bone marrow transplantation [BMT]) or B10.D2 mice (allogeneic BMT). All studies were approved by the institutional review board.
GVHD skin score
Clinical cGVHD score was previously described29 : healthy appearance = 0; skin lesions with alopecia ≤1 cm2 in area = 1; 1 to 2 cm2 = 2; 2 to 5 cm2 = 3; 5 to 10 cm2 = 4; 10 to 15 cm2 = 5; 15 to 20 cm2 = 6; >20 cm2 = 7. Additionally, animals were assigned 0.4 points for skin disease (lesions or scaling) on tail and 0.3 points each for lesions on ears and paws (minimum score = 0, maximum score = 8). Final scores for dead animals were kept in the data set for the remaining time points.
Histologic analysis
The skin and lung were fixed in 10% formalin and embedded in paraffin. Sections (6 μm thick) were stained with hematoxylin and eosin and Masson’s trichrome. Skin histopathology was scored by a dermatopathologist (blinded to experimental groups) on the basis of epidermal interface changes, dermal collagen thickness, mononuclear cell inflammation, subdermal fat loss, and follicular dropout, with scores from 0 to 2 for each category (total score, 0 to 10).30 Masson’s trichrome staining was used to detect the collagen fibers and collagen deposition. Collagen deposition was quantified on trichrome-stained sections as the ratio of blue-stained area to total stained area by using Adobe Photoshop CS4 analysis tools.
Flow cytometry
The following monoclonal antibodies (mAbs) were used: FITC-, PE-, PE-Cy5-, PE-Cy7-, PerCP-Cy5.5-, APC-, APC-PECy7-, or Pacific Blue-conjugated mAbs to mouse Thy1.2 (30-H12), B220 (30-F11), CD4 (RM4-5), CD8 (53-6.7), CD11b (M1-70), CD19 (1D3), CD1d (1B1), CD5 (30-H12), and CD21 (7G6) (BioLegend, San Diego, CA), and Ly9.1 (30C7) (BD Biosciences, San Jose, CA). LIVE/DEAD Fixable Aqua Dead cell (Invitrogen, Grand Island, NY) was used to detect dead cells.
Splenic and skin single-cell suspensions were stained for 20 minutes for multicolor immunofluorescence analysis by using mAbs at predetermined optimal concentrations on a FACSCanto II flow cytometer (BD Biosciences).29 Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Intracellular cytokine staining
B cells and monocytes/macrophages were stimulated for 5 hours with lipopolysaccharide (LPS; 10 μg/mL; Sigma-Aldrich, St. Louis, MO), phorbol myristate acetate (PMA; 50 ng/mL; Sigma-Aldrich), ionomycin (500 ng/mL; Sigma-Aldrich), and brefeldin A (3 μM; BioLegend) for detection of cytokine production. T cells were stimulated for 4 hours with PMA (50 ng/mL), ionomycin (1 μg/mL), and brefeldin A (3 μM). After cell-surface staining, the cells were washed, fixed, and permeabilized using the Cytofix/Cytoperm Kit (BD Biosciences), followed by staining with anti-IL-10 (JES5-16E3) or anti-IL-6 (MP5-20F3) mAbs (BioLegend), or anti–tumor necrosis factor alpha (TNF-α) (MP6-XT22), anti–interferon gamma (IFN-γ; XMG1.2), anti-IL-17A (TC11-18H10.1), or anti-granzyme B (GB11) mAbs (BioLegend) or anti-IL-13 (eBio13A) mAb (eBioscience, San Jose, CA). Skin single-cell suspensions without stimulation were stained with anti-CD11b mAb, followed by intracellular staining with anti–latency-associated peptide (LAP) mAb (TW7-16B4; BioLegend).
Cell sorting and adoptive transfer experiments
Wild-type B10.D2 mice were used as B-cell donors. Splenic B cells were first enriched by using CD19 mAb-coated microbeads (Miltenyi Biotec). In addition, CD1dhiCD5+ and CD1dlowCD5− (CD1dloCD5−) B cells were isolated by using a JSAN Desktop Cell Sorter (Bay Bioscience, Kobe, Japan) with purities of 85% to 90%. After purification, 1 × 106 cells were immediately transferred intravenously into recipients.
In vitro T-cell, B-cell, and monocyte/macrophage coculture assays
Splenic B cells were stimulated with LPS (10 μg/mL) for 1 hour. CD11b+ cells were isolated by CD11b mAb-coated microbeads (Miltenyi Biotec) from spleens of CD19−/− donor-transplanted mice 14 days after BMT. LPS-stimulated B cells (1 × 106) were cocultured with 1 × 106 CD11b+ cells for 24 hours, with LPS (10 μg/mL), PMA (50 ng/mL), ionomycin (500 ng/mL), and brefeldin A (3 μM) added during the final 5 hours of culture.
Splenic CD1dhiCD5+ or CD1dloCD5− B cells were stimulated with LPS (10 μg/mL) for 5 hours. T cells were purified by a magnetically activated cell sorter from spleens of CD19−/− donor-transplanted mice 14 days after BMT, and labeled with carboxyfluorescein succinimidyl ester (CFSE). TCD splenocytes from wild-type BALB/c mice were irradiated with 3,000 cGy. CFSE-labeled T cells (4 × 105 cells/mL) and irradiated TCD splenocytes (4 × 105 cells/mL) were cultured alone or with LPS-stimulated CD1dhiCD5+ or CD1dloCD5− B cells (4 × 105 cells/mL) in the presence of anti-CD3 (0.5 μg/mL; BioLegend) and anti-CD28 (0.5 μg/mL; BioLegend) mAbs for 72 hours. Proliferation was measured by CFSE dilution. For cytokine analysis, T cells were cocultured as above for 72 hours, with PMA (50 ng/mL), ionomycin (1000 ng/mL), and brefeldin A (3 μM) added during the final 4 hours of culture, followed by intracellular cytokine staining.
Cytometric bead array
CD11b+ cells and T cells were cocultured as described above and with recombinant IL-10 (0.5 ng/mL; BioLegend) or anti-mouse IL-10 receptor (IL-10R) mAb (30 μg/mL; 1B1.3e; BioLegend), followed by adding LPS, PMA, and ionomycin during the final 5 hours or PMA and ionomycin during the final 4 hours for CD11b+-cell coculture or T-cell coculture, respectively. Cytokine concentrations in supernatant were measured by cytometric bead array inflammatory kit (BD Biosciences).
Statistics
All data are shown as mean ± standard error of the mean. The significance of differences between sample means was determined with the Student t test.
Results
Increased percentage of CD1dhiCD5+ cells and B10 cells during Scl-cGVHD development
To examine changes in splenic immune cells, T cells, monocytes/macrophages, and B cells were analyzed in syngeneic and allogeneic BMT by flow cytometry every 7 days after BMT. The percentage of Thy1.2+ T cells decreased gradually after 7 days (Figure 1A), and the ratio of CD8+:CD4+ T cells was increased beginning 14 days after BMT in the allogeneic BMT group (Figure 1D). The splenic CD11b+ monocyte/macrophage population was markedly expanded in the early stage (days 7 and 14) and in the later stage (days 35 and 42) of GVHD (Figure 1B). A lower percentage and number of B cells were present in allogeneic BMT mice compared with syngeneic BMT mice (Figure 1C). In comparison with syngeneic BMT, the percentage of CD1dhiCD5+ B cells was significantly increased after allogeneic BMT (Figure 1E). Similarly, frequencies of splenic IL-10–producing B cells (B10 cells) were also significantly elevated on days 14, 21, and 28 (Figure 1F). Thus, CD1dhiCD5+ B cells and B10 cells were increased during Scl-cGVHD development.
Changes in splenic immune cells during Scl-cGVHD development. Female BALB/c recipients were irradiated (800 cGy) and transplanted with 10 × 106 TCD BM cells and 10 × 106 unfractionated splenocytes from either male B10.D2 (allogeneic) or male BALB/c (syngeneic) donors. Spleen samples were collected every 7 days after BMT. Single-cell suspensions were stained for surface markers and to differentiate live cells from dead cells. (A) Percentage and number of Thy1.2+ T cells among total live splenocytes, (B) percentages and numbers of CD11b+ monocytes/macrophages among total live splenocytes, (C) B220+ B cells among total live splenocytes, (D) ratio of CD8+:CD4+ T cells, and (E) percentage of CD1dhiCD5+ among (F) CD19+ B cells analyzed every 7 days after BMT by flow cytometry. Splenic single-cell suspensions were cultured with PMA, ionomycin, LPS, and brefeldin A for 5 hours. B10 cell (splenic IL-10–producing B cells) frequencies were determined by intracellular staining with IL-10; 3 to 5 mice per group. *P < .05; **P < .01; ***P < .001.
Changes in splenic immune cells during Scl-cGVHD development. Female BALB/c recipients were irradiated (800 cGy) and transplanted with 10 × 106 TCD BM cells and 10 × 106 unfractionated splenocytes from either male B10.D2 (allogeneic) or male BALB/c (syngeneic) donors. Spleen samples were collected every 7 days after BMT. Single-cell suspensions were stained for surface markers and to differentiate live cells from dead cells. (A) Percentage and number of Thy1.2+ T cells among total live splenocytes, (B) percentages and numbers of CD11b+ monocytes/macrophages among total live splenocytes, (C) B220+ B cells among total live splenocytes, (D) ratio of CD8+:CD4+ T cells, and (E) percentage of CD1dhiCD5+ among (F) CD19+ B cells analyzed every 7 days after BMT by flow cytometry. Splenic single-cell suspensions were cultured with PMA, ionomycin, LPS, and brefeldin A for 5 hours. B10 cell (splenic IL-10–producing B cells) frequencies were determined by intracellular staining with IL-10; 3 to 5 mice per group. *P < .05; **P < .01; ***P < .001.
Augmented GVHD severity and fibrosis scores in CD19−/− donor-transplanted recipients
As described previously,18,19 the numbers of IL-10–producing B cells were markedly decreased in CD19−/− mice on BALB/c and B10.D2 backgrounds compared with their wild-type counterparts (P < .01; Figure 2A). To investigate whether CD19 deficiency on B cells of recipients or donors has effects on Scl-cGVHD, CD19−/− BALB/c or CD19−/− B10.D2 mice were used as recipients or donors, respectively. When compared with the control group, there was no significant difference in skin score (Figure 2B), histopathologic skin score (Figure 2C), or fibrosis areas in the skin and lung (Figure 2D-E) in the CD19−/− recipient group. Thus, CD19 expression in recipients did not influence Scl-cGVHD. By contrast, CD19−/− donor cells significantly promoted Scl-cGVHD as evidenced by an increased average skin score compared with the control group (P < .01 and P < .05 on days 21 and 42, respectively; Figure 2F). Histopathologic skin scores and fibrosis areas in the skin and lungs in the CD19−/− donor-transplanted group were consistently significantly higher than in the control group (P < .05; Figure 2G-I). Therefore, CD19 deficiency in donors, but not in recipients, augmented Scl-cGVHD.
Augmented GVHD severity and fibrosis scores in CD19−/− donor-transplanted recipients. (A) Representative histograms and numbers of IL-10–producing B220+ B cells in wild-type (WT) BALB/c, CD19−/− BALB/c, WT B10.D2, and CD19−/− B10.D2 groups (three mice in each group). (B) Female wild-type BALB/c (▪, Control) or CD19−/− BALB/c (▿, CD19−/− Recipient) mice were transplanted with TCD BM and splenic T and B cells from wild-type CD19+/+ B10.D2 mice. (C) Skin scores were monitored every 3 days after BMT. Histopathologic scores and the ratio of trichrome area:total area in (D) the skin and in (E) the lung were analyzed 42 days after BMT. (F) Female wild-type BALB/c mice were transplanted with TCD BM and splenic T cells and B cells from male wild-type B10.D2 (▪, Control) or CD19−/− B10.D2 (△, CD19−/− Donor) mice. Skin scores were monitored every 3 days after BMT. (G) Histopathologic scores and the ratio of trichrome area:total area in (H) the skin and in (I) the lung were evaluated 42 days after BMT. Representative photographs and microphotographs were taken 42 days after BMT; 4 to 6 mice per group. *P < .05; **P < .01; ***P < .001.
Augmented GVHD severity and fibrosis scores in CD19−/− donor-transplanted recipients. (A) Representative histograms and numbers of IL-10–producing B220+ B cells in wild-type (WT) BALB/c, CD19−/− BALB/c, WT B10.D2, and CD19−/− B10.D2 groups (three mice in each group). (B) Female wild-type BALB/c (▪, Control) or CD19−/− BALB/c (▿, CD19−/− Recipient) mice were transplanted with TCD BM and splenic T and B cells from wild-type CD19+/+ B10.D2 mice. (C) Skin scores were monitored every 3 days after BMT. Histopathologic scores and the ratio of trichrome area:total area in (D) the skin and in (E) the lung were analyzed 42 days after BMT. (F) Female wild-type BALB/c mice were transplanted with TCD BM and splenic T cells and B cells from male wild-type B10.D2 (▪, Control) or CD19−/− B10.D2 (△, CD19−/− Donor) mice. Skin scores were monitored every 3 days after BMT. (G) Histopathologic scores and the ratio of trichrome area:total area in (H) the skin and in (I) the lung were evaluated 42 days after BMT. Representative photographs and microphotographs were taken 42 days after BMT; 4 to 6 mice per group. *P < .05; **P < .01; ***P < .001.
Splenic MZ, CD1dhiCD5+, and Bregs are decreased in CD19−/− donor-transplanted GVHD mice
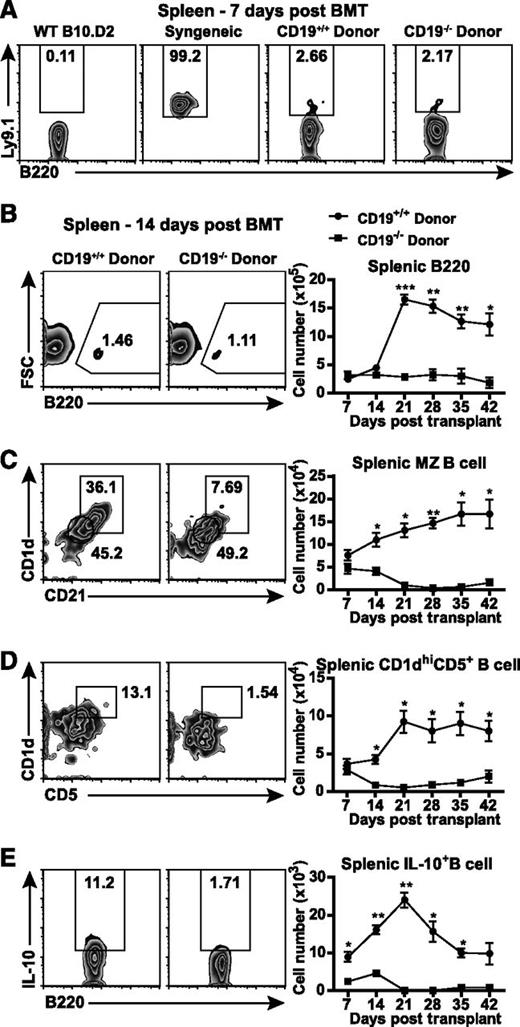
We further analyzed the numbers of splenic B cells, MZ B cells, CD1dhiCD5+ B cells, and B10 cells during Scl-cGVHD development. Ly9.1+ host B cells were found at low frequencies in both CD19+/+ and CD19−/− transplanted groups (2.23% ± 0.01% vs 2.10% ± 0.02%, not significant; Figure 3A), suggesting that the majority of B cells are composed of donor B cells. During the early stage, the number of B220+ B cells remained unchanged in CD19+/+ and CD19−/− donor-transplanted groups, whereas it was significantly increased in CD19+/+ donor-transplanted recipients during the later stage (P < .001 and P < .05 on days 21 and 42, respectively; Figure 3B).
Decreased splenic regulatory B cells in CD19−/− donor-transplanted GVHD mice. Female wild-type BALB/c recipients were irradiated (800 cGy) and transplanted with TCD BM and splenic T and B cells from male wild-type B10.D2 or CD19−/− B10.D2 mice. (A) Frequencies of host B cells were identified by Ly9.1. Numbers of (B) splenic B cells (B220+), (C) marginal zone (MZ) B cells (CD1d+CD21+B220+), and (D) CD1dhighCD5+ B cells (CD1dhiCD5+B220+) were analyzed every 7 days after BMT. Splenic single-cell suspensions from CD19+/+ and CD19−/− donor-transplanted GVHD groups were cultured with PMA, ionomycin, LPS, and brefeldin A for 5 hours. (E) The number of splenic IL-10–producing B220+ B cells (Breg cells) was determined by intracellular staining every 7 days after BMT; 4 to 5 mice per group. *P < .05; **P < .01; ***P < .001.
Decreased splenic regulatory B cells in CD19−/− donor-transplanted GVHD mice. Female wild-type BALB/c recipients were irradiated (800 cGy) and transplanted with TCD BM and splenic T and B cells from male wild-type B10.D2 or CD19−/− B10.D2 mice. (A) Frequencies of host B cells were identified by Ly9.1. Numbers of (B) splenic B cells (B220+), (C) marginal zone (MZ) B cells (CD1d+CD21+B220+), and (D) CD1dhighCD5+ B cells (CD1dhiCD5+B220+) were analyzed every 7 days after BMT. Splenic single-cell suspensions from CD19+/+ and CD19−/− donor-transplanted GVHD groups were cultured with PMA, ionomycin, LPS, and brefeldin A for 5 hours. (E) The number of splenic IL-10–producing B220+ B cells (Breg cells) was determined by intracellular staining every 7 days after BMT; 4 to 5 mice per group. *P < .05; **P < .01; ***P < .001.
Although there were no significant differences in the numbers of CD1d+CD21+ MZ and CD1dhiCD5+ B cells between the two groups on day 7, the development of MZ B cells and CD1dhiCD5+ B cells was significantly suppressed in CD19−/− donor-transplanted mice in both the early and late stages of Scl-cGVHD (P < .05; Figure 3C-D). The most remarkable difference was observed in B10 cells, since the number of B10 cells in CD19−/− donor-transplanted mice was significantly lower than in CD19+/+ donor-transplanted mice (Figure 3E). Splenic B10 frequencies were also significantly lower in CD19−/− donor-transplanted mice when compared with CD19+/+ donor-transplanted mice. Among B220+ cells, the percentages of IL-10–producing B cells were 0.75% ± 0.24% and 0.43% ± 0.08% in CD19−/− donor-transplanted mice, and 9.19% ± 0.96% and 4.28% ± 0.61% in CD19+/+ donor-transplanted group, on days 14 and 21, respectively (P < .05; data not shown). Collectively, disturbed reconstitution of Bregs in CD19−/− donor-transplanted mice may augment Scl-cGVHD.
Increased splenic T cells and cytotoxic CD8+ T cells, and reduced IL-6–producing monocytes and Th1 cells in CD19−/− donor-transplanted GVHD mice during the early stage
The course of Scl-cGVHD can be divided into early (cytotoxic: before day 20 of BMT) and later (sclerotic: after day 20) stages.31,32 When compared with the control group, increased frequency of Thy1.2+ T cells (Figure 4A), number of splenic Thy1.2+ T cells, and a higher ratio of CD8+:CD4+ T cells were observed in the early stage of CD19−/− donor-transplanted mice (P < .01; Figure 4B-C). The frequency of granzyme B–producing CD8+ T cells in total T cells was significantly higher in CD19−/− donor-transplanted mice when compared with that in CD19+/+ donor-transplanted mice (Figure 4D). Therefore, CD8+ T cells, especially granzyme B–producing cytotoxic CD8+ T cells, were increased in CD19−/− donor-transplanted mice.
Splenic cytotoxic CD8+ T cells, IL-6–producing CD11b+ cells, and TNF-α– and IFN-γ–producing CD4+ T cells are increased in CD19−/− donor-transplanted GVHD mice. (A) Percentages of CD11b+ and Thy1.2+ cells within a live gate in CD19+/+ and CD19−/− donor-transplanted groups. (B) The number of splenic T cells (Thy1.2+) and (C) the ratio of splenic CD8+:CD4+ T cells was analyzed 14 days after BMT. Splenic single-cell suspensions from CD19+/+ donor and CD19−/− donor-transplanted groups were cultured with PMA, ionomycin, LPS, and brefeldin A for 5 hours for detection of IL-6–producing CD11b+ cells, or with PMA, ionomycin, and brefeldin A for 4 hours for detecting granzyme B–producing CD8+ T cells and TNF-α– and IFN-γ–producing CD4+ T cells. (D) Percentages of cytotoxic CD8+ T cells (Granzyme B–producing CD8+ T cells) among T cells (Thy1.2+). (E) From within a splenic live gate, percentages of CD11b+ and IL-6+ cells are shown in CD19+/+ and CD19−/− donor-transplanted groups. (F) Percentage of IL-6–producing CD11b+ cells among total live cells. (G) Percentage of TNF-α–producing CD4+ T cells and (H) percentage of IFN-γ–producing CD4+ T cells among CD4+ T cells; 4 to 5 mice per group. *P < .05; **P < .01. LPS-stimulated B cells purified from wild-type or CD19−/− B10.D2 spleens were cocultured with purified CD11b+ cells from CD19−/− donor-transplanted mice 14 days after BMT with/without IL-10 or anti–IL-10R antibody. (I) Representative histograms and bar graphs showed percentage of IL-6– or TNF-α–producing CD11b+ cells from CD11b+ cells. (J) Bar graphs indicate concentrations of IL-6 and TNF-α in supernatant after a 24-hour coculture. Experiments were performed in triplicate. *P < .05; **P < .01.
Splenic cytotoxic CD8+ T cells, IL-6–producing CD11b+ cells, and TNF-α– and IFN-γ–producing CD4+ T cells are increased in CD19−/− donor-transplanted GVHD mice. (A) Percentages of CD11b+ and Thy1.2+ cells within a live gate in CD19+/+ and CD19−/− donor-transplanted groups. (B) The number of splenic T cells (Thy1.2+) and (C) the ratio of splenic CD8+:CD4+ T cells was analyzed 14 days after BMT. Splenic single-cell suspensions from CD19+/+ donor and CD19−/− donor-transplanted groups were cultured with PMA, ionomycin, LPS, and brefeldin A for 5 hours for detection of IL-6–producing CD11b+ cells, or with PMA, ionomycin, and brefeldin A for 4 hours for detecting granzyme B–producing CD8+ T cells and TNF-α– and IFN-γ–producing CD4+ T cells. (D) Percentages of cytotoxic CD8+ T cells (Granzyme B–producing CD8+ T cells) among T cells (Thy1.2+). (E) From within a splenic live gate, percentages of CD11b+ and IL-6+ cells are shown in CD19+/+ and CD19−/− donor-transplanted groups. (F) Percentage of IL-6–producing CD11b+ cells among total live cells. (G) Percentage of TNF-α–producing CD4+ T cells and (H) percentage of IFN-γ–producing CD4+ T cells among CD4+ T cells; 4 to 5 mice per group. *P < .05; **P < .01. LPS-stimulated B cells purified from wild-type or CD19−/− B10.D2 spleens were cocultured with purified CD11b+ cells from CD19−/− donor-transplanted mice 14 days after BMT with/without IL-10 or anti–IL-10R antibody. (I) Representative histograms and bar graphs showed percentage of IL-6– or TNF-α–producing CD11b+ cells from CD11b+ cells. (J) Bar graphs indicate concentrations of IL-6 and TNF-α in supernatant after a 24-hour coculture. Experiments were performed in triplicate. *P < .05; **P < .01.
IL-6 plays an important role in the pathogenesis of Scl-cGVHD.29 We noted elevated expansion of splenic monocytes/macrophages (CD11b+ cells) in allogeneic BMT mice (Figure 1B). On day 14 after BMT, CD11b+ cells produced IL-6 at a higher rate than CD11b− cells in both groups (Figure 4E). There was a significantly higher percentage of IL-6–producing CD11b+ cells among total splenocytes in CD19−/− donor-transplanted mice compared with CD19+/+ donor GVHD mice (P < .05; Figure 4F). Furthermore, CD19+/+ donor-transplanted mice showed significantly reduced percentages of TNF-α− and IFN-γ–producing CD4+ T cells 14 days after BMT when compared with CD19−/− donor-transplanted mice (P < .01; Figure 4G-H).
The influence of CD19+/+ B cells on cytokine production by CD11b+ cells was assessed in vitro. LPS-stimulated CD19+/+ B cells decreased IL-6 and TNF-α production in GVHD-derived CD11b+ monocytes/macrophages when compared with LPS-stimulated CD19−/− B cells (58.3% ± 0.36% vs 66.7% ± 1.85% and 60.7% ± 1.43% vs 76.3% ± 0.88%; P < .01; Figure 4I) or GVHD-derived monocytes/macrophages alone (Figure 4I). The inhibitory effect of LPS-stimulated CD19+/+ B cells on cytokine production by CD11b+ cells was reduced by adding anti–IL-10R mAb (Figure 4J). Furthermore, IL-10 suppressed IL-6 and TNF-α production by GVHD CD11b+ cells in vitro (Figure 4J). Therefore, CD19 expression by donor B cells correlates with reduced IL-6 production by monocytes/macrophages.
Increased cutaneous CD11b+LAP+ cells in the CD19−/− donor-transplanted group
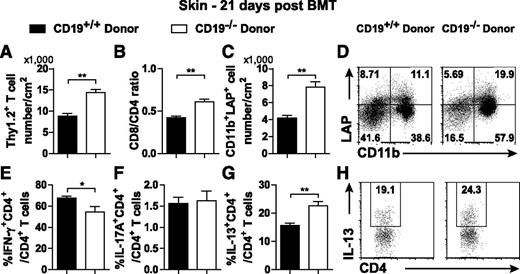
Th2 and profibrotic cytokines produced by infiltrating immune cells promote fibrosis in Scl-cGVHD.31,33-35 T-cell infiltration was increased 14 days (data not shown) and 21 days after CD19−/− donor transplantation (P < .01; Figure 5A). Although donor CD4+ T cells are required for the initiation of cGVHD,35 CD8+ T cells also contribute to progression of fibrosis in Scl-cGVHD.36 The ratio of CD8+:CD4+ T cells in the skin was higher in the CD19−/− donor-transplanted group than in the CD19+/+ donor-transplanted group 14 days (data not shown) and 21 days after BMT (P = .003; Figure 5B).
Increased CD11b+LAP+ cells and IL-13–producing CD4+ T cells and reduced IFN-γ–producing CD4+ T cells in the skin of CD19−/− donor-transplanted mice. A skin cell suspension was generated by digesting a 3 × 3-cm piece of depilated skin and was stained for surface markers and to differentiate live cells from dead cells. (A) The number of skin T cells (Thy1.2+) and (B) ratio of CD8+:CD4+ T cells were analyzed 21 days after BMT. A skin single-cell suspension was intracellularly stained with LAP (TGF-β1). (C) The number of CD11b+LAP+ cells, and (D) representative histograms from a live cell gate show the percentage of CD11b+LAP+ cells. Skin single-cell suspensions from CD19+/+ donor and CD19−/− donor-transplanted groups were cultured with PMA, ionomycin, and brefeldin A for 4 hours for detection of cytokine-producing T cells. Percentages of (E) IFN-γ–, (F) IL-17A–, and (G) IL-13–producing CD4+ T cells in total CD4+ T cells were analyzed. (H) Representative histograms from the CD4+ gate show the percentage of IL-13–producing CD4+ T cells; 4 to 5 mice per group. *P < .05; **P < .01.
Increased CD11b+LAP+ cells and IL-13–producing CD4+ T cells and reduced IFN-γ–producing CD4+ T cells in the skin of CD19−/− donor-transplanted mice. A skin cell suspension was generated by digesting a 3 × 3-cm piece of depilated skin and was stained for surface markers and to differentiate live cells from dead cells. (A) The number of skin T cells (Thy1.2+) and (B) ratio of CD8+:CD4+ T cells were analyzed 21 days after BMT. A skin single-cell suspension was intracellularly stained with LAP (TGF-β1). (C) The number of CD11b+LAP+ cells, and (D) representative histograms from a live cell gate show the percentage of CD11b+LAP+ cells. Skin single-cell suspensions from CD19+/+ donor and CD19−/− donor-transplanted groups were cultured with PMA, ionomycin, and brefeldin A for 4 hours for detection of cytokine-producing T cells. Percentages of (E) IFN-γ–, (F) IL-17A–, and (G) IL-13–producing CD4+ T cells in total CD4+ T cells were analyzed. (H) Representative histograms from the CD4+ gate show the percentage of IL-13–producing CD4+ T cells; 4 to 5 mice per group. *P < .05; **P < .01.
Activated monocyte/macrophage-secreted transforming growth factor beta (TGF-β) is a key mediator of fibrosis.37-39 When compared with the CD19+/+ donor-transplanted group, the percentage and number of skin CD11b+LAP+ cells were significantly increased in the CD19−/− donor-transplanted group (Figure 5C-D). Therefore, donor CD19 expression may contribute to suppression of TGF-β1–producing monocytes/macrophages in the skin.
Increased cutaneous Th2 cells in the CD19−/− donor-transplanted group
In Scl-cGVHD cutaneous tissue, there is a mixed Th1/Th2-like cytokine profile with a Th1-like predominance during the early stage and Th2-like profile at the later stage.38 When compared with syngeneic BMT mice, both IFN-γ–producing CD4 + T cells and IL-13–producing CD4+ T cells were clearly increased in the skin of allogeneic BMT mice (data not shown). Nonetheless, the CD19−/− donor GVHD group showed a modest, but significant, decrease in the percentage of skin IFN-γ–producing CD4+ T cells 21 days after BMT (P = .045; Figure 5E). There was no significant change in the percentage of IL-17A–producing CD4+ T cells in either group (Figure 5F). In contrast, the percentage of IL-13–producing CD4+ T cells was significantly increased in the skin of the CD19−/− donor GVHD group 21 days after BMT (P = .0024; Figure 5G-H). Therefore, CD19 deficiency in donor B cells promoted Th2-cell infiltration and reduced Th1-cell infiltration in the skin in the later stage of Scl-cGVHD.
Early adoptive transfer of CD1dhiCD5+ B cells reduces the severity of CD19−/− donor-induced Scl-cGVHD
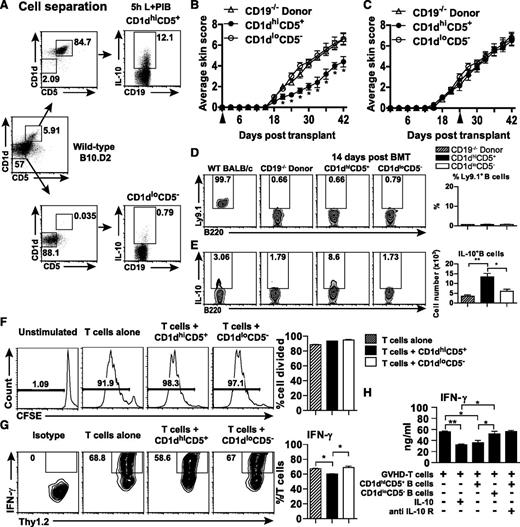
The CD1dhiCD5+ B cell population was able to produce higher levels of IL-10 than CD1dloCD5− B cells (Figure 6A). To evaluate the role of IL-10–producing B cells in the course of Scl-cGVHD, splenic CD1dhiCD5+ B cells and CD1dloCD5− B cells from wild-type B10.D2 mice were transferred into CD19−/− donor-transplanted mice on BMT day or on day 21. Early adoptive transfer of CD1dhiCD5+ B cells (day of BMT) reduced the skin score of CD19−/− donor-induced Scl-cGVHD in comparison with mice given CD1dloCD5− B cells and compared with CD19−/− donor-transplanted mice (Figure 6B). Adoptive transfer of CD21hiCD23lo MZ B cells from wild-type mice was also capable of reducing the severity of Scl-cGVHD, albeit less effectively (data not shown). In contrast, there were no significant changes in skin score when CD1dhiCD5+ B cells were transferred later (day 21; Figure 6C). Host B cells were mostly absent 14 days after BMT. However, the IL-10–producing B cells were significantly improved in CD1dhiCD5+ group when compared with CD19−/− donor and CD1dloCD5− groups (P < .05; Figure 6E). These results demonstrate that early transfer of CD1dhiCD5+ B cells reduces Scl-cGVHD severity due to Bregs reconstitution.
Early adoptive transfer of CD1dhiCD5+ B cells reduced CD19−/− donor-induced Scl-cGVHD severity. (A) Representative B10-cell purification results from an adoptive transfer experiment. Purified splenic B cells from wild-type male B10.D2 were separated into CD1dhiCD5+CD19+ and CD1dloCD5−CD19+ B-cell populations. The isolated cells were cultured with PMA, ionomycin, LPS, and brefeldin A for 5 hours. IL-10–producing B-cell frequencies in the stimulated cell cultures were determined by intracellular staining with IL-10. (B) CD19−/− donor-transplanted female recipients were also given phosphate-buffered saline, CD1dhiCD5+ B cells, or CD1dloCD5− B cells on the day of BMT or (C) on day 21 after BMT+- Skin scores were monitored every 3 days after BMT. (D) Percentages of host B cells (Ly.9.1+B220+) and (E) percentages and numbers of IL-10–producing B cells in each group are presented in representative histograms and bar graphs. (F-H) B10 cells altered the T-cell IFN-γ production profile but did not affect T-cell proliferation. (F) Representative histograms showing the proliferation of T cells when T cells and irradiated TCD splenocytes were cocultured without the presence of anti-CD3 and anti-CD28 (Unstimulated), or with the presence of anti-CD3 and anti-CD28 (T cells alone), or with either CD1dhiCD5+ (T cells + CD1dhiCD5+) or CD1dloCD5− (T cells + CD1dloCD5−) B cells in the presence of soluble anti-CD3 and anti-CD28. Proliferation was measured by CFSE dilution. LPS-stimulated CD1dhiCD5+ or CD1dloCD5− B cells isolated from wild-type B10.D2 spleens were cocultured with purified T cells from CD19−/− donor-transplanted mice 14 days after BMT in presence of irradiated BALB/c TCD splenocytes and soluble anti-CD3 and anti-CD28 for 72 hours, and with/without IL-10 or anti-IL-10R antibody. (G) Representative histograms and bar graphs showed percentage of IFN-γ–producing T cells. (H) Bar graph indicates concentrations of IFN-γ in supernatant after a 72-hour coculture. The experiments were performed in triplicate. *P < .05; **P < .01.
Early adoptive transfer of CD1dhiCD5+ B cells reduced CD19−/− donor-induced Scl-cGVHD severity. (A) Representative B10-cell purification results from an adoptive transfer experiment. Purified splenic B cells from wild-type male B10.D2 were separated into CD1dhiCD5+CD19+ and CD1dloCD5−CD19+ B-cell populations. The isolated cells were cultured with PMA, ionomycin, LPS, and brefeldin A for 5 hours. IL-10–producing B-cell frequencies in the stimulated cell cultures were determined by intracellular staining with IL-10. (B) CD19−/− donor-transplanted female recipients were also given phosphate-buffered saline, CD1dhiCD5+ B cells, or CD1dloCD5− B cells on the day of BMT or (C) on day 21 after BMT+- Skin scores were monitored every 3 days after BMT. (D) Percentages of host B cells (Ly.9.1+B220+) and (E) percentages and numbers of IL-10–producing B cells in each group are presented in representative histograms and bar graphs. (F-H) B10 cells altered the T-cell IFN-γ production profile but did not affect T-cell proliferation. (F) Representative histograms showing the proliferation of T cells when T cells and irradiated TCD splenocytes were cocultured without the presence of anti-CD3 and anti-CD28 (Unstimulated), or with the presence of anti-CD3 and anti-CD28 (T cells alone), or with either CD1dhiCD5+ (T cells + CD1dhiCD5+) or CD1dloCD5− (T cells + CD1dloCD5−) B cells in the presence of soluble anti-CD3 and anti-CD28. Proliferation was measured by CFSE dilution. LPS-stimulated CD1dhiCD5+ or CD1dloCD5− B cells isolated from wild-type B10.D2 spleens were cocultured with purified T cells from CD19−/− donor-transplanted mice 14 days after BMT in presence of irradiated BALB/c TCD splenocytes and soluble anti-CD3 and anti-CD28 for 72 hours, and with/without IL-10 or anti-IL-10R antibody. (G) Representative histograms and bar graphs showed percentage of IFN-γ–producing T cells. (H) Bar graph indicates concentrations of IFN-γ in supernatant after a 72-hour coculture. The experiments were performed in triplicate. *P < .05; **P < .01.
CD1dhiCD5+ B cells do not inhibit GVHD T-cell proliferation but do regulate T-cell IFN-γ production
To investigate the mechanisms underlying suppression of GVHD by B10 cells in vivo, T-cell/B-cell cocultures in the presence of antigen-presenting cells were performed in vitro. Incubation of GVHD T cells with B cells (CD1dhiCD5+ or CD1dloCD5−) or without B cells did not alter T-cell proliferation (Figure 6F). By contrast, GVHD T cells cultured with CD1dhiCD5+ B cells showed significantly reduced IFN-γ production compared with GVHD T cells cocultured with CD1dloCD5− B cells (Figure 6G-H). The inhibitory effect of CD1dhiCD5+ B cells on T-cell IFN-γ production was reduced by adding anti-IL-10R mAb (Figure 6H). Furthermore, IL-10 suppressed IFN-γ production by GVHD T cells in vitro (Figure 6H).
Discussion
We herein demonstrate that Breg reconstitution is critical for the suppression of Scl-cGVHD. Although the percentage of splenic B cells was low during Scl-cGVHD development (Figure 1C), Breg percentages were markedly increased in wild-type donor-transplanted mice (Figure 1E-F). In contrast, the reconstitution of B10 cells was disturbed in CD19−/− donor-transplanted mice (Figure 3). Additionally, Scl-cGVHD severity and fibrosis were accelerated in CD19−/− donor-transplanted mice (Figure 2), which was lessened by adoptive transfer of CD1dhiCD5+ B cells from wild-type mice (Figure 6). Although a previous study indicated that host B cells suppress GVHD,8 CD19 expression in recipients did not alter the course or severity of Scl-cGVHD (Figure 2). This study demonstrates, for the first time, that Bregs play a critical suppressive role in immune-mediated fibrotic diseases. In contrast to our study, several other studies have shown that donor B cells have disease-promoting roles in Scl-cGVHD.5 The apparent discrepancy between the other studies and ours can be explained by the use of CD19−/− mice in our study, because CD19−/− mice are likely to have pathogenic B cells,18,19 but they lack MZ B cells, which are major sources of B-cell–derived IL-10. Collectively, donor B cells have pathogenic and regulatory roles in the development of cGVHD.
This study indicates that an important interaction exists between B cells and CD11b+ cells in Scl-cGVHD. In fact, the expansion of the monocyte/macrophage population was observed in the spleen during the early stage of disease (Figure 1B). Early blockade of IL-6 signaling by anti–IL-6 receptor mAb reduced Scl-cGVHD severity and fibrosis through an increase in regulatory T-cell numbers.29 Our study demonstrates that IL-6 is mainly produced by splenic monocytes/macrophages (Figure 4E). Further, IL-6–producing CD11b+ cells were increased in the CD19−/− donor-transplanted group (Figure 4F). By contrast, CD19+/+ B cells were capable of reducing IL-6 production in CD11b+ cells in vitro (Figure 4I-J). Furthermore, CD19+/+ donor B cells were found to reduce the initial inflammatory reaction of Scl-cGVHD through B10-cell–dependent suppression of CD11b+ cells. Although B10 cells have been considered to control T-cell responses, B10 cells are also likely to modulate functions of monocytes/macrophages, especially cytokine production.
Monocyte activation by host-reactive T cells is considered to be an initiating event of fibrotic changes in Scl-cGVHD.32 Increased cutaneous T-cell infiltration was also noted in CD19−/− donor-transplanted mice during the early stage (Figure 5A) and may be a result of impaired suppressive functions in donor CD19−/− B cells. Therefore, B cells may also regulate monocyte activation indirectly via T-cell activation. Skin-infiltrating monocytes produce TGF-β1, resulting in collagen upregulation and leading to skin fibrosis.31 TGF-β is secreted by many immune cell types, including monocytes/macrophages, in a latent form in which it is complexed with latent TGF-β binding protein, LAP.40 Increased CD11b+LAP+ cell numbers were observed in the skin of CD19−/− donor-transplanted mice on day 21 after BMT (Figure 5C-D) and may additionally contribute to progression of skin fibrosis. Furthermore, TGF-β1 can induce the upregulation of MCP-1 and RANTES. MCP-1, in turn, can increase TGF-β secretion from macrophages.41,42
In addition to TGF-β, other cytokines also play pivotal roles in fibrosis. For example, IL-13, IL-10, and IL-4 released by activated T cells stimulate fibroblasts to produce collagen. In this study, IL-13–producing CD4+ T cells were increased in the skin of CD19−/− donor-transplanted mice at the later stage (Figure 5G). In contrast, the cutaneous infiltration of IFN-γ–producing CD4+ T cells was decreased in the CD19−/− donor-transplanted group (Figure 5E). This contrasts with the increased percentage of IFN-γ–producing CD4+ T cells on day 14 in the spleens of CD19−/− donor-transplanted mice. It has been reported that cytokine profiles shift over the course of fibrosis in Scl-cGVHD, changing from Th1 predominance in the early phase to a Th2 profile in the later phase.38 Thus, our finding is consistent with this profile of cytokine expression, and excessive Th2 cytokine production in the skin of CD19−/− donor-transplanted mice may promote fibrosis in the later stage.
Recently, B10 cells have been identified within a unique CD1dhiCD5+ B cell subset.27 Although early adoptive transfer of these CD1dhiCD5+ B cells from wild-type B10.D2 mice into CD19−/− donor-transplanted mice significantly improved the average skin score, later adoptive transfer did not alter Scl-cGVHD severity (Figure 6B-C). From these results, it can be hypothesized that donor Bregs suppress the immune reaction more dominantly during the early stage than in the later stage. However, CD1dhiCD5+ B cells from wild-type B10.D2 mice did not directly suppress GVHD-derived T-cell proliferation (Figure 6F). Instead, when these regulatory cells were cocultured with T cells, there was a significant reduction in IFN-γ (Figure 6G-H).
Although the precise mechanisms of B10 cell activation in cGVHD remain unclear, optimal expansion of B10 cells has been considered to require the initial stimulation of toll-like receptors followed by B-cell reactivity and CD40 engagements.43 Because apoptotic cells are reported to enhance Breg functions,44 they may serve as endogenous toll-like receptors ligands. Furthermore, a recent study has shown that B10 cell maturation into functional IL-10–producing cells requires IL-21 and CD40-dependent cognate interactions with T cells and that IL-21 receptor and CD40 signals can efficiently drive B10 cell development and expansion in vitro,45 suggesting the requirement of the interaction with conventional antigen-specific T cells. Conversely, recent studies have demonstrated that invariant natural killer T cells can provide cognate antigen-specific help to CD1d+ B cells via CD1d and IL-21.46,47 Additionally, increased levels of B-cell activating factor have also been reported in cGVHD.48 B-cell activating factor treatment in vivo increases the number of B10 cells.49 Finally, IL-10 secretion by B10 cells is likely to facilitate B10-cell expansion in an autocrine fashion. These mechanisms may collectively contribute to B10-cell expansion in the spleen in cGVHD.
In conclusion, we have demonstrated that the reconstitution of B10 cells from donor B cells exerts suppressive functions in the development of Scl-cGVHD through the reconstitution of B10 cells. Development of B10-cell–based therapy may be promising for treatment of Scl-cGVHD and other fibrotic diseases including SSc in humans.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Matsubara and Y. Yamada for technical assistance.
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and from the Ministry of Health, Labour and Welfare of Japan.
Authorship
Contribution: D.L.H. designed and performed research, collected and analyzed data, and wrote the paper. T.M. designed research and collected and analyzed data and wrote the paper. G.J. performed research. Y.H., M.H., K.T., and T.F.T. designed research and wrote the paper. M.F. designed research and analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takashi Matsushita, Department of Dermatology, Faculty of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kanazawa 920-8641, Japan; e-mail: t-matsushita@derma.m.kanazawa-u.ac.jp; and Manabu Fujimoto, Department of Dermatology, Faculty of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kanazawa 920-8641, Japan; e-mail: fujimoto-m@umin.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal