Key Points
Anti–third-party Tcm kill malignant B cells in a T-cell receptor–independent mechanism while sparing naive B cells.
Abstract
Generation of T cells endowed with graft-versus-leukemia (GVL) and depleted of graft-versus-host (GVH) activity represents a highly desirable goal in bone marrow transplantation (BMT). Here, we demonstrate that donor anti–third-party CD8 T cells with central memory phenotype (Tcm) exhibit marked GVL reactivity through a unique T-cell receptor–independent mechanism. Thus, in a residual disease mouse model, Tcm therapy following autologous BMT led to significant survival prolongation, with 30% to 40% of the treated mice displaying long-term tumor-free survival. A more impressive finding was that infusion of donor Tcm in an allogeneic model rapidly eliminated residual lymphoma cells and led to long-term survival of 100% in the absence of GVH disease. Collectively, the strong GVL reactivity of anti–third-party Tcm, coupled with their demonstrated enhancement of bone marrow allografting, suggests that the use of Tcm therapy in conjunction with allogeneic T-cell–depleted BMT could be of particular benefit in patients with B-cell malignancies who cannot tolerate intensive myeloablative conditioning.
Introduction
The vital role of donor T cells in promoting engraftment and mediating graft-versus-leukemia (GVL) reactivity of allogeneic bone marrow (BM) transplants was established more than 2 decades ago upon the introduction of T-cell depletion for the prevention of graft-versus-host disease (GVHD).1,2 We have recently shown that host T-cell–mediated rejection of T-cell–depleted BM transplants (TDBMT) can be overcome in a mouse model by adding to the transplant inoculum activated anti–third-party donor CD8+ T cells with central memory phenotype (Tcm); these cells can home to the recipient’s lymph nodes and specifically delete host anti–donor T-cell clones.3,4 Importantly, these Tcm were shown to be depleted of graft-versus-host reactivity by virtue of their initial stimulation against third-party cells under cytokine deprivation.
In the present study, we addressed a second attribute of anti–third-party Tcm, namely their potential GVL reactivity, which could be very valuable for patients undergoing bone marrow transplantation (BMT) following reduced intensity conditioning (RIC).
The possibility that Tcm might exhibit GVL has been indicated initially by our previous unexpected observation in the human setting that both allogeneic and autologous anti–third-party CD8+ cytotoxic T lymphocytes (CTLs) exhibit in vitro significant killing of B-cell chronic lymphocytic leukemia (B-CLL)5 and B-cell non-Hodgkin lymphoma (B-NHL) cells6 while sparing acute myeloid leukemia blasts.5 The killing of B-cell tumors by anti–third-party CTLs was shown to involve a unique T-cell receptor (TCR)-independent 2-step mechanism. First, long-lasting conjugates are formed between the CTL and the tumor cell. These conjugates are rapidly formed through binding of intercellular adhesion molecule 1 (ICAM-1) on tumor cells by leukocyte function-associated antigen 1 (LFA-1) expressed on effector T cells. Second, a slower process of major histocompatibility complex I (MHC-I)–dependent apoptosis is mediated by binding of the MHC-I α2/3 constant region on the tumor cells to the CD8 molecule on the CTL membrane.
However, considering the nonconventional characteristics of this mechanism, it could be argued that this type of killing represents an artificial phenomenon with very little relevance if any to clinical settings. Thus, it was critical to evaluate in an appropriate mouse model whether murine anti–third-party Tcm can mediate significant GVL reactivity in vivo, in addition to their potent tolerizing activity.
Initially, we verified in vitro that mouse anti–third-party Tcm are endowed with antilymphoma reactivity through a TCR-independent mechanism, as was previously shown for their human counterparts. Subsequently, we tested their antilymphoma reactivity in a model simulating minimal residual disease following BMT using bioluminescence imaging (BLI). Strikingly, we discovered that both syngeneic and allogeneic Tcm were able to efficiently eliminate lymphoma cells. This effect was achieved without any GVHD and while sparing naive B cells.
Thus, together with their ability to markedly enhance BM allografting, anti–third-party Tcm can uniquely address both the challenge of engraftment following RIC and the problem of relapse commonly associated with RIC protocols. This novel cell therapy could be highly attractive, particularly for elderly patients with B-CLL and other B-cell malignancies who might not tolerate aggressive conditioning.
Methods
Animals
For detailed information on mouse strains used, see “supplemental Methods.” Institutional review board approvals were as follows: Institutional Animal Care and Use Committee (IACUC) application number 00520111-4 “TCM GVL in-vitro”; IACUC application number 00510111-3 “In-vivo GVL effect of anti third-party TCMs”; IACUC application number 02850711-1 “Humoral response after treatment with anti third party Tcm.”
Flow cytometric analysis
For detailed information on antibodies (Abs) used and fluorescence-activated cell sorting (FACS) analysis, see “supplemental Methods.”
Lymphoma cell lines
A20 lymphoma cells and A20 cells transduced with a luciferase reporter gene (A20 luc)7 were cultured in RPMI supplemented with 10% fetal calf serum (FCS) and antibiotics.
BCL1-luc cells7 were thawed and washed twice with RPMI supplemented with 10% FCS before injection.
Preparation of host nonreactive anti–third-party cells
Anti–third-party Tcm were grown as previously described.3 Briefly, splenocytes from the donor mouse strain (4 × 106 cells/mL) were cultured at a 1:1 ratio against irradiated (20 Gy) third-party splenocytes (donor, third-party, and host mice were MHC-I disparate) for 60 hours under exogenous cytokine deprivation at 37°C in a 5% CO2 incubator. Subsequently, the cells were fractionated on Ficoll-Paque Plus (Amersham Pharmacia Biotech, AB) and CD8+ cells were positively selected using Magnetic Particles (BD Pharmingen) and cultured (1 × 106 cells/mL) with rhIL-15 (20 ng/mL; R&D systems) in an Ag-free environment (in the absence of stimulators) at 37°C in a 5% CO2 incubator. To attain a purified population at the end of the culture (day 16), the Tcm were positively selected for L-selectin (CD62L, MACS Cell Separation; Miltenyi Biotec, Bergisch Gladbach, Germany). Cell phenotype was then analyzed by flow cytometry (FACScan; Becton Dickinson) for cell size and expression of the activation marker (CD44) and L-selectin (CD62L) within the CD8 compartment.
Mixed lymphocyte reaction killing assay
Lymphoma cells were obtained by Ficoll density gradient centrifugation, after which they were labeled according to the manufacturer’s instructions with 0.15 µg/mL CalceinAM (Invitrogen, Carlsbad, CA), a vital dye that is released upon cell death. Cells were brought to a concentration of 1 × 106 cells/mL in RPMI supplemented with 10% FCS and antibiotics. Next, 2.5 × 105 Calcein-labeled lymphoma cells were incubated with or without anti–third-party Tcm at the indicated ratio for 16 hours in 24-well plates. Cells were recovered and analyzed for survival by measuring the number of surviving Calcein-stained lymphoma cells by FACS. To obtain absolute values of cells, samples were resuspended in a constant volume and flow cytometric counts for each sample were obtained during a predetermined period of time and compared with flow cytometric counts obtained with a fixed volume and fixed numbers of input cells.8 Survival rates are presented relative to the survival of lymphoma cells alone. The percentage of lymphoma cell killing was calculated by the formula:
Inhibition of B-cell lymphoma killing by blocking Abs
Anti–third-party Tcm or lymphoma cells were preincubated for 30 minutes in a minimal volume with the indicated neutralizing Ab at the indicated concentrations. Blocked cells were then incubated with the other nonblocked component of the mixed lymphocyte reaction for 16 hours at a 1:5 ratio in favor of the anti–third-party cells. Lymphoma cell survival was analyzed by FACS. The following neutralizing Abs were used: LFA-1 (CD11a) blocking Ab (clone M17/4; Biolegend), ICAM-1 (CD54) blocking Ab (clone YN1/1.7.4; Biolegend), anti–CD8a blocking Ab (clone 53-6.7; Biolegend).
GVL mouse models
The A20-luc model.
Host mice (BALB/c; 12-13 weeks of age) were exposed to a single dose of lethal total body irradiation (TBI; 8 Gy) on day −1. The following day (day 0), the mice were intravenously administered a transplant of 3 × 106 syngeneic (BALB/c) or allogeneic (B6) nude BM cells with or without 5 × 103 A20-luc cells. On day +1, selected mice received anti–third-party Tcm cells in the indicated amounts. Tumor localization, migratory patterns of A20 cells, and tumor load were monitored using an in vivo imaging system.
The BCL1-luc model.
Host mice (BALB/c; 12 weeks of age) were exposed to a single dose of lethal TBI (8Gy) on day −1. The following day (day 0), the mice were intravenously administered a transplant of 3 × 106 syngeneic (BALB/c) nude BM cells with or without 1 × 103 BCL1-luc cells. On day +1, selected mice received anti–third-party Tcm cells in the indicated amounts. Tumor localization, migratory patterns of BCL1 cells, and tumor load were monitored using an in vivo imaging system.
In vivo imaging
The in vivo GVL model was established as described in the previous section. At the indicated times posttransplant, the mice were anesthetized with ketamine (100 mg/kg; Kepro, Holland, Netherlands) and xylazine (20 mg/kg; Kepro) injected intraperitoneally. Mice were then injected intraperitoneally with an aqueous solution of D-luciferin (150 mg/kg Cat#XR-1001, Xenogen; 30 mg/mL in phosphate-buffered saline) 10 minutes prior to imaging. The mice were then monitored using the optical whole-body imaging system (IVIS 100, Xenogen) coupled with a Pixelfly QE (PCO, Kelheim, Germany) charge-coupled device camera. Image processing and data analysis were performed using Living Image 3.2 software.
GVHD evaluation
Mice receiving allogeneic transplants were evaluated for symptoms of GVHD; the mice were monitored for survival and for external signs of GVHD including ruffled fur, hunched back, and weight loss.
Purification of naive CD8 T and B cells and lipopolysaccharide activation
For detailed information, see “supplemental Methods.”
Measurement of antigen-specific immunoglobulins by ELISA
Anti-TNP antibodies in the serum of immunized mice were measured by enzyme-linked immunosorbent assay (ELISA). ELISA plates (Nunc; Maxisorp, Rochester, NY) were coated with 10 μg/mL TNP bovine serum albumin prepared as previously described.9 Doubling dilutions of serum were made and incubated for 30 min at 37°C. Following washing in phosphate-buffered saline/0.05% Tween-20, plates were incubated with horseradish-peroxidase–conjugated polyclonal goat anti-mouse Fab (Jackson ImmunoResearch Laboratories, West Grove, PA). Antibody binding was detected by addition of TMB substrate (Sigma, St. Louis, MO) to the washed plates and the optical density at 630 nm filter was measured 10 minutes after substrate addition using an ELISA reader (Bio-Tek, Winooski, VT).
Statistical analysis
The analysis of survival data was performed using Kaplan-Meier curves (log-rank test). Comparison of means was conducted using the Student t test.
Results
Anti–third-party CD8 Tcm induce apoptosis of lymphoma cells ex vivo through a TCR-independent mechanism mediated via LFA-1/ICAM-1 interactions
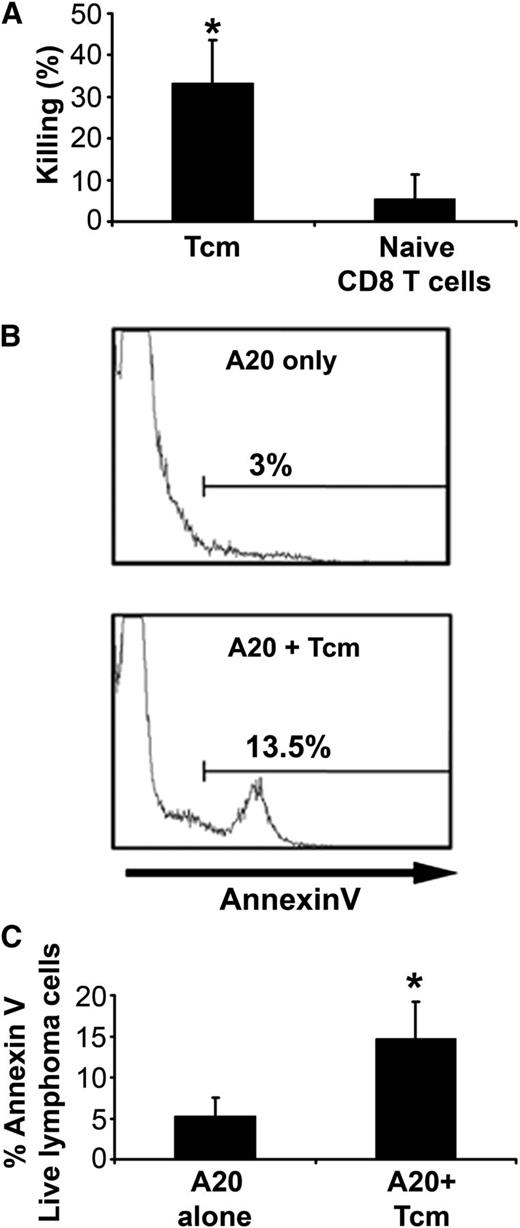
To verify whether mouse A20 lymphoma cells can serve as a target for TCR-independent killing by anti–third-party Tcm, we initially tested their ex vivo cytotoxic capacity in short-term cultures. As can be seen in Figure 1A, (BALB/cxB6)F1-derived anti–third-party Tcm, which are not alloreactive against BALB/c parental cells, effectively killed A20 target cells derived from BALB/c origin (33.2% ± 10.4%), in contrast to nonactivated naive CD8 T cells, which barely killed the A20 targets (5.4% ± 5.9%). These results are similar to our previous results using human cells in which autologous anti–third-party CTLs were found to be effective in eradicating pathological cells.5,6 In accordance with their human counterparts, the Tcm-induced death of the mouse lymphoma cells was through apoptosis. Thus, the proportion of A20 lymphoma cells binding Annexin V was significantly increased upon interaction with F1 anti–third-party Tcm, from 5.3% ± 2.2% to 14.7% ± 4.5%, respectively (Figure 1B-C).
Apoptosis induction in mouse lymphoma cells by anti–third-party Tcm through a nonalloreactive mechanism. CalceinAM-prelabeled murine A20 lymphoma cells (BALB/c origin) were incubated for 16 hours with or without a 5-fold excess of F1 (CB6-H2bd) –derived anti–third-party Tcm or naive CD8 T cells. (A) Numbers of viable Calcein+ lymphoma cells were determined by flow cytometry. Percent killing was calculated as described in “Methods.” Results shown represent average ± SD of 3 independent experiments. *P value < .05 compared with percent killing displayed by naive CD8 T cells. (B-C) Apoptosis was evaluated by using flow cytometry for Calcein and Annexin V+ fluorescence. (B) Representative results demonstrating live A20 lymphoma cells (gating on Calcein+ cells) undergoing apoptosis (Annexin V+) in the presence or absence of anti–third-party Tcm. (C) Quantification of results, shown in panel B, demonstrating apoptosis induction in A20 cells by anti–third-party Tcm. Results represent mean ± SD of 3 independent experiments, each in triplicate. *P value < .05 compared with percent Annexin V+ on A20 cells alone.
Apoptosis induction in mouse lymphoma cells by anti–third-party Tcm through a nonalloreactive mechanism. CalceinAM-prelabeled murine A20 lymphoma cells (BALB/c origin) were incubated for 16 hours with or without a 5-fold excess of F1 (CB6-H2bd) –derived anti–third-party Tcm or naive CD8 T cells. (A) Numbers of viable Calcein+ lymphoma cells were determined by flow cytometry. Percent killing was calculated as described in “Methods.” Results shown represent average ± SD of 3 independent experiments. *P value < .05 compared with percent killing displayed by naive CD8 T cells. (B-C) Apoptosis was evaluated by using flow cytometry for Calcein and Annexin V+ fluorescence. (B) Representative results demonstrating live A20 lymphoma cells (gating on Calcein+ cells) undergoing apoptosis (Annexin V+) in the presence or absence of anti–third-party Tcm. (C) Quantification of results, shown in panel B, demonstrating apoptosis induction in A20 cells by anti–third-party Tcm. Results represent mean ± SD of 3 independent experiments, each in triplicate. *P value < .05 compared with percent Annexin V+ on A20 cells alone.
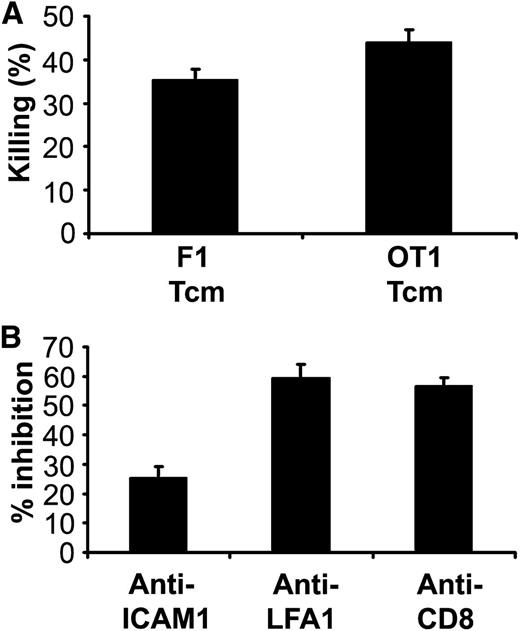
Next, we examined whether murine anti–third-party Tcm, similarly to human anti–third-party CTLs, eliminate lymphoma cells via a TCR-independent mechanism. To that end, we used an OT-I Rag−/− transgenic mouse strain whose T cells solely express a TCR transgene specific for the SIINFEKL peptide of ovalbumin presented on H2Kb; this target antigen is not expressed by A20 lymphoma cells. As shown in Figure 2A, Tcm derived from OT-I Rag−/− mice induced killing of lymphoma cells (43.9% ± 3%) in comparable levels to that exhibited by nonalloreactive (BALB/cxB6)F1-derived anti–third-party Tcm (35.3% ± 2.4%), confirming that this killing is indeed TCR independent.
Killing of murine lymphoma cells by anti–third-party Tcm is TCR independent and mediated by ICAM-1/LFA-1 interactions and engagement of CD8 molecules. (A) CalceinAM-prelabeled murine A20 lymphoma cells were incubated with or without OT1/Rag−/− (transgenic TCR)- or F1 (CB6)-derived anti–third-party Tcm for 16 hours at a 5:1 effector/lymphoma cell ratio. Numbers of viable cells were determined by flow cytometry. Percent killing was calculated as described in “Methods.” Results shown represent average ± SD of triplicates in 1 representative experiment out of 2 performed. (B) CalceinAM-labeled murine A20 lymphoma cells were preincubated for 1 hour with or without 50 μg/mL anti–ICAM-1 blocking Ab. F1-derived anti–third-party Tcm were preincubated for 1 hour with or without anti–LFA-1 or anti-CD8 blocking Abs. Following culture for 16 hours at a 5:1 Tcm/lymphoma cell ratio, the number of viable cells was determined by flow cytometry. Results shown represent average ± SD of triplicates in 1 representative experiment out of 3 performed.
Killing of murine lymphoma cells by anti–third-party Tcm is TCR independent and mediated by ICAM-1/LFA-1 interactions and engagement of CD8 molecules. (A) CalceinAM-prelabeled murine A20 lymphoma cells were incubated with or without OT1/Rag−/− (transgenic TCR)- or F1 (CB6)-derived anti–third-party Tcm for 16 hours at a 5:1 effector/lymphoma cell ratio. Numbers of viable cells were determined by flow cytometry. Percent killing was calculated as described in “Methods.” Results shown represent average ± SD of triplicates in 1 representative experiment out of 2 performed. (B) CalceinAM-labeled murine A20 lymphoma cells were preincubated for 1 hour with or without 50 μg/mL anti–ICAM-1 blocking Ab. F1-derived anti–third-party Tcm were preincubated for 1 hour with or without anti–LFA-1 or anti-CD8 blocking Abs. Following culture for 16 hours at a 5:1 Tcm/lymphoma cell ratio, the number of viable cells was determined by flow cytometry. Results shown represent average ± SD of triplicates in 1 representative experiment out of 3 performed.
To define whether similar to human anti–third-party CTLs,6 LFA-1, ICAM-1, and CD8 are involved in the killing mechanism, we used blocking Abs. As demonstrated in Figure 2B, preincubation of lymphoma cells with anti–ICAM-1 neutralizing Ab or preincubation of the anti–third-party Tcm with anti–LFA-1 or anti-CD8 neutralizing Ab inhibited the killing by 25.4% ± 3.9%, 59.5% ± 4.4%, and 56.9% ± 2.8%, respectively.
Taken together, these results verify the relevance of the mouse model to our earlier observation that human anti–third-party CTLs kill malignant B cells ex vivo through a TCR-independent mechanism mediated by apoptosis upon initial conjugate formation via LFA-1/ICAM-1 interactions. The possibility that this TCR-independent killing was mediated by a contaminating population of natural killer (NK) or natural killer T (NKT) cells was ruled out by staining B6-derived anti–third-party Tcm at the end of their preparation process using a pan-NK and NKT marker Ab (NK1.1).10 FACS analysis shows that there is no distinct population of NK or NΚT cells in the generated anti–third-party Tcm preparation (supplemental Figure 1A). Moreover, the possible involvement of the NKG2D receptor, an important receptor for the recognition of malignant cells by NK cells, NKT cells, and certain subsets of γδ T cells,11-13 was negated by our finding that addition of NKG2D blocking Ab did not inhibit the killing of lymphoma cells by anti–third-party Tcm (supplemental Figure 1B).
Syngeneic anti–third-party Tcm mediate effective lymphoma regression in vivo and contribute to prolongation of survival in mice with minimal residual disease
Following the verification that mouse anti–third-party Tcm exhibit TCR-independent killing of A20 lymphoma cells ex vivo, we proceeded to test their potential GVL activity in vivo in a mouse model specifically designed to study minimal residual disease. This model simulates the common clinical condition found following autologous or allogeneic BM transplantation in patients with B-CLL or other types of lymphoma. For this purpose, we used an A20 B-cell lymphoma cell line clone expressing a luciferase reporter gene stably integrated into the genome (A20-luc)7 enabling us to monitor by bioluminescence imaging (BLI) the fate of the lymphoma cells7 following adoptive transfer of anti third-party Tcm cells.
Initially, we tested the ability of syngeneic (H-2d) anti–third-party Tcm to eradicate lymphoma in vivo, excluding the contribution of possible alloreactive mechanisms. Thus, BALB/c recipient mice were lethally irradiated with 8Gy TBI (day −1) and infused the following day with 3 × 106 BM cells from syngeneic BALB/c-NUDE donors in the presence or absence of 5 ×103 A20-luc lymphoma cells (day 0). On the next day, mice were treated intravenously with syngeneic anti–third-party Tcm. Disease burden was assessed by weekly BLI beginning on day 14. As can be seen in Figure 3A, mice that received syngeneic TDBM and were not treated with anti–third-party Tcm all succumbed to lymphoma (tumor overload) before day 30, with median survival of 22 days (Figure 3B). In contrast, adoptive transfer of 5 × 106 syngeneic anti–third-party Tcm after syngeneic TDBM transplantation resulted in marked reduction of tumor burden as reflected by BLI and prolongation of survival to a median of 47 days (P < .0001; data not shown). Further improvement was found with increased Tcm doses, leading to 28.5% and 42.9% survival at 100 days posttransplant, with median survival of 49 and 80 days upon infusion of 1 × 107 or 2 × 107 Tcm, respectively (Figure 3B). A similar trend of tumor attenuation in vivo upon infusion of syngeneic Tcm was also found with an additional B-cell tumor cell line, namely BCL1 (supplemental Figure 2A-B; P < .001).
Inhibition of tumor relapse by syngeneic anti–third-party Tcm after syngeneic BMT. Lethally irradiated (8Gy TBI) BALB/c mice (H-2d) received an intravenous transplant of 3 × 106 syngeneic T-depleted BM cells (BALB/c nude [H-2d]) in the presence or absence of 5 × 103A20-luc lymphoma cells (day 0). On the following day, mice were intravenously injected with 10 × 106 (n = 7) or 2 × 107 (n = 5) BALB/c-derived anti–third-party Tcm or left untreated (A20 only) (n = 7). (A) Tumor growth was monitored by BLI from day 14 at weekly intervals. (B) Survival curves of the animals from the various treatment groups are shown.
Inhibition of tumor relapse by syngeneic anti–third-party Tcm after syngeneic BMT. Lethally irradiated (8Gy TBI) BALB/c mice (H-2d) received an intravenous transplant of 3 × 106 syngeneic T-depleted BM cells (BALB/c nude [H-2d]) in the presence or absence of 5 × 103A20-luc lymphoma cells (day 0). On the following day, mice were intravenously injected with 10 × 106 (n = 7) or 2 × 107 (n = 5) BALB/c-derived anti–third-party Tcm or left untreated (A20 only) (n = 7). (A) Tumor growth was monitored by BLI from day 14 at weekly intervals. (B) Survival curves of the animals from the various treatment groups are shown.
Allogeneic anti–third-party Tcm eradicate residual disease with no signs of GVHD
We next tested the potential GVL reactivity of fully allogeneic Tcm after allogeneic TDBM transplantation. Thus, BALB/c recipient mice were lethally irradiated with 8Gy TBI (day −1), and on day 0, 3 × 106 T-cell–depleted allogeneic BM (TDBM) cells from a B6-nude donor were infused in the presence or absence of 5 × 103 A20-luc lymphoma cells. On the following day, mice were treated with 5 × 106 donor-type B6 anti–third-party Tcm (A20+Tcm). Disease burden was then assessed by weekly BLI from day 14 to day 100. As can be seen in Figure 4A, BLI images demonstrated that TDBM-transplanted mice not treated with Tcm developed significant disease burden, and all the mice in this control group succumbed to the disease before day 28 (Figure 4B). Strikingly, all mice receiving allogeneic anti–third-party Tcm were clear of any detectable tumor during the entire observation period, and all of these mice survived more than 100 days post-BMT. Importantly, the infusion of Tcm was not associated with any symptoms of GVHD. Thus, while cleansed of residual A20 lymphoma cells, the weight (supplemental Figure 3) and overall appearance of mice receiving allogeneic anti–third-party Tcm were similar to that exhibited by the control group receiving a transplant of allogeneic nude TDBM in the absence of Tcm or lymphoma cells.
Inhibition of tumor relapse by adoptive transfer of allogeneic anti–third-party Tcm. Lethally irradiated (8Gy TBI) BALB/c (H-2d) mice received an intravenous transplant of 3 × 106 allogeneic TDBM cells (B6 nude [H-2b]) in the presence or absence of 5 × 103A20-luc lymphoma cells (day 0). Mice were then (day +1) intravenously injected with 5 × 106 B6-derived anti–third-party Tcm (A20+ Allo Tcm; n = 7) or left untreated (A20 only; n = 8). (A) Tumor growth was monitored by BLI from day 13 at weekly intervals. (B) Survival rates of the animals in the different treatment groups are shown.
Inhibition of tumor relapse by adoptive transfer of allogeneic anti–third-party Tcm. Lethally irradiated (8Gy TBI) BALB/c (H-2d) mice received an intravenous transplant of 3 × 106 allogeneic TDBM cells (B6 nude [H-2b]) in the presence or absence of 5 × 103A20-luc lymphoma cells (day 0). Mice were then (day +1) intravenously injected with 5 × 106 B6-derived anti–third-party Tcm (A20+ Allo Tcm; n = 7) or left untreated (A20 only; n = 8). (A) Tumor growth was monitored by BLI from day 13 at weekly intervals. (B) Survival rates of the animals in the different treatment groups are shown.
The reduced GVL efficacy of syngeneic anti–third-party Tcm compared with their allogeneic counterparts is associated with lymphoma cell infiltration to the CNS
The comparison between syngeneic and allogeneic Tcm in our GVL model suggests that while effective elimination of peripheral tumor burden in nearly all mice tested was attained by both treatments (Figures 3A and 4A), the overall survival of syngeneic recipients was markedly inferior compared with mice receiving allogeneic Tcm (Figures 3B and 4B). This discrepancy could be explained in part by the apparent slower GVL kinetics exhibited by syngeneic compared with allogeneic Tcm (Figures 3A and 4A), likely enabling infiltration of lymphoma cells into the central nervous system (CNS) sanctuary not accessible to the Tcm due to its unique immune-privileged state.14 Indeed, although clear of lymphoma signal in anterior view, this was indicated by the observation that recipients of syngenic Tcm suffered from paralysis in their hindlimbs and further clarified by a posterior view examination revealing lymphoma signal located in the head and the spinal column areas (Figure 5). Gross pathological evaluation, upon dissecting and separating the CNS from the cranium and the spinal column, revealed lymphoma cells residing within the CNS and not in the protective bones (Figure 5).
CNS infiltration by lymphoma cells in mice receiving syngeneic anti–third-party Tcm. Lethally irradiated (8Gy TBI) BALB/c mice (H-2d) received an intravenous transplant of 3 × 106 syngeneic TDBM cells (BALB/c nude [H-2d]) in the presence 5 × 103A20-luc lymphoma cells (day 0). Mice were then intravenously injected with 1 × 107 BALB/c-derived (syngeneic) anti–third-party Tcm (A20+Tcm; n = 6). Mice suffering from hindlimb paralysis were monitored by BLI (upper panel, anterior view; middle panel, posterior view). The mice were then killed and brain, spinal cord, cranium, and spinal column were dissected and analyzed by BLI (lower panel).
CNS infiltration by lymphoma cells in mice receiving syngeneic anti–third-party Tcm. Lethally irradiated (8Gy TBI) BALB/c mice (H-2d) received an intravenous transplant of 3 × 106 syngeneic TDBM cells (BALB/c nude [H-2d]) in the presence 5 × 103A20-luc lymphoma cells (day 0). Mice were then intravenously injected with 1 × 107 BALB/c-derived (syngeneic) anti–third-party Tcm (A20+Tcm; n = 6). Mice suffering from hindlimb paralysis were monitored by BLI (upper panel, anterior view; middle panel, posterior view). The mice were then killed and brain, spinal cord, cranium, and spinal column were dissected and analyzed by BLI (lower panel).
The enhanced survival attained with allogeneic Tcm without any manifestation of CNS metastasis strongly suggests that the allogeneic treatment is advantageous, possibly as a result of an additional alloreactive GVL effect that, together with the TCR-independent mechanism of killing, is capable of eradicating residual disease before it can disseminate into the CNS.
Anti–third-party Tcm spare normal B cells
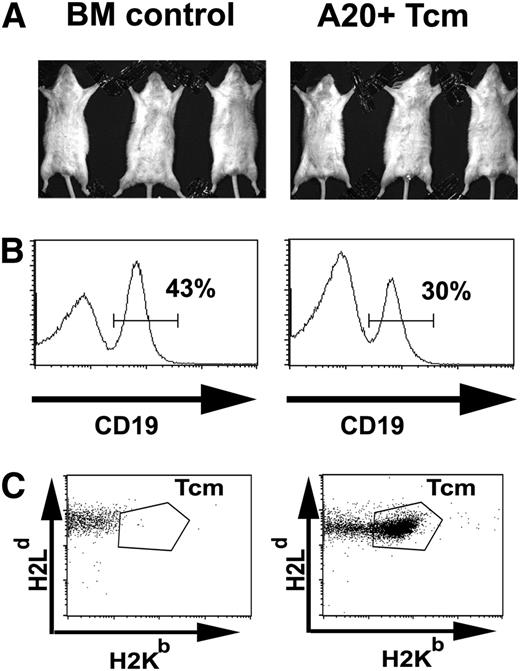
A major issue in cell therapy of cancer is the level of antitumor specificity exhibited by the infused cells. Thus, it was important to define in vivo the potential killing of normal host B-cell subpopulations. To address this question, a syngeneic minimal residual disease model was established as described above, except that 2 × 107 F1-derived anti–third-party Tcm that can be monitored by their H2b expression was used instead of syngeneic Tcm. To investigate whether Tcm can adversely affect host B cells in vivo, B-cell levels were defined 3 weeks after transplantation in mice responding beneficially to F1 Tcm treatment. As can be seen in Figure 6 A-B, while mice responding to the Tcm treatment did not exhibit any signal of residual disease, the size of their B-cell population in the spleen was comparable to that found in the BM control group not receiving Tcm (31.3% ± 9.6% vs 38.6% ± 5.6%, respectively). Importantly, at this time point, the Tcm still persisted in the treated mice (Figure 6C), indicating that while Tcm induce marked killing of lymphoma cells, they do not adversely affect normal B cells, which persist in the treated mice.
Anti–third-party Tcm eradicate lymphoma cells while sparing normal B cells in vivo. Lethally irradiated BALB/c mice (H-2d) received an intravenous transplant of 3 × 106 syngeneic BALB/c nude BM in the absence (BM control) or presence of 5 × 103A20-luc lymphoma cells (day 0). After 1 day, the mice were treated intravenously with 2 × 107 (C57BL/6xBALB/c) F1-derived anti–third-party Tcm (A20+Tcm). (A) On day 21, the mice were monitored for disease burden by BLI and mice responding beneficially to the Tcm treatment were chosen for further analysis (right panel, A20+ Tcm, n = 3). This is in comparison with mice that did not receive either A20 or Tcm (left panel, BM control, n = 3). (B) B-cell abundance. Mice were sacrificed and mononuclear cells were purified from the spleens and analyzed by FACS. (C) Tcm persistence. On day 21, peripheral blood levels of Tcm were analyzed by FACS to measure H2Kb H2Dd double-positive cells in the CD8+ T-cell subpopulation. For panels B and C, representative plots are displayed.
Anti–third-party Tcm eradicate lymphoma cells while sparing normal B cells in vivo. Lethally irradiated BALB/c mice (H-2d) received an intravenous transplant of 3 × 106 syngeneic BALB/c nude BM in the absence (BM control) or presence of 5 × 103A20-luc lymphoma cells (day 0). After 1 day, the mice were treated intravenously with 2 × 107 (C57BL/6xBALB/c) F1-derived anti–third-party Tcm (A20+Tcm). (A) On day 21, the mice were monitored for disease burden by BLI and mice responding beneficially to the Tcm treatment were chosen for further analysis (right panel, A20+ Tcm, n = 3). This is in comparison with mice that did not receive either A20 or Tcm (left panel, BM control, n = 3). (B) B-cell abundance. Mice were sacrificed and mononuclear cells were purified from the spleens and analyzed by FACS. (C) Tcm persistence. On day 21, peripheral blood levels of Tcm were analyzed by FACS to measure H2Kb H2Dd double-positive cells in the CD8+ T-cell subpopulation. For panels B and C, representative plots are displayed.
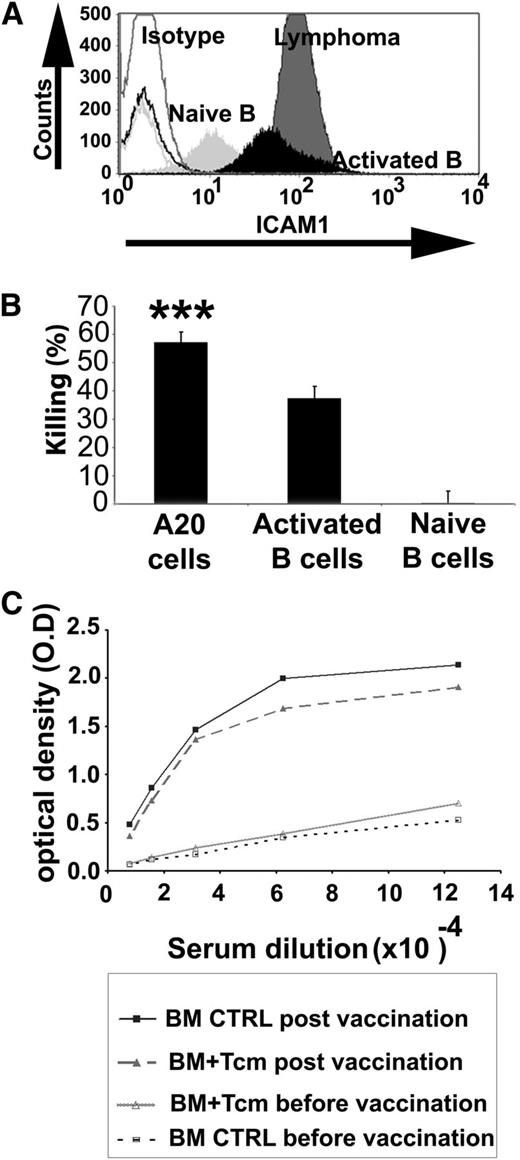
The enhanced sensitivity of lymphoma cells to killing by anti–third-party Tcm can be explained, at least in part, by their relative high expression of ICAM-1 due to the malignant activation process15,16 (Figure 6A). In line with this hypothesis, naive B cells, which express low levels of ICAM-1, were not killed by the Tcm. In contrast, nonmalignant lipopolysaccharide (LPS)-activated B cells exhibited upregulation of ICAM-1 (Figure 7A) and showed enhanced susceptibility to killing by the Tcm (Figure 7B), although at a significantly reduced level compared with the killing of lymphoma cells (Figure 7B). Clearly, as ICAM-1 is also expressed by many other cell types that are not susceptible to killing by Tcm, other molecular changes are likely critical for this unique TCR-independent killing mechanism and for the observed disparity between normal and malignant cells.
Anti–third-party Tcm eradicate lymphoma cells and artificially activated B cells in vitro but do not significantly impair the humoral response in vivo. (A) ICAM-1 expression on the different B-cell types was analyzed by flow cytometry. A representative experiment is shown out of 3 performed. Isotype controls (Isotype, nonfilled histograms), naive B cells (Naive B, light gray), activated B cells (Activated B, black), and A20 lymphoma cells (Lymphoma, dark gray). (B) In vitro killing assay. Naive B cells purified from BALB/c spleen using magnetic beads, activated B cells, obtained by further stimulation with 2 mg/mL LPS for 24 hours, or A20 cells were incubated for 16 hours with syngeneic anti–third-party Tcm. The number of viable B cells was determined by flow cytometry. Results shown represent average ± SD of triplicates in 1 representative experiment out of 3 performed. ***P value < .001 compared with percent killing displayed using naive B cells as targets. (C) Lethally irradiated BALB/c mice received 3 × 106 syngeneic nude BM cells in the absence (BM control n = 6) or presence of 10 × 106 syngeneic Tcm (BM+Tcm n = 6). At 5 weeks posttransplant, recipient mice were vaccinated with TNP-KLH in CFA as described in “Methods.” Anti-TNP antibodies in the serum were measured by ELISA. Results represent average serum levels of anti-TNP antibodies in each group, reflected by optical density (O.D) values at 630 nm. CTRL, control.
Anti–third-party Tcm eradicate lymphoma cells and artificially activated B cells in vitro but do not significantly impair the humoral response in vivo. (A) ICAM-1 expression on the different B-cell types was analyzed by flow cytometry. A representative experiment is shown out of 3 performed. Isotype controls (Isotype, nonfilled histograms), naive B cells (Naive B, light gray), activated B cells (Activated B, black), and A20 lymphoma cells (Lymphoma, dark gray). (B) In vitro killing assay. Naive B cells purified from BALB/c spleen using magnetic beads, activated B cells, obtained by further stimulation with 2 mg/mL LPS for 24 hours, or A20 cells were incubated for 16 hours with syngeneic anti–third-party Tcm. The number of viable B cells was determined by flow cytometry. Results shown represent average ± SD of triplicates in 1 representative experiment out of 3 performed. ***P value < .001 compared with percent killing displayed using naive B cells as targets. (C) Lethally irradiated BALB/c mice received 3 × 106 syngeneic nude BM cells in the absence (BM control n = 6) or presence of 10 × 106 syngeneic Tcm (BM+Tcm n = 6). At 5 weeks posttransplant, recipient mice were vaccinated with TNP-KLH in CFA as described in “Methods.” Anti-TNP antibodies in the serum were measured by ELISA. Results represent average serum levels of anti-TNP antibodies in each group, reflected by optical density (O.D) values at 630 nm. CTRL, control.
However, considering our finding that B cells that were artificially activated in vitro by LPS were also killed to some extent by the Tcm, it could be argued that Tcm therapy might be associated with an adverse effect on posttransplant humoral responses. To address this possibility, 5 weeks after syngeneic TDBMT and adoptive transfer of 107 syngeneic anti–third-party Tcm, recipient mice were challenged with a common TNP-KLH/CFA vaccine. Ten days after vaccination, serum was collected and the level of anti-TNP antibodies was measured. As can be seen in Figure 7C, a significant elevation in anti-TNP antibodies was found in recipients of Tcm compared with their levels prior to the vaccination, although an insignificant reduced trend was seen compared with control mice receiving TDBM without Tcm. Similar results were found when allogeneic Tcm and TDBMT were used (data not shown).
Importantly, when vaccinated at 5 months posttransplant, anti-TNP Ab levels in mice treated with Tcm were comparable to those found in control group, which received BM in the absence of Tcm (supplemental Figure 4). Thus, while exhibiting strong GVL effect against lymphoma B cells, Tcm therapy was associated with very mild and statistically insignificant short-term impact on primary humoral response.
Discussion
The most common cause of death after allogeneic BMT is relapse of the primary disease, occurring in nearly 40% of the patients who suffer from hematologic malignancies.17 Donor-lymphocyte infusion offers an important approach to address this challenge; however, the high risk for GVHD associated with donor-lymphocyte infusion dampens the wide use of this mode of therapy.18
For patients with advanced B-CLL or NHL, the most common treatments are either autologous BMT after intensive conditioning or allogeneic BMT following RIC.19-21 The former is limited to patients who can tolerate the harsh conditioning, and therefore excludes many elderly patients who make up the majority of the B-NHL population. Thus, a recent report from the Center for International Blood and Marrow Transplant Research supports the use of the latter for such patients.22 However, the risk of organ toxicity and infections associated with graft rejection or GVHD following allogeneic transplantation is still a major concern in this setting.23,24 Thus, the use of RIC that is followed by TDBMT, which results in a low rate of chronic GVHD, is currently evaluated in major centers.25 In this context, enhanced relapse rates likely represent the major risk due to the small number of donor T cells in the inoculum. Our present study clearly demonstrates that this major concern could potentially be overcome by donor anti–third-party Tcm, which exhibit striking antilymphoma reactivity both in vitro and in vivo.
Furthermore, our demonstration that administration of donor Tcm in the allogeneic setting led to tumor elimination and overall survival of 100% of the mice while having very little adverse effect on the level of normal B cells and their ability to generate a primary humoral response suggests that Tcm therapy could be advantageous compared with BMT following rituximab-based conditioning or treatment with T cells transfected with chimeric TCR directed against CD19,26 both of which were found to be associated in B-cell lymphoma patients, with markedly reduced B-cell counts and immunoglobulin G levels for 1 to 2 years following BMT.27,28
In patients who can tolerate intensive conditioning, a second treatment modality that could be considered based on our data is the use of Tcm in the context of autologous BMT. This procedure, which is free of graft rejection or GVHD risk, is associated with less transplant-related mortality but is clearly more prone to relapse compared with allogeneic T-cell–replete transplants. Therefore, the use of Tcm therapy could be attractive even though it was associated in our model with protracted relapse as a consequence of tumor cells harbored in the CNS.
This superior GVL reactivity of allogeneic Tcm could be mediated in part by potential contamination in Tcm preparations with residual alloreactive naive T cells, which might exist even in the absence of clinical manifestation of GVHD. Likewise, BM-derived donor-type alloreactive NK cells generated de novo after transplantation could potentially exert GVL reactivity without inducing GVHD and strengthen the TCR-independent GVL mechanism of Tcm operational in the syngeneic setting.
However, the inferior efficacy of syngenic Tcm in clearing A20 tumor cells in the CNS might be less problematic in humans, as CNS involvement is known to occur only in the minority of lymphoma patients.29 Furthermore, it can be addressed in the human setting by prophylaxis with methotroxate.30 Thus, the full potential of Tcm therapy should also be considered for patients undergoing autologous BMT.
Notably, considering that inbred mice kept under sterile conditions are less prone to GVHD compared with humans, translation of this approach to the clinical allogeneic setting might require additional allodepleting steps,31,32 even though recipients of allogeneic Tcm did not exhibit symptoms of GVHD in the present study or in other mouse models.3,33
In conclusion, our study demonstrates that anti–third-party Tcm kill malignant B cells in a TCR-independent mechanism while sparing naive B cells. Using in vivo imaging, we were able to simulate and monitor the common threat of postchemotherapy minimal residual disease. This model was used to demonstrate the effectiveness of activated anti–third-party Tcm in overcoming residual disease, both in a syngeneic and an allogeneic setting. The former, which is associated with less transplant-related mortality, could be advantageous if CNS involvement can be controlled, whereas the latter might prove more effective overall if using adequate T-cell depletion to prevent GVHD. Furthermore, considering our previous demonstration of tolerance induction by these cells,3 the Tcm can offer a “double-supportive effect” of enhancing BM allografts and, at the same time, inducing GVL reactivity without causing GVHD. Such cell therapy could be highly attractive for patients with B-CLL and other B-cell malignancies who might not tolerate aggressive conditioning due to age or performance status. Further clinical evaluation of both treatment modalities is warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from Cell Source and from Roberto and Renata Ruhman.
Authorship
Contribution: A.L., E.O., and Y.R. designed and performed the research, analyzed data, wrote the manuscript, and approved the final version of the manuscript; N.O.-G. and A.C.-F. designed and performed the research, and analyzed data; R.A. and B.N. performed research; Y.E. and S.R.-Z. designed the research; M.E. and R.S.N. contributed vital new reagents; and D.H. designed the research and analyzed data.
Conflict-of-interest disclosure: Y.R. serves as a consultant to Cell Source. The remaining authors declare no competing financial interests.
Correspondence: Yair Reisner, Department of Immunology, Weizmann Institute of Science, 234 Herzl St, Rehovot, Israel 7600; e-mail: yair.reisner@weizmann.ac.il.
References
Author notes
A.L. and E.O. contributed equally to this study.

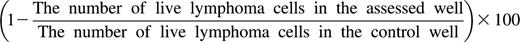
![Figure 3. Inhibition of tumor relapse by syngeneic anti–third-party Tcm after syngeneic BMT. Lethally irradiated (8Gy TBI) BALB/c mice (H-2d) received an intravenous transplant of 3 × 106 syngeneic T-depleted BM cells (BALB/c nude [H-2d]) in the presence or absence of 5 × 103A20-luc lymphoma cells (day 0). On the following day, mice were intravenously injected with 10 × 106 (n = 7) or 2 × 107 (n = 5) BALB/c-derived anti–third-party Tcm or left untreated (A20 only) (n = 7). (A) Tumor growth was monitored by BLI from day 14 at weekly intervals. (B) Survival curves of the animals from the various treatment groups are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/15/10.1182_blood-2012-06-432443/4/m_3033f3.jpeg?Expires=1767725519&Signature=r2hyMubsHy00n2O6JL3amP3FQeRb4kIe3M3KTBtPeXMd4uYP8VHnAgcG34NT62Dmo7UORHIsFoZ4ZOVk2~aAyB-bs955iU4eeexN~Wulwa49VG9hIjt7XeBmKkta22cHldtB01bdWkrS6PhCtLgHJPaOKghX60nj9HS4HndWSN~fH-kxZqblZA9O4w15ajMzptnBWyjpKzTqwwzQXpDWdj-ylc0qoL4WWlVDAwOmixrmxX8mVP6elHFMiS3gCvTAaOtDC3LBlYxQkRl4O4H5IOcVVJHXXFxheIzeUD5B8VKGWccM9XIEtHU5MYw3pUWCRjweswk~i934MjaS08x9sg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Inhibition of tumor relapse by adoptive transfer of allogeneic anti–third-party Tcm. Lethally irradiated (8Gy TBI) BALB/c (H-2d) mice received an intravenous transplant of 3 × 106 allogeneic TDBM cells (B6 nude [H-2b]) in the presence or absence of 5 × 103A20-luc lymphoma cells (day 0). Mice were then (day +1) intravenously injected with 5 × 106 B6-derived anti–third-party Tcm (A20+ Allo Tcm; n = 7) or left untreated (A20 only; n = 8). (A) Tumor growth was monitored by BLI from day 13 at weekly intervals. (B) Survival rates of the animals in the different treatment groups are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/15/10.1182_blood-2012-06-432443/4/m_3033f4.jpeg?Expires=1767725519&Signature=BCK8k8LL-SnjfjzJ-eP3-dYA9sF2p0fP33QnK66s0HHbGolX1cqtG8-Ge49weDZXfbTCZo9oSstFTP6NAnXsdy9BolvRc7IfthAiuMfzdi9BznsNUcwNxCQ-NbE30mYsLO~nOPy4UaKjSFVZkQSI1Wdm5Jvu8nxtCmZL4CX-FtzTdfrQTB~YKXdsHL6pDrtA~VJ1rtckGtZ97yxyY8H7sMPL8-4yfVyQ9f48VtH9F6gEez2w29Q2aCnJB~eWpq9Y7E5I64t8RiCXHz1vIDt5PH--SdxaGpX1x0LKIDOm~zRF303M7u5zUG8YZlYJUUJ6kWkXINQWPrtJ59OCJYqypQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CNS infiltration by lymphoma cells in mice receiving syngeneic anti–third-party Tcm. Lethally irradiated (8Gy TBI) BALB/c mice (H-2d) received an intravenous transplant of 3 × 106 syngeneic TDBM cells (BALB/c nude [H-2d]) in the presence 5 × 103A20-luc lymphoma cells (day 0). Mice were then intravenously injected with 1 × 107 BALB/c-derived (syngeneic) anti–third-party Tcm (A20+Tcm; n = 6). Mice suffering from hindlimb paralysis were monitored by BLI (upper panel, anterior view; middle panel, posterior view). The mice were then killed and brain, spinal cord, cranium, and spinal column were dissected and analyzed by BLI (lower panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/15/10.1182_blood-2012-06-432443/4/m_3033f5.jpeg?Expires=1767725519&Signature=eajlion2lVidWqR3gPwpH4O~fqVOg4sGQfqoFG2oTXjb8qeICuvUFCCdAFgSWWdVOlk2fu6QJ0vrf~lZ7ThjSLPBezuE9YBqcUvmpraiAAu~PYEI4Q2g5-WicndJ6HmRJYfNRZA7JZzWPQvSLmoGTZ1IB~tphQy14ip4Qs-Vc2tigSFmOdN1CiVPRWi0u7wrFH9o15ijYTVh3H5rBe4tr0WZj8e1dmzs-MsGJM-rvnpeRCMvh5cBV1mJ8wGkGfV5~0obQ0kR8JrtYkH7ey4ZMZDp2sbmUFG0z6yiqDdD1nYE9WkvSzi20BaxM5d7D3pqk04whvGzIyNrsdQeqhM68A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)