Key Points
Coincident with major changes in cancer treatments, the occurrence of tAML has changed significantly with time.
The risks for tAML should be weighed against the benefits of chemotherapy.
Abstract
Therapy-related acute myeloid leukemia (tAML) is a rare but highly fatal complication of cytotoxic chemotherapy. Despite major changes in cancer treatment, data describing tAML risks over time are sparse. Among 426 068 adults initially treated with chemotherapy for first primary malignancy (9 US population-based cancer registries, 1975-2008), we identified 801 tAML cases, 4.70 times more than expected in the general population (P < .001). Over time, tAML risks increased after chemotherapy for non-Hodgkin lymphoma (n = 158; Poisson regression Ptrend < .001), declined for ovarian cancer (n = 72; Ptrend < .001), myeloma (n = 62; Ptrend = .02), and possibly lung cancer (n = 65; Ptrend = .18), and were significantly heterogeneous for breast cancer (n = 223; Phomogeneity = .005) and Hodgkin lymphoma (n = 58; Phomogeneity = .007). tAML risks varied significantly by age at first cancer and latency and were nonsignificantly heightened with radiotherapy for lung, breast, and ovarian cancers. We identified newly emerging elevated tAML risks in patients treated with chemotherapy since 2000 for esophageal, cervical, prostate, and possibly anal cancers; and since the 1990s for bone/joint and endometrial cancers. Using long-term, population-based data, we observed significant variation in tAML risk with time, consistent with changing treatment practices and differential leukemogenicity of specific therapies. tAML risks should be weighed against the benefits of chemotherapy, particularly for new agents and new indications for standard agents.
Introduction
With nearly 12 million cancer survivors in the United States today,1 understanding adverse effects of cancer treatments has significant clinical and public health implications. The development of therapy-related acute myeloid leukemia (tAML) has long been recognized as a rare but highly fatal complication of cytotoxic chemotherapy, with varying leukemogenicity among individual agents.2-5 Radiotherapy has been implicated in tAML as well, but the risks are substantially lower than with chemotherapy.6-8
Approaches to cancer treatment have evolved considerably for the last several decades, with the introduction of numerous new drugs (including rapidly expanding use of molecularly targeted agents in the past decade), changes in treatment protocols, increasing use of (neo)adjuvant therapy, and use of maintenance therapy or repeated courses of treatment. Most previous studies of tAML risks have derived from clinical trials, hospital series, or case reports, which provide valuable information on tAML risks associated with specific chemotherapeutic agents but comprise selected patient populations who were diagnosed during a limited period. Despite dramatic changes in cancer treatment, no large-scale study quantifying tAML risks after chemotherapy across multiple types of first primary malignancies among adult cancer patients treated in the US general population has been published since 1984.9 In addition, data on the risks for tAML in the current treatment era are sparse, even from clinic-based studies, although several case reports and small studies suggest increased tAML risks in cancer sites with expanded use of chemotherapy in the last decade.
We therefore investigated changes in tAML risk with time among patients reported to have received chemotherapy as part of initial cancer treatment, using data from population-based cancer registries in the Surveillance, Epidemiology and End Results Program of the United States. To identify patients at highest risk, we assessed tAML risks by calendar period, age at diagnosis, time since diagnosis (latency), and type of first primary malignancy.
Methods
Study population
Patients eligible for this study were adults 20 to 84 years old who were diagnosed with a first primary malignancy during 1975-2008 and who received initial treatment with chemotherapy (including immunotherapy and tyrosine kinase inhibitors), as reported in 9 registries of the Surveillance, Epidemiology and End Results (SEER) Program of the United States (Connecticut, Hawaii, Iowa, New Mexico, and Utah; metropolitan areas of Atlanta, Georgia; Detroit, Michigan; San Francisco/Oakland, California; and Seattle/Puget Sound, Washington). Together, these registries account for 9.5% of the US population, with similar distributions with respect to poverty and level of education, although the SEER population is somewhat more urban and has a higher proportion of foreign-born individuals.1 In addition, SEER is known to underascertain initial chemotherapy, and data on subsequent therapy are not available (supplemental Figure 1).
All first primary malignancies were included except leukemias, because of likely under-reporting of multiple leukemias. In addition, patients were restricted to ages 20 to 84 years at diagnosis because of different treatment approaches for children and young adults and likely underascertainment of second malignancies in elderly patients (≥85 years). Initial chemotherapy was defined in SEER as receipt of chemotherapy within 4 months of initiation of treatment (<1988) or inclusion of chemotherapy in the first treatment course (≥1988); data on specific drugs, doses, and subsequent treatments of recurrent/progressive disease were not available.
Statistical analysis
Patients were observed from the date of the first diagnosis of a primary malignancy until the occurrence of a second primary malignancy, attained age 85 years, death, loss to follow-up (n = 10 683, 2.5%), or end of the study (December 31, 2008), whichever occurred first. Cases of AML (International Classification of Diseases for Oncology, Third Edition morphology codes 9840, 9861, 9866-9867, 9870-9874, 9891, 9895-9897, 9910, 9920, and 9930-9931) that developed at least 1 year after the first diagnosis of a primary malignancy were classified as tAML according to the World Health Organization classification10 because all patients in the cohort received prior chemotherapy. The first year of follow-up was excluded because the time to development of tAML typically is 1 year or longer.5
Comparisons with the general population.
Standardized incidence ratios (SIRs) were calculated as the ratio of the observed-to-expected (O/E) number of AML (SEER*Stat software, version 7.0.5). The expected number of AML was computed from age-, race-, sex-, and calendar year–specific incidence rates of AML from the general SEER population, multiplied by the appropriate person-years at risk. We calculated exact, 2-sided, Poisson-based 95% confidence intervals (CIs) about the SIRs. We also computed the excess absolute risk (EAR) [(O−E)*10 000/person-years at risk] to estimate the number of excess cancers beyond that expected per 10 000 persons per year.
Internal comparisons within cohorts.
For first primary sites with at least 50 tAML cases, we conducted multivariate Poisson regression analyses comparing excess relative risks (ERR=RR–1) by categories of calendar year, age at first primary diagnosis, and initial radiotherapy, where RR denotes the risk relative to a specified referent group (eg, earliest calendar period). The ERR for a category defined by the combination of several variables such as age, latency, and calendar year was expressed as the product of ERRs for each of the variables (specified in Figures 1 and 2; supplemental Tables 1-3). With this multiplicative model, the ratios of ERRs comparing categories within each variable (eg, comparing calendar-year categories) are adjusted for the other variables and do not depend on these variables. Expected values of tAML on the basis of the general population were used as an offset in the Poisson model to provide indirect adjustment for sex, race, attained age, and attained calendar year.11 Poisson models also were adjusted for age at first diagnosis of primary malignancy, receipt of initial radiotherapy, latency, sex (when appropriate), and disease stage (for lung and bronchus, female breast, and ovary). We also compared EARs by categories of age using similar methods. Parameter estimates, hypothesis tests, and CIs were based on likelihood ratio methods. Two-sided P values and CIs were used throughout. Analyses were implemented with the AMFIT module of the software package EPICURE (version 1.8).
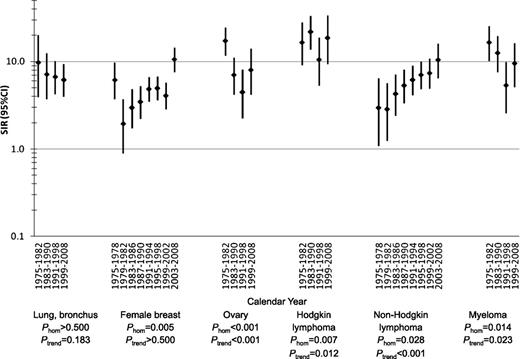
SIRs for tAML by calendar year after initial chemotherapy treatment of selected first primary malignancies in adulthood, 9 SEER registries, 1975-2008.P-values were derived from Poisson regression models adjusted for age at first primary malignancy diagnosis, receipt of initial radiotherapy, sex (for lung and bronchus, Hodgkin lymphoma, non-Hodgkin lymphoma, and myeloma), stage (for lung and bronchus, female breast, and ovary), and latency (overall: 1.0-4.9, 5.0-9.9, 10+ years; for lung and bronchus, 1-4.9, 5+ years; 1-4.9 years: 1.0-2.9, 3.0-4.9 years). CI, confidence interval; Phom, p for homogeneity; tAML, therapy-related acute myeloid leukemia; SIR, standardized incidence ratio; SEER, Surveillance, Epidemiology and End Results.
SIRs for tAML by calendar year after initial chemotherapy treatment of selected first primary malignancies in adulthood, 9 SEER registries, 1975-2008.P-values were derived from Poisson regression models adjusted for age at first primary malignancy diagnosis, receipt of initial radiotherapy, sex (for lung and bronchus, Hodgkin lymphoma, non-Hodgkin lymphoma, and myeloma), stage (for lung and bronchus, female breast, and ovary), and latency (overall: 1.0-4.9, 5.0-9.9, 10+ years; for lung and bronchus, 1-4.9, 5+ years; 1-4.9 years: 1.0-2.9, 3.0-4.9 years). CI, confidence interval; Phom, p for homogeneity; tAML, therapy-related acute myeloid leukemia; SIR, standardized incidence ratio; SEER, Surveillance, Epidemiology and End Results.
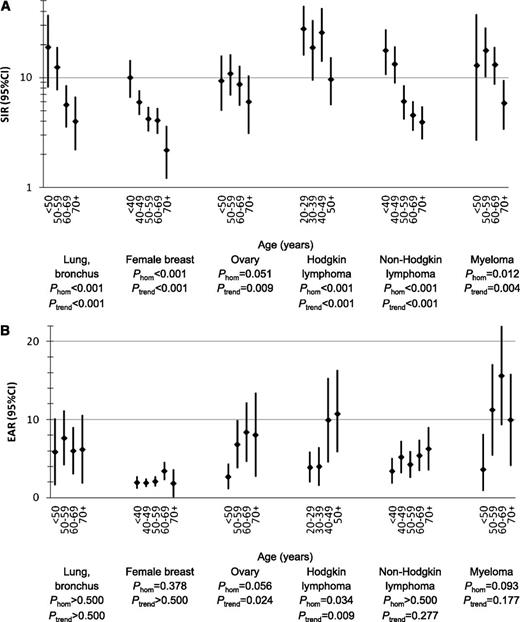
SIRs and EARs of tAML by age (years) after initial chemotherapy treatment of selected first primary malignancies in adulthood, 9 SEER registries, 1975-2008.P-values were derived from Poisson regression models adjusted for year of first primary malignancy diagnosis, receipt of initial radiotherapy, sex (for lung and bronchus, Hodgkin lymphoma, non-Hodgkin lymphoma, and myeloma), stage (for lung and bronchus, female breast, and ovary), and latency (1.0-4.9, 5.0-9.9, 10+ years; for lung and bronchus: 1-4.9, 5+ years). CI, confidence interval; EAR, excess absolute risk; Phom, p for homogeneity; tAML, therapy-related acute myeloid leukemia; SIR, standardized incidence ratio; SEER, Surveillance, Epidemiology and End Results.
SIRs and EARs of tAML by age (years) after initial chemotherapy treatment of selected first primary malignancies in adulthood, 9 SEER registries, 1975-2008.P-values were derived from Poisson regression models adjusted for year of first primary malignancy diagnosis, receipt of initial radiotherapy, sex (for lung and bronchus, Hodgkin lymphoma, non-Hodgkin lymphoma, and myeloma), stage (for lung and bronchus, female breast, and ovary), and latency (1.0-4.9, 5.0-9.9, 10+ years; for lung and bronchus: 1-4.9, 5+ years). CI, confidence interval; EAR, excess absolute risk; Phom, p for homogeneity; tAML, therapy-related acute myeloid leukemia; SIR, standardized incidence ratio; SEER, Surveillance, Epidemiology and End Results.
Results
Initial chemotherapy was reported in 426 068 adults diagnosed with a first primary malignancy during 1975-2008 (Table 1). Chemotherapy use was reported most frequently (≥60% of patients) for anal cancer, ovarian cancer, Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), and myeloma (supplemental Figure 1). Patients nearly always (≥85%) also received initial radiotherapy for cancers of the buccal cavity/pharynx, esophagus, anus, larynx, cervix, and brain. The proportion of patients reported to have received chemotherapy (with or without radiotherapy) increased during 1975-2008 for many malignancies, particularly cancers of the buccal cavity/pharynx, esophagus, stomach, colon, rectum, anus, larynx, lung, female breast, cervix, and brain, as well as HL.
Selected characteristics of patients who received initial chemotherapy for first primary malignancy in adulthood, 9 SEER registries, 1975-2008
| First primary malignancy . | Total (N) . | Also received initial radiotherapy (%) . | Women (%) . | Mean age at diagnosis (y) . | Mean person-years at risk (y) . |
|---|---|---|---|---|---|
| Total* | 426 068 | 45 | 63 | 57.3 | 4.9 |
| Buccal cavity, pharynx | 9903 | 92 | 22 | 56.8 | 3.5 |
| Esophagus | 5535 | 88 | 23 | 63.1 | 2.2 |
| Stomach | 7707 | 50 | 32 | 61.3 | 2.4 |
| Colon | 38 064 | 8 | 49 | 62.5 | 4.5 |
| Rectum, rectosigmoid junction | 22 757 | 74 | 39 | 61.1 | 4.4 |
| Anus, anal canal, anorectum | 3476 | 96 | 62 | 58.6 | 5.4 |
| Biliary tract | 4015 | 36 | 41 | 60.9 | 1.8 |
| Larynx | 2679 | 92 | 23 | 59.8 | 3.5 |
| Lung, bronchus | 51 096 | 64 | 45 | 62.7 | 1.7 |
| Small-cell | 16 923 | 64 | 49 | 63.2 | 1.6 |
| Non–small-cell | 33 712 | 65 | 43 | 62.5 | 1.7 |
| Bones and joints | 955 | 28 | 39 | 38.3 | 6.4 |
| Melanoma of the skin | 1369 | 15 | 38 | 51.5 | 6.1 |
| Soft tissue (including heart) | 2268 | 60 | 44 | 48.1 | 5.2 |
| Female breast | 121 864 | 54 | 100 | 52.6 | 6.7 |
| Cervix | 4282 | 93 | 100 | 49.7 | 4.1 |
| Endometrium | 4683 | 52 | 100 | 61.3 | 3.8 |
| Ovary | 23 180 | 4 | 100 | 59.0 | 4.9 |
| Prostate | 3195 | 49 | 0 | 66.5 | 4.6 |
| Testis | 4418 | 8 | 0 | 32.8 | 11.4 |
| Urinary bladder | 9703 | 17 | 24 | 66.0 | 5.7 |
| Brain, central nervous system | 6241 | 94 | 40 | 48.6 | 2.8 |
| Hodgkin lymphoma | 10 976 | 39 | 43 | 39.6 | 8.6 |
| Non-Hodgkin lymphoma | 46 566 | 24 | 45 | 59.0 | 5.9 |
| Myeloma | 16 164 | 29 | 46 | 64.8 | 3.1 |
| First primary malignancy . | Total (N) . | Also received initial radiotherapy (%) . | Women (%) . | Mean age at diagnosis (y) . | Mean person-years at risk (y) . |
|---|---|---|---|---|---|
| Total* | 426 068 | 45 | 63 | 57.3 | 4.9 |
| Buccal cavity, pharynx | 9903 | 92 | 22 | 56.8 | 3.5 |
| Esophagus | 5535 | 88 | 23 | 63.1 | 2.2 |
| Stomach | 7707 | 50 | 32 | 61.3 | 2.4 |
| Colon | 38 064 | 8 | 49 | 62.5 | 4.5 |
| Rectum, rectosigmoid junction | 22 757 | 74 | 39 | 61.1 | 4.4 |
| Anus, anal canal, anorectum | 3476 | 96 | 62 | 58.6 | 5.4 |
| Biliary tract | 4015 | 36 | 41 | 60.9 | 1.8 |
| Larynx | 2679 | 92 | 23 | 59.8 | 3.5 |
| Lung, bronchus | 51 096 | 64 | 45 | 62.7 | 1.7 |
| Small-cell | 16 923 | 64 | 49 | 63.2 | 1.6 |
| Non–small-cell | 33 712 | 65 | 43 | 62.5 | 1.7 |
| Bones and joints | 955 | 28 | 39 | 38.3 | 6.4 |
| Melanoma of the skin | 1369 | 15 | 38 | 51.5 | 6.1 |
| Soft tissue (including heart) | 2268 | 60 | 44 | 48.1 | 5.2 |
| Female breast | 121 864 | 54 | 100 | 52.6 | 6.7 |
| Cervix | 4282 | 93 | 100 | 49.7 | 4.1 |
| Endometrium | 4683 | 52 | 100 | 61.3 | 3.8 |
| Ovary | 23 180 | 4 | 100 | 59.0 | 4.9 |
| Prostate | 3195 | 49 | 0 | 66.5 | 4.6 |
| Testis | 4418 | 8 | 0 | 32.8 | 11.4 |
| Urinary bladder | 9703 | 17 | 24 | 66.0 | 5.7 |
| Brain, central nervous system | 6241 | 94 | 40 | 48.6 | 2.8 |
| Hodgkin lymphoma | 10 976 | 39 | 43 | 39.6 | 8.6 |
| Non-Hodgkin lymphoma | 46 566 | 24 | 45 | 59.0 | 5.9 |
| Myeloma | 16 164 | 29 | 46 | 64.8 | 3.1 |
The study population was restricted to patients who were diagnosed with a first primary malignancy between ages 20 and 84 years and survived at least 1 year after diagnosis. Cohort follow-up was censored at age 85 years because of underascertainment of second malignancies among older patients.
All first primary malignancies excluding leukemia and nonmelanoma skin cancer. First primary malignancies after which <3 cases of tAML were observed among patients with initial chemotherapy were included in the “total” but are not presented separately in this table (eg, pancreas, kidney, thyroid).
We identified 801 cases of tAML among patients treated with initial chemotherapy, compared with 170.5 cases of AML expected in the general population, representing a 4.70-fold increased risk (SIR; 95% CI, 4.38-5.04) and an excess of 3.04 cases per 10 000 person-years (EAR; Table 2). tAML risks were especially pronounced for patients treated with chemotherapy for cancers of the bones and joints, soft tissue, ovary, and lung, as well as HL and myeloma. Nearly half of the tAMLs occurred after breast cancer and NHL (breast: n = 223, SIR=4.60, EAR=2.15; NHL: n = 158, SIR=5.85, EAR=4.81). On the basis of fewer than 10 cases, tAML risks also were significantly elevated after chemotherapy for cancers of the esophagus, anus, biliary tract, cervix, endometrium, testes, brain, and prostate. Despite frequent use of chemotherapy (≥25% of patients) for cancers of the stomach, colon, and rectum, tAML risks were not significantly elevated.
SIRs for tAML overall and by latency after initial chemotherapy treatment of first primary malignancy in adulthood, 9 SEER registries, 1975-2008
| First primary malignancy . | . | . | . | . | By latency . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall . | 1.0-4.9 y . | 5.0-9.9 y . | ≥10.0 y . | ||||||||||
| N . | EAR/10 000* . | SIR* . | (95% CI) . | N . | SIR* . | (95% CI) . | N . | SIR* . | (95% CI) . | N . | SIR* . | (95% CI) . | |
| Total† | 801 | 3.04 | 4.70‡ | (4.38-5.04) | 487 | 6.07‡ | (5.54-6.64) | 223 | 4.62‡ | (4.03-5.26) | 91 | 2.17‡ | (1.74-2.66) |
| Buccal cavity, pharynx | 5 | 0.58 | 1.67 | (0.54-3.90) | 4 | 2.37 | (0.64-6.06) | <3 | 1.22 | (0.03-6.77) | 0 | § | (0.00-7.69) |
| Esophagus | 6 | 3.65 | 3.84‡ | (1.41-8.36) | 4 | 3.87‡ | (1.06-9.92) | <3 | 2.66 | (0.07-14.81) | <3 | 6.55 | (0.17-36.52) |
| Stomach | 5 | 1.56 | 2.36 | (0.77-5.50) | 3 | 2.43 | (0.50-7.11) | <3 | 4.33 | (0.52-15.66) | 0 | § | (0.00-8.64) |
| Colon | 25 | 0.2 | 1.16 | (0.75-1.71) | 11 | 1.06 | (0.53-1.89) | 7 | 1.07 | (0.43-2.20) | 7 | 1.50 | (0.60-3.10) |
| Rectum, rectosigmoid junction | 15 | 0.25 | 1.20 | (0.67-1.98) | 7 | 1.11 | (0.45-2.29) | 4 | 1.05 | (0.29-2.68) | 4 | 1.68 | (0.46-4.31) |
| Anus, anal canal, anorectum | 6 | 2.21 | 3.24‡ | (1.19-7.04) | 3 | 3.70 | (0.76-10.80) | <3 | 3.43 | (0.42-12.39) | <3 | 2.18 | (0.06-12.12) |
| Biliary tract | 4 | 4.58 | 5.52‡ | (1.50-14.13) | 3 | 5.77‡ | (1.19-16.86) | <3 | 7.59 | (0.19-42.29) | 0 | § | (0.00-50.48) |
| Larynx | 3 | 2.14 | 3.02 | (0.62-8.81) | 0 | § | (0.00-6.54) | 3 | 10.33‡ | (2.13-30.19) | 0 | § | (0.00-26.26) |
| Lung, bronchus | 65 | 6.49 | 6.82‡ | (5.26-8.69) | 54 | 7.96‡ | (5.98-10.39) | 9 | 4.70‡ | (2.15-8.91) | <3 | 2.38 | (0.29-8.61) |
| Small-cell | 32 | 11.13 | 11.04‡ | (7.55-15.58) | 27 | 14.28‡ | (9.41-20.78) | 3 | 4.51 | (0.93-13.17) | <3 | 5.84 | (0.71-21.09) |
| Non–small-cell | 32 | 4.35 | 4.87‡ | (3.33-6.88) | 26 | 5.37‡ | (3.51-7.87) | 6 | 4.84‡ | (1.78-10.54) | 0 | § | (0.00-7.55) |
| Bones and joints | 4 | 6.21 | 19.25‡ | (5.25-49.30) | <3 | 28.53‡ | (3.45-103.05) | <3 | 40.65‡ | (4.92-146.86) | 0 | § | (0.00-41.71) |
| Melanoma of the skin | 3 | 2.78 | 4.50 | (0.93-13.15) | <3 | 4.84 | (0.12-26.99) | 0 | § | (0.00-25.44) | <3 | 6.35 | (0.77-22.92) |
| Soft tissue (including heart) | 11 | 8.67 | 14.41‡ | (7.20-25.79) | 7 | 23.86‡ | (9.59-49.16) | 3 | 14.63‡ | (3.02-42.74) | <3 | 3.78 | (0.10-21.06) |
| Female breast | 223 | 2.15 | 4.60‡ | (4.02-5.25) | 160 | 8.60‡ | (7.32-10.05) | 40 | 2.70‡ | (1.93-3.68) | 23 | 1.53 | (0.97-2.29) |
| Cervix | 6 | 2.92 | 7.29‡ | (2.67-15.86) | 4 | 9.36‡ | (2.55-23.96) | <3 | 8.60‡ | (1.04-31.07) | 0 | § | (0.00-22.56) |
| Endometrium | 9 | 4.16 | 5.97‡ | (2.73-11.33) | 6 | 7.80‡ | (2.86-16.98) | <3 | 2.55 | (0.06-14.20) | <3 | 5.78 | (0.70-20.87) |
| Ovary | 72 | 5.66 | 8.68‡ | (6.79-10.94) | 44 | 12.07‡ | (8.77-16.20) | 22 | 10.81‡ | (6.77-16.37) | 6 | 2.30 | (0.84-5.00) |
| Prostate | 8 | 3.62 | 2.95‡ | (1.27-5.80) | 7 | 5.01‡ | (2.01-10.32) | <3 | 1.11 | (0.03-6.17) | 0 | § | (0.00-8.87) |
| Testis | 8 | 1.34 | 6.34‡ | (2.74-12.48) | 4 | 16.01‡ | (4.36-40.99) | 3 | 10.64‡ | (2.19-31.11) | <3 | 1.37 | (0.03-7.62) |
| Urinary bladder | 12 | 0.58 | 1.36 | (0.70-2.37) | 5 | 1.38 | (0.45-3.21) | 4 | 1.57 | (0.43-4.03) | 3 | 1.13 | (0.23-3.30) |
| Brain, central nervous system | 6 | 3.04 | 9.42‡ | (3.46-20.50) | 4 | 11.27‡ | (3.07-28.86) | <3 | 14.00‡ | (1.70-50.59) | 0 | § | (0.00-26.45) |
| Hodgkin lymphoma | 58 | 5.8 | 16.65‡ | (12.64-21.52) | 29 | 24.34‡ | (16.30-34.96) | 22 | 22.84‡ | (14.31-34.58) | 7 | 5.27‡ | (2.12-10.85) |
| Non-Hodgkin lymphoma | 158 | 4.81 | 5.85‡ | (4.97-6.83) | 76 | 6.13‡ | (4.83-7.67) | 59 | 7.44‡ | (5.66-9.59) | 23 | 3.44‡ | (2.18-5.16) |
| Myeloma | 62 | 11.16 | 10.43‡ | (7.99-13.37) | 37 | 8.75‡ | (6.16-12.05) | 22 | 17.18‡ | (10.77-26.01) | 3 | 6.90‡ | (1.42-20.17) |
| First primary malignancy . | . | . | . | . | By latency . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall . | 1.0-4.9 y . | 5.0-9.9 y . | ≥10.0 y . | ||||||||||
| N . | EAR/10 000* . | SIR* . | (95% CI) . | N . | SIR* . | (95% CI) . | N . | SIR* . | (95% CI) . | N . | SIR* . | (95% CI) . | |
| Total† | 801 | 3.04 | 4.70‡ | (4.38-5.04) | 487 | 6.07‡ | (5.54-6.64) | 223 | 4.62‡ | (4.03-5.26) | 91 | 2.17‡ | (1.74-2.66) |
| Buccal cavity, pharynx | 5 | 0.58 | 1.67 | (0.54-3.90) | 4 | 2.37 | (0.64-6.06) | <3 | 1.22 | (0.03-6.77) | 0 | § | (0.00-7.69) |
| Esophagus | 6 | 3.65 | 3.84‡ | (1.41-8.36) | 4 | 3.87‡ | (1.06-9.92) | <3 | 2.66 | (0.07-14.81) | <3 | 6.55 | (0.17-36.52) |
| Stomach | 5 | 1.56 | 2.36 | (0.77-5.50) | 3 | 2.43 | (0.50-7.11) | <3 | 4.33 | (0.52-15.66) | 0 | § | (0.00-8.64) |
| Colon | 25 | 0.2 | 1.16 | (0.75-1.71) | 11 | 1.06 | (0.53-1.89) | 7 | 1.07 | (0.43-2.20) | 7 | 1.50 | (0.60-3.10) |
| Rectum, rectosigmoid junction | 15 | 0.25 | 1.20 | (0.67-1.98) | 7 | 1.11 | (0.45-2.29) | 4 | 1.05 | (0.29-2.68) | 4 | 1.68 | (0.46-4.31) |
| Anus, anal canal, anorectum | 6 | 2.21 | 3.24‡ | (1.19-7.04) | 3 | 3.70 | (0.76-10.80) | <3 | 3.43 | (0.42-12.39) | <3 | 2.18 | (0.06-12.12) |
| Biliary tract | 4 | 4.58 | 5.52‡ | (1.50-14.13) | 3 | 5.77‡ | (1.19-16.86) | <3 | 7.59 | (0.19-42.29) | 0 | § | (0.00-50.48) |
| Larynx | 3 | 2.14 | 3.02 | (0.62-8.81) | 0 | § | (0.00-6.54) | 3 | 10.33‡ | (2.13-30.19) | 0 | § | (0.00-26.26) |
| Lung, bronchus | 65 | 6.49 | 6.82‡ | (5.26-8.69) | 54 | 7.96‡ | (5.98-10.39) | 9 | 4.70‡ | (2.15-8.91) | <3 | 2.38 | (0.29-8.61) |
| Small-cell | 32 | 11.13 | 11.04‡ | (7.55-15.58) | 27 | 14.28‡ | (9.41-20.78) | 3 | 4.51 | (0.93-13.17) | <3 | 5.84 | (0.71-21.09) |
| Non–small-cell | 32 | 4.35 | 4.87‡ | (3.33-6.88) | 26 | 5.37‡ | (3.51-7.87) | 6 | 4.84‡ | (1.78-10.54) | 0 | § | (0.00-7.55) |
| Bones and joints | 4 | 6.21 | 19.25‡ | (5.25-49.30) | <3 | 28.53‡ | (3.45-103.05) | <3 | 40.65‡ | (4.92-146.86) | 0 | § | (0.00-41.71) |
| Melanoma of the skin | 3 | 2.78 | 4.50 | (0.93-13.15) | <3 | 4.84 | (0.12-26.99) | 0 | § | (0.00-25.44) | <3 | 6.35 | (0.77-22.92) |
| Soft tissue (including heart) | 11 | 8.67 | 14.41‡ | (7.20-25.79) | 7 | 23.86‡ | (9.59-49.16) | 3 | 14.63‡ | (3.02-42.74) | <3 | 3.78 | (0.10-21.06) |
| Female breast | 223 | 2.15 | 4.60‡ | (4.02-5.25) | 160 | 8.60‡ | (7.32-10.05) | 40 | 2.70‡ | (1.93-3.68) | 23 | 1.53 | (0.97-2.29) |
| Cervix | 6 | 2.92 | 7.29‡ | (2.67-15.86) | 4 | 9.36‡ | (2.55-23.96) | <3 | 8.60‡ | (1.04-31.07) | 0 | § | (0.00-22.56) |
| Endometrium | 9 | 4.16 | 5.97‡ | (2.73-11.33) | 6 | 7.80‡ | (2.86-16.98) | <3 | 2.55 | (0.06-14.20) | <3 | 5.78 | (0.70-20.87) |
| Ovary | 72 | 5.66 | 8.68‡ | (6.79-10.94) | 44 | 12.07‡ | (8.77-16.20) | 22 | 10.81‡ | (6.77-16.37) | 6 | 2.30 | (0.84-5.00) |
| Prostate | 8 | 3.62 | 2.95‡ | (1.27-5.80) | 7 | 5.01‡ | (2.01-10.32) | <3 | 1.11 | (0.03-6.17) | 0 | § | (0.00-8.87) |
| Testis | 8 | 1.34 | 6.34‡ | (2.74-12.48) | 4 | 16.01‡ | (4.36-40.99) | 3 | 10.64‡ | (2.19-31.11) | <3 | 1.37 | (0.03-7.62) |
| Urinary bladder | 12 | 0.58 | 1.36 | (0.70-2.37) | 5 | 1.38 | (0.45-3.21) | 4 | 1.57 | (0.43-4.03) | 3 | 1.13 | (0.23-3.30) |
| Brain, central nervous system | 6 | 3.04 | 9.42‡ | (3.46-20.50) | 4 | 11.27‡ | (3.07-28.86) | <3 | 14.00‡ | (1.70-50.59) | 0 | § | (0.00-26.45) |
| Hodgkin lymphoma | 58 | 5.8 | 16.65‡ | (12.64-21.52) | 29 | 24.34‡ | (16.30-34.96) | 22 | 22.84‡ | (14.31-34.58) | 7 | 5.27‡ | (2.12-10.85) |
| Non-Hodgkin lymphoma | 158 | 4.81 | 5.85‡ | (4.97-6.83) | 76 | 6.13‡ | (4.83-7.67) | 59 | 7.44‡ | (5.66-9.59) | 23 | 3.44‡ | (2.18-5.16) |
| Myeloma | 62 | 11.16 | 10.43‡ | (7.99-13.37) | 37 | 8.75‡ | (6.16-12.05) | 22 | 17.18‡ | (10.77-26.01) | 3 | 6.90‡ | (1.42-20.17) |
Exact cell counts with <3 patients are suppressed to protect patient confidentiality.
SIRs and EARs are unadjusted.
All first primary malignancies excluding leukemia and nonmelanoma skin cancer.
Indicates P < .05.
Indicates SIR not presented because of zero observed cases.
tAML risks tended to decline with increasing time since initial cancer diagnosis (Table 2). tAML risks were particularly high in the first 5 years and then declined substantially after lung, breast, and endometrial cancers. There was no evidence of significantly elevated tAML risks at 10 years or longer after any nonhematologic malignancy, whereas significantly elevated three- to sixfold increased risks persisted for 10 years or longer after HL, NHL, and myeloma.
We conducted more detailed analyses for malignancies associated with at least 50 tAMLs, using multivariate Poisson regression models to evaluate observed differences in SIRs and EARs, with adjustment for age and other key confounding variables (specified in Figures 1 and 2). Analyses by calendar period revealed striking changes in tAML risk during 1975-2008 (Figure 1; supplemental Table 1). SIRs for tAML increased in more recent years after initial chemotherapy for NHL (multivariate Poisson Ptrend < .001), whereas SIRs declined after ovarian cancer (Ptrend < .001), myeloma (Ptrend = .023), and possibly lung cancer (Ptrend = .183). After breast cancer, tAML SIRs were highest during the earliest period (1975-1978), significantly lower in the 1980s, and nonsignificantly higher during the 1990s (Phomogeneity = .005). The elevated SIR in the most recent period (2003-2008) mainly reflects the high tAML risks concentrated in the initial years after breast cancer diagnosis and was not significantly higher than previous years in multivariate Poisson regression analyses that included adjustment for latency. For HL, SIRs also varied substantially with time (Phomogeneity = .007; Ptrend = .012), with high risks during 1975-1990, lower risks during 1991-1998, and a nonsignificant increase during 1999-2008. For each of the 6 first primary malignancies, changes in tAML risk by calendar period were consistent when restricted to cases occurring within 5 years after the first primary malignancy diagnosis (supplemental Table 1), and the EAR patterns by calendar year were similar to those for SIRs (data not shown).
In analyses by age, SIRs for tAML were highest among patients treated at younger ages across the 6 first primary malignancies evaluated, although SIRs remained significantly elevated across all age groups (multivariate Poisson comparing older age groups with youngest age group, Ptrend < .01; Figure 2A; supplemental Table 2). In contrast, EARs were significantly higher at older compared with younger age groups after chemotherapy for ovarian cancer (Ptrend = .024, Phomogeneity = .058) and HL (Ptrend = .009; Phomogeneity = .034), but EARs did not differ significantly by age after lung cancer, breast cancer, NHL, or myeloma (Figure 2B; supplemental Table 2). Comparing EARs across all 6 first primary malignancies within each age group, the EAR was consistently lowest for breast cancer; intermediate for lung cancer, ovarian cancer, and NHL; and highest for HL and myeloma.
Combination radiotherapy and chemotherapy nonsignificantly heightened risks for tAML compared with chemotherapy alone for cancers of the lung (ERR ratio, 1.99; 95% CI, 0.97-4.96), breast (ERR ratio, 1.38; 95% CI, 0.98-1.98), and ovary (ERR ratio, 2.02; 95% CI, 0.89-4.10), but not for HL, NHL, or myeloma (Table 3). Changes in tAML risk by calendar period appeared unrelated to use of chemotherapy and radiotherapy vs chemotherapy alone.
SIRs for tAML, overall and by calendar time, by initial chemotherapy with or without radiotherapy for selected first primary malignancies in adulthood, 9 SEER registries, 1975-2008
| . | . | . | . | By calendar period . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Total . | 1975-1982 . | 1983-1990 . | 1991-1998 . | 1999-2008 . | ||||||
| First primary malignancy . | N . | SIR* . | ERR ratio (95% CI)† . | N . | SIR* . | N . | SIR* . | N . | SIR* . | N . | SIR* . |
| Lung, bronchus | |||||||||||
| Chemotherapy without radiotherapy | 14 | 4.24‡ | 1.00 (referent) | <3 | 3.13 | 3 | 4.48 | 6 | 6.16‡ | 4 | 3.00 |
| Chemotherapy with radiotherapy | 51 | 8.17‡ | 1.99 (0.97-4.96) | 6 | 15.06‡ | 9 | 8.93‡ | 17 | 6.91‡ | 19 | 8.01‡ |
| Female breast | |||||||||||
| Chemotherapy without radiotherapy | 96 | 3.70‡ | 1.00 (referent) | 20 | 3.42‡ | 23 | 2.80‡ | 32 | 4.18‡ | 21 | 4.98‡ |
| Chemotherapy with radiotherapy | 127 | 5.64‡ | 1.38 (0.98-1.98) | 8 | 4.33‡ | 16 | 4.22‡ | 50 | 5.55‡ | 53 | 6.73‡ |
| Ovary | |||||||||||
| Chemotherapy without radiotherapy | 63 | 8.00‡ | 1.00 (referent) | 24 | 15.54‡ | 17 | 6.87‡ | 11 | 4.59‡ | 11 | 7.52‡ |
| Chemotherapy with radiotherapy | 9 | 21.80‡ | 2.02 (0.89-4.10) | 7 | 28.30‡ | <3 | 11.78 | 0 | § | <3 | 32.22 |
| Hodgkin lymphoma | |||||||||||
| Chemotherapy without radiotherapy | 38 | 16.08‡ | 1.00 (referent) | 9 | 15.68‡ | 18 | 24.87‡ | 4 | 5.57‡ | 7 | 20.14‡ |
| Chemotherapy with radiotherapy | 20 | 17.85‡ | 0.89 (0.47-1.60) | 5 | 18.42‡ | 4 | 14.26‡ | 7 | 21.41‡ | 4 | 16.57‡ |
| Non-Hodgkin lymphoma | |||||||||||
| Chemotherapy without radiotherapy | 130 | 6.32‡ | 1.00 (referent) | 14 | 3.74‡ | 31 | 5.17‡ | 52 | 7.90‡ | 33 | 7.80‡ |
| Chemotherapy with radiotherapy | 28 | 4.33‡ | 0.61 (0.36-0.96) | 0 | § | 6 | 3.69‡ | 7 | 3.01‡ | 15 | 10.43‡ |
| Myeloma | |||||||||||
| Chemotherapy without radiotherapy | 46 | 10.26‡ | 1.00 (referent) | 15 | 16.19‡ | 14 | 12.32‡ | 7 | 5.01‡ | 10 | 9.77‡ |
| Chemotherapy with radiotherapy | 16 | 10.95‡ | 1.02 (0.52-1.85) | 5 | 17.90‡ | 5 | 13.33‡ | 3 | 6.41‡ | 3 | 8.85‡ |
| . | . | . | . | By calendar period . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Total . | 1975-1982 . | 1983-1990 . | 1991-1998 . | 1999-2008 . | ||||||
| First primary malignancy . | N . | SIR* . | ERR ratio (95% CI)† . | N . | SIR* . | N . | SIR* . | N . | SIR* . | N . | SIR* . |
| Lung, bronchus | |||||||||||
| Chemotherapy without radiotherapy | 14 | 4.24‡ | 1.00 (referent) | <3 | 3.13 | 3 | 4.48 | 6 | 6.16‡ | 4 | 3.00 |
| Chemotherapy with radiotherapy | 51 | 8.17‡ | 1.99 (0.97-4.96) | 6 | 15.06‡ | 9 | 8.93‡ | 17 | 6.91‡ | 19 | 8.01‡ |
| Female breast | |||||||||||
| Chemotherapy without radiotherapy | 96 | 3.70‡ | 1.00 (referent) | 20 | 3.42‡ | 23 | 2.80‡ | 32 | 4.18‡ | 21 | 4.98‡ |
| Chemotherapy with radiotherapy | 127 | 5.64‡ | 1.38 (0.98-1.98) | 8 | 4.33‡ | 16 | 4.22‡ | 50 | 5.55‡ | 53 | 6.73‡ |
| Ovary | |||||||||||
| Chemotherapy without radiotherapy | 63 | 8.00‡ | 1.00 (referent) | 24 | 15.54‡ | 17 | 6.87‡ | 11 | 4.59‡ | 11 | 7.52‡ |
| Chemotherapy with radiotherapy | 9 | 21.80‡ | 2.02 (0.89-4.10) | 7 | 28.30‡ | <3 | 11.78 | 0 | § | <3 | 32.22 |
| Hodgkin lymphoma | |||||||||||
| Chemotherapy without radiotherapy | 38 | 16.08‡ | 1.00 (referent) | 9 | 15.68‡ | 18 | 24.87‡ | 4 | 5.57‡ | 7 | 20.14‡ |
| Chemotherapy with radiotherapy | 20 | 17.85‡ | 0.89 (0.47-1.60) | 5 | 18.42‡ | 4 | 14.26‡ | 7 | 21.41‡ | 4 | 16.57‡ |
| Non-Hodgkin lymphoma | |||||||||||
| Chemotherapy without radiotherapy | 130 | 6.32‡ | 1.00 (referent) | 14 | 3.74‡ | 31 | 5.17‡ | 52 | 7.90‡ | 33 | 7.80‡ |
| Chemotherapy with radiotherapy | 28 | 4.33‡ | 0.61 (0.36-0.96) | 0 | § | 6 | 3.69‡ | 7 | 3.01‡ | 15 | 10.43‡ |
| Myeloma | |||||||||||
| Chemotherapy without radiotherapy | 46 | 10.26‡ | 1.00 (referent) | 15 | 16.19‡ | 14 | 12.32‡ | 7 | 5.01‡ | 10 | 9.77‡ |
| Chemotherapy with radiotherapy | 16 | 10.95‡ | 1.02 (0.52-1.85) | 5 | 17.90‡ | 5 | 13.33‡ | 3 | 6.41‡ | 3 | 8.85‡ |
Exact cell counts with <3 patients are suppressed to protect patient confidentiality. Includes first primary malignancies after which at least 50 tAMLs were reported.
SIRs are unadjusted.
Derived from Poisson regression models adjusted for age at first diagnosis of primary malignancy, receipt of initial radiotherapy, sex (for lung and bronchus, Hodgkin lymphoma, non-Hodgkin lymphoma, and myeloma), stage (for lung and bronchus, female breast, ovary), and latency (overall: 1.0-4.9, 5.0-9.9, 10+ years, except for lung and bronchus: 1-4.9, 5+ years).
Indicates P < .05.
Indicates SIR not presented because of zero observed cases.
Because of changing treatment approaches and increases in chemotherapy use with time, we sought to identify newly emerging tAML risks among patients treated in the current era. On the basis of small numbers (≤5), significantly increased tAML risks were observed for patients during 1999-2008 that were not evident before 1999 for cancers of the esophagus (SIR = 6.71), anus (SIR = 6.66), cervix (SIR = 10.72), and prostate (SIR = 9.58); and beginning in the 1990s for cancers of the bones and joints (SIR1991-1998 = 49.33, SIR1999-2008 = 40.55) and endometrium (SIR1991-1998 = 8.89, SIR1999-2008 = 7.57). To increase statistical power, we conducted a secondary analysis in 380 811 patients treated with initial chemotherapy in 16 SEER registries during 2001-2008, which also showed increased risks for each of these sites, except for anal cancer (Table 4).
SIRs for tAML after initial chemotherapy treatment of first primary malignancy in adulthood, 16 SEER registries, 2001-2008
| . | Total patients diagnosed . | Patients who received initial chemotherapy . | tAML among patients who received initial chemotherapy . | |
|---|---|---|---|---|
| First primary malignancy . | N . | N (%) . | N . | SIR* (95% CI) . |
| Total† | 1 333 224 | 380 811 (29) | 408 | 5.72‡ (5.18-6.31) |
| Buccal cavity, pharynx | 32 540 | 12 390 (38) | 5 | 2.24 (0.73-5.23) |
| Esophagus | 7422 | 5147 (69) | 6 | 6.17‡ (2.26-13.42) |
| Stomach | 14 154 | 6868 (49) | 3 | 2.55 (0.53-7.47) |
| Colon | 93 133 | 33 660 (36) | 9 | 1.01 (0.46-1.91) |
| Rectum, rectosigmoid junction | 44 208 | 22 839 (52) | 4 | 0.66 (0.18-1.70) |
| Anus, anal canal, anorectum | 5086 | 3884 (76) | 3 | 3.63 (0.75-10.62) |
| Biliary tract | 14 453 | 5836 (40) | <3 | 2.62 (0.32-9.48) |
| Larynx | 11 324 | 3160 (28) | <3 | 3.14 (0.38-11.35) |
| Lung, bronchus | 92 408 | 46 811 (51) | 35 | 5.11‡ (3.56-7.10) |
| Small-cell | 10 770 | 9505 (88) | 15 | 13.06‡ (7.31-21.54) |
| Non–small-cell | 79 381 | 37 008 (47) | 18 | 3.18‡ (1.88-5.02) |
| Bones and joints | 2301 | 687 (30) | 5 | 98.90‡ (32.11-230.79) |
| Melanoma of the skin | 69 942 | 1137 (2) | <3 | 5.90 (0.15-32.85) |
| Soft tissue (including heart) | 8945 | 2016 (23) | 6 | 23.50‡ (8.62-51.15) |
| Female breast | 255 909 | 116 243 (45) | 151 | 7.86‡ (6.66-9.22) |
| Cervix | 17 012 | 7148 (42) | 4 | 4.98‡ (1.36-12.75) |
| Endometrium | 46 740 | 5258 (11) | 5 | 6.19‡ (2.01-14.45) |
| Ovary | 20 707 | 14 970 (72) | 11 | 4.43‡ (2.21-7.92) |
| Prostate | 300 016 | 1959 (1) | 6 | 7.92‡ (2.91-17.25) |
| Testis | 12 417 | 3252 (26) | 4 | 18.69‡ (5.09-47.85) |
| Urinary bladder | 56 823 | 6048 (11) | <3 | 1.10 (0.13-3.97) |
| Brain, central nervous system | 12 870 | 6283 (49) | 3 | 7.38‡ (1.52-21.56) |
| Hodgkin lymphoma | 10 314 | 8710 (84) | 22 | 22.59‡ (14.15-34.20) |
| Non-Hodgkin lymphoma | 57 476 | 35 282 (61) | 81 | 8.56‡ (6.80-10.64) |
| DLBCL | 18 567 | 15 790 (85) | 31 | 7.34‡ (4.99-10.42) |
| Follicular lymphoma | 13 692 | 7780 (57) | 17 | 8.18‡ (4.77-13.10) |
| Myeloma | 15 732 | 9753 (62) | 15 | 6.58‡ (3.68-10.86) |
| . | Total patients diagnosed . | Patients who received initial chemotherapy . | tAML among patients who received initial chemotherapy . | |
|---|---|---|---|---|
| First primary malignancy . | N . | N (%) . | N . | SIR* (95% CI) . |
| Total† | 1 333 224 | 380 811 (29) | 408 | 5.72‡ (5.18-6.31) |
| Buccal cavity, pharynx | 32 540 | 12 390 (38) | 5 | 2.24 (0.73-5.23) |
| Esophagus | 7422 | 5147 (69) | 6 | 6.17‡ (2.26-13.42) |
| Stomach | 14 154 | 6868 (49) | 3 | 2.55 (0.53-7.47) |
| Colon | 93 133 | 33 660 (36) | 9 | 1.01 (0.46-1.91) |
| Rectum, rectosigmoid junction | 44 208 | 22 839 (52) | 4 | 0.66 (0.18-1.70) |
| Anus, anal canal, anorectum | 5086 | 3884 (76) | 3 | 3.63 (0.75-10.62) |
| Biliary tract | 14 453 | 5836 (40) | <3 | 2.62 (0.32-9.48) |
| Larynx | 11 324 | 3160 (28) | <3 | 3.14 (0.38-11.35) |
| Lung, bronchus | 92 408 | 46 811 (51) | 35 | 5.11‡ (3.56-7.10) |
| Small-cell | 10 770 | 9505 (88) | 15 | 13.06‡ (7.31-21.54) |
| Non–small-cell | 79 381 | 37 008 (47) | 18 | 3.18‡ (1.88-5.02) |
| Bones and joints | 2301 | 687 (30) | 5 | 98.90‡ (32.11-230.79) |
| Melanoma of the skin | 69 942 | 1137 (2) | <3 | 5.90 (0.15-32.85) |
| Soft tissue (including heart) | 8945 | 2016 (23) | 6 | 23.50‡ (8.62-51.15) |
| Female breast | 255 909 | 116 243 (45) | 151 | 7.86‡ (6.66-9.22) |
| Cervix | 17 012 | 7148 (42) | 4 | 4.98‡ (1.36-12.75) |
| Endometrium | 46 740 | 5258 (11) | 5 | 6.19‡ (2.01-14.45) |
| Ovary | 20 707 | 14 970 (72) | 11 | 4.43‡ (2.21-7.92) |
| Prostate | 300 016 | 1959 (1) | 6 | 7.92‡ (2.91-17.25) |
| Testis | 12 417 | 3252 (26) | 4 | 18.69‡ (5.09-47.85) |
| Urinary bladder | 56 823 | 6048 (11) | <3 | 1.10 (0.13-3.97) |
| Brain, central nervous system | 12 870 | 6283 (49) | 3 | 7.38‡ (1.52-21.56) |
| Hodgkin lymphoma | 10 314 | 8710 (84) | 22 | 22.59‡ (14.15-34.20) |
| Non-Hodgkin lymphoma | 57 476 | 35 282 (61) | 81 | 8.56‡ (6.80-10.64) |
| DLBCL | 18 567 | 15 790 (85) | 31 | 7.34‡ (4.99-10.42) |
| Follicular lymphoma | 13 692 | 7780 (57) | 17 | 8.18‡ (4.77-13.10) |
| Myeloma | 15 732 | 9753 (62) | 15 | 6.58‡ (3.68-10.86) |
Exact cell counts with <3 patients are suppressed to protect patient confidentiality.
SIRs are unadjusted.
All first primary malignancies excluding leukemia and nonmelanoma skin cancer.
Indicates P < .05.
Discussion
This comprehensive, population-based evaluation of 426 068 adults treated with chemotherapy during 3 decades revealed substantial changes in tAML risk with time by first primary cancer, after accounting for sporadic occurrences of AML based in the general population and eliminating the selection bias seen in hospital-based series. In particular, we observed increases with time in tAML risk after NHL; decreases after diagnosis of ovarian cancer, myeloma, and possibly lung cancer; and significant heterogeneity after diagnosis of breast cancer and HL. For the first time, we demonstrate newly emerging risks for tAML since 2000 after chemotherapy for cancers of the esophagus, cervix, prostate, and possibly anus; and since the 1990s for cancers of the bones and joints and endometrium. Although SEER lacks data on specific drugs and doses, our results are consistent with changing treatment practices and differential leukemogenicity of specific treatments, with increasing tAML risks after chemotherapy likely associated with increasing use of (neo)adjuvant therapy, multiple courses of therapy, duration/intensity of treatment, and newly introduced agents.
Breast cancer patients comprised 28% of the tAML cases in our study. Consistent with previous studies, we observed especially high tAML risks during 1975-1978, when melphalan was widely used, and much lower risks through the 1980s, coincident with the shift to less leukemogenic cyclophosphamide-based chemotherapy.12 The nonsignificant increase in tAML risks for women diagnosed in the 1990s coincided with the increasing use of anthracyclines; dose-dense regimens; growth factors; and/or, less frequently, use of autologous stem cell transplantation.13-15 Multivariate analyses revealed that the strikingly high SIR for women treated during 2003-2008 mainly reflects the high tAML risks concentrated in the initial years after breast cancer diagnosis. Longer-term follow-up will be needed to better characterize tAML risks among women with current treatments, including taxanes and molecularly targeted agents.16
Previous studies have reported elevated risk for tAML after NHL treated with cyclophosphamide and other alkylating agents, topoisomerase inhibitors, and purine analogues.17,18 Our novel finding of a steady increase in tAML risks after chemotherapy for NHL during the last 3 decades may be explained by more widespread use of subsequent therapy for persistent or relapsed/refractory NHL. This also may explain the similar tAML risks reported after aggressive and indolent lymphomas,19 as well as the persistence of tAML risk that we observed at 10 years or longer after initial cancer diagnosis. The particularly high risk for tAML in the current treatment era (SIR, 10.47) warrants further investigation into potential risks associated with cumulative doses of therapies given for disease relapse/progression, newly introduced cytotoxic and targeted therapies, dose-dense regimens,20 radioimmunotherapy,21 direct or indirect effects of growth factors, as well as immunosuppressive effects of these therapies.22
In contrast, we observed declines in tAML risk with time after chemotherapy for ovarian cancer, myeloma, and possibly lung cancer. Although these 3 malignancies have relatively poor survival outcomes, the risk for tAML remained significantly increased even in the recent period. For ovarian cancer, the striking tAML risk during 1975-1982 and subsequent decline in that risk are consistent with the shift from melphalan to platinum-based chemotherapy coincident with declining use of full pelvic irradiation, as reported previously.23 Of note is that tAML risks appeared relatively constant during the remainder of the study period (1983-2008) as standard treatments shifted from cisplatin to carboplatin and taxanes were introduced. Our findings, restricted to patients known to have received chemotherapy and adjustment for confounders with multivariate analyses, differed from an earlier SEER analysis indicating lower tAML risk after ovarian cancer diagnosed since 1996 compared with previous years.24
Patients with myeloma face some of the highest risks for tAML, likely because melphalan was the mainstay of treatment of much of the study period for younger patients and remains the mainstay for older patients who are not transplant candidates.25,26 The explanation for the observed decline in tAML risk we observed with time is unknown but may be the result of decreases in melphalan dosing and/or duration of therapy.25 The decline was observed in all age groups, even with the introduction of high-dose melphalan followed by autologous stem cell transplantation at ∼1990. The higher SIR in the most recent period warrants further research in light of the introduction of novel agents in the past decade (eg, thalidomide, lenalidomide, bortezomib) used with or without melphalan.25-27
Although the risk for therapy-related leukemia after lung cancer is well established,28-30 we observed a nonsignificant decline in tAML risk with time, possibly reflecting the modest advances in treatment of lung cancer, with platinum-based therapy remaining the cornerstone of treatment since the 1980s and efficacy of second-line therapy only demonstrated in 2000.31 Additional study is needed to assess tAML risks associated with use of second- and third-line agents as well as maintenance therapy. Limited survival duration and the use of topoisomerase inhibitors, particularly for small-cell lung cancer, may account for the substantially increased tAML risks within 5 years after lung cancer diagnosis.
We observed a peak in tAML risk after HL diagnosis in the 1980s, consistent with the high tAML risks reported with the introduction of MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) for HL at ∼1970.32,33 The subsequent decline in tAML risk in the 1990s, also consistent with previous reports, has been attributed to the change to ABVD (Adriamycin, bleomycin, vinblastine, and dacarbazine) as standard chemotherapy, fewer cycles of chemotherapy, and smaller radiotherapy fields and lower doses.33 The persistently elevated tAML risks among HL patients treated in the era of ABVD or alternative regimens34,35 underscore the continued need to develop less toxic treatment.
The highest EARs for tAML were observed after HL and myeloma and the lowest EARs after breast cancer, which is consistent with known differences in the leukemogenicity of specific agents (eg, melphalan and mechlorethamine are more potent leukemogens than cyclophosphamide).5 Although these patterns generally were consistent across all age groups, age was an important modifier of tAML risk. Higher SIRs among younger patients may reflect the low background rate of de novo AML in younger adults in the general population or an intrinsic susceptibility to leukemogenic exposures at a younger age. In contrast, the EARs for tAML were significantly higher at older ages after ovarian cancer and HL, possibly reflecting more intensive and/or prolonged treatment of patients with poorer survival duration.
A unique feature of our study is the ability to quantify risks for tAML after chemotherapy in the current treatment era. We report newly emerging tAML risks coincident with the expanding use of chemotherapy for cancers of the esophagus, anus, cervix, endometrium, and prostate, which have been suggested previously in case reports.36-38 For esophageal, anal, cervical, and endometrial cancers, the emerging tAML risks could be related to the addition of platinum-based regimens to standard treatment approaches for certain patients,39-41 whereas for prostate cancer, risks may be associated with an increasing role of chemotherapy in the (neo)adjuvant setting and for the treatment of castration-resistant disease.42 We also observed an increased risk for tAML after bone and joint cancers since the 1990s, which may be associated with the addition of ifosfamide and etoposide to standard chemotherapy.43 Changes in the intensity and/or duration of treatment along with use of adjunctive supportive care (eg, growth factors) may further contribute to this risk. We did not observe elevated tAML risks after chemotherapy for stomach, colorectal, or bladder cancers. However, the expanding use of platinum-based chemotherapy for stomach, colorectal, and bladder cancers (eg, oxaliplatin, cisplatin) and topoisomerase I inhibitors (eg, irinotecan) for colorectal cancer in the last decade44-46 underscores the importance of continued surveillance for tAML risks in these patients.
The primary limitation of our study was the lack of data on specific drugs, doses, and subsequent treatments of recurrent/progressive disease. Furthermore, SEER does not completely capture all initial chemotherapy because some treatment may be given outside of hospitals or medical centers that regularly report to registries, and the degree of underascertainment may vary by calendar period and first primary malignancy. Thus, our results may not have been representative of the full population of patients who received chemotherapy. Nevertheless, the observed trends in chemotherapy use were consistent with more detailed studies based on subsequent abstraction of medical records and physician verification of treatment (supplemental Figure 1).47-49 Additional limitations included a lack of central review of tAML diagnoses, changes in the definition of AML in the World Health Organization classification, and exclusion of therapy-related myelodysplastic syndromes (which were not reportable to SEER before 2001). However, the effect of these limitations should be minimized because of our comparison of tAML incidence with AML in the general population. Interpretation of tAML risks after certain malignancies (eg, testicular and bladder cancers, melanoma) was limited by the small number of observed cases. Finally, we lacked data on other potential risk factors for AML, such as tobacco use, which might interact with chemotherapy.
In summary, our comprehensive, population-based survey of 426 068 adults treated with chemotherapy during 3 decades reveals significant changes in tAML risk after specific malignancies and quantifies the risk for tAML in the current treatment era. The patterns of risk generally are consistent with changes in treatment regimens with known cytotoxic agents that have leukemogenic potential, although other factors, such as the intensity of treatment and use of growth factors, also may have played a role. With an increasing number of patients receiving cytotoxic agents in the last decade, the overall incidence of tAML likely will increase. Further research is warranted to assess leukemogenicity of new targeted and immunomodulatory agents introduced into the clinic, and increasing use of (neo)adjuvant and maintenance therapies. As such, second malignancies including tAML should be considered as end points in prospective clinical studies of new agents or new uses of standard agents. In addition, patients at the highest risk for tAML should be identified so that such a risk and other treatment sequelae can be weighed against the established benefits of chemotherapy (with or without radiotherapy), particularly for cancer sites associated with favorable long-term survival duration, and prevention efforts implemented where possible.
Presented in abstract form at the 4th International Symposium on Secondary Leukemia and Leukemogenesis, Rome, Italy, March 26, 2011.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Authorship
Contribution: L.M.M., G.M.D., M.A.T., C.J.K., J.F.F., and R.E.C. designed the research; L.M.M., E.S.G., and R.E.C. conducted the statistical analysis; L.M.M. wrote the manuscript; and L.M.M., G.M.D., M.A.T., C.J.K., K.O., E.S.G., J.F.F., and R.E.C. contributed to interpretation of the data and critical evaluation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lindsay M. Morton, Radiation Epidemiology Branch, Division of Cancer Epidemiology and Genetics; National Cancer Institute/NIH/DHHS; 6120 Executive Blvd/EPS 7040/MSC#7238; Rockville, MD 20892; e-mail: mortonli@mail.nih.gov.