Abstract
Vaccination is among the most efficient forms of immunotherapy. Although sometimes inducing lifelong protective B-cell responses, T-cell–mediated immunity remains challenging. Targeting antigen to dendritic cells (DCs) is an extensively explored concept aimed at improving cellular immunity. The identification of various DC subsets with distinct functional characteristics now allows for the fine-tuning of targeting strategies. Although some of these DC subsets are regarded as superior for (cross-) priming of naive T cells, controversies still remain about which subset represents the best target for immunotherapy. Because targeting the antigen alone may not be sufficient to obtain effective T-cell responses, delivery systems have been developed to target multiple vaccine components to DCs. In this Perspective, we discuss the pros and cons of targeting DCs: if targeting is beneficial at all and which vaccine vehicles and immunization routes represent promising strategies to reach and activate DCs.
Introduction
Classic vaccines are among the most cost-effective public health interventions and provide a good example of effective immunotherapy. Their development has been one of trial and error spanning several centuries. Initially, farm animals and humans were inoculated with serous fluid from infected individuals to protect against infectious diseases.1 In the late 18th century, Jenner published a relatively safe immunization strategy, using cowpox to provide cross-immunity against smallpox in humans.2 Many governments worldwide rapidly implemented this procedure, acknowledging its potential to reduce the devastating effect of epidemics on the general population. In the 19th century, Pasteur generated artificially weakened pathogens and used them for vaccination against rabies and anthrax.1 Adjuvants were introduced in the 20th century by Ramon, who showed that vaccine efficacy was enhanced by the addition of substances such as bread crumbs, tapioca, starch oil, or saponin.3 Aluminum salts (alum) were among the first adjuvants to be applied and remain, to date, the most common adjuvant in prophylactic vaccines.4 For decades, alum was the only adjuvant licensed for human use, but its mechanisms of action are only now being understood.5
Despite successful application in many vaccines, the use of alum is limited to vaccines aiming to induce Th2-type immunity. These classic prophylactic vaccines focus mainly on the induction of long-lived T-helper cell–dependent IgG responses. However, therapeutic vaccines for treatment of chronic infections and cancer require strong proinflammatory CD4+ and CD8+ T-cell responses.6 Advanced knowledge in the molecular and cellular mechanisms underlying effective immune responses has revolutionized vaccine development over the past decades. Last year’s Nobel Laureates Beutler, Hoffman, and Steinman made seminal contributions to the two pillars that form the basis of present-day rational vaccine design. Together, with important work by Medzhitov and Janeway, Beutler and Hoffman discovered how Toll-like receptors (TLRs) activate immune cells. This resulted in the broad range of TLR agonists that are currently explored in clinical trials. Steinman discovered the dendritic cell (DC), the key antigen presenting cell (APC) orchestrating adaptive immune responses that is particularly important in effectuating potent CD4+ and CD8+ T-cell responses. Steinman’s work formed the basis for cellular vaccines, such as the licensed Sipuleucel-T,7 and for vaccines specifically targeting antigens to DC surface receptors.8
Although it seems logical in vaccine design to focus on DCs as the most potent APCs, the identification of various subsets complicate the choice.9 Immunologists are vigorously attempting to unravel the biological properties of these subsets to learn how to best reach and activate them, and thus to improve vaccine design. In this Perspective, we discuss recent findings and provide guiding principles for the development of novel vaccine strategies.
Pathogen recognition receptors for the targeting of DC subsets
Most classical vaccines are administered intramuscularly or subcutaneously, where they attract various types of APCs. Upon activation, these APCs begin migrating to the lymph nodes to activate T cells. Depending on the vaccine formulation, particular APC subsets release specific cytokines that contribute to the polarization and fine-tuning of T-cell immunity.10 The concept of direct targeting of DC subsets in situ overcomes the need for cell migration and facilitates the instant delivery of antigen to (cross-) presenting resident DC subsets in the spleen and the lymph nodes. Thus, vaccine components are delivered directly to those APCs that are most potent in mediating CD4+ and CD8+ T-cell immunity. To this end, researchers exploit the differential expression of both intra- and extracellular receptors by DC subsets (Table 1). Many of these receptors are pathogen recognition receptors (PRRs), including C-type lectin receptors (CLRs) and TLRs. While CLRs function mainly as the address label to reach a specific subset, TLRs are used as a target for cell activation.
DC subsets and their properties are grouped by the most prominently associated induced T-cell response
| Immune response . | DC subset . | Uptake receptor . | TLR . | Cross-presentation . | Comments . |
|---|---|---|---|---|---|
| Th1 | BDCA3+ H CD8α+ M | Clec9A, LangerinM, DEC-205, Clec12A, DCAR1M | 1, 2, 3, 4M, 6, 8, 9M | ++ | Treg47 |
| Langerhans | Langerin, DEC-205, Dectin1, Dectin2, DCIRH | 1H, 2, 3, 4M, 6H,M?, 9M | + | CTL induction or licensing97 Th2, Th17, Th22 | |
| Dermal CD1a+ H Dermal CD103+ M | Langerin, DEC-205, MGLH, DCIRH | 3M, 4H | ++ | Th1798 | |
| Th2 | BDCA1+ H CD8α− M | DCIR2M, Clec12A, DCIR, Dectin-1H, DEC-205H, mMGL1M, 2M, | 1, 2, 3H, 4, 5, 6, 7, 8, 9M | + | |
| Th1/Th2 | moDCH | DC-SIGN, DEC-205, MR, DCIR | 1, 2, 3, 4, 5, 6, 8, 10 | ++ | Th17 |
| IFN-I | pDC | Siglec-H, BST-2, BDCA-2H, Clec9AM, Clec12A, Dectin-2, DEC-205H, DCIRH, Dectin-1M, mMGL1M | 1H,M?, 6H,M?, 7, 9 | + | Activation of myeloid DC and NK, B and T cells |
| Immune response . | DC subset . | Uptake receptor . | TLR . | Cross-presentation . | Comments . |
|---|---|---|---|---|---|
| Th1 | BDCA3+ H CD8α+ M | Clec9A, LangerinM, DEC-205, Clec12A, DCAR1M | 1, 2, 3, 4M, 6, 8, 9M | ++ | Treg47 |
| Langerhans | Langerin, DEC-205, Dectin1, Dectin2, DCIRH | 1H, 2, 3, 4M, 6H,M?, 9M | + | CTL induction or licensing97 Th2, Th17, Th22 | |
| Dermal CD1a+ H Dermal CD103+ M | Langerin, DEC-205, MGLH, DCIRH | 3M, 4H | ++ | Th1798 | |
| Th2 | BDCA1+ H CD8α− M | DCIR2M, Clec12A, DCIR, Dectin-1H, DEC-205H, mMGL1M, 2M, | 1, 2, 3H, 4, 5, 6, 7, 8, 9M | + | |
| Th1/Th2 | moDCH | DC-SIGN, DEC-205, MR, DCIR | 1, 2, 3, 4, 5, 6, 8, 10 | ++ | Th17 |
| IFN-I | pDC | Siglec-H, BST-2, BDCA-2H, Clec9AM, Clec12A, Dectin-2, DEC-205H, DCIRH, Dectin-1M, mMGL1M | 1H,M?, 6H,M?, 7, 9 | + | Activation of myeloid DC and NK, B and T cells |
Expression of uptake receptors and TLRs as well as cross-presentation potential is of further importance in the choice of suitable targets. M, mouse; H, human
CLRs facilitate receptor-mediated endocytosis by binding to carbohydrate ligands.11 Although the first targeting experiments involving MHC class II molecules and Fc receptors were carried out in the late 1980s,12,13 the field really kicked off at the beginning of the new millennium with the discovery of many new CLRs on DCs. It was at this time that Steinman and his colleagues first described the targeting properties of antibodies, which recognize the CLR Dec205.14,15 Many more groups subsequently followed this approach and studied a plethora of receptors present on APCs as possible targets for antigen uptake and subsequent (cross-) presentation (Table 2).16-21 A prominent example is the CLR Clec9a that has not only been exploited for antigen targeting, but was recently also shown to bind to filamentous actin released by dying and necrotic cells.22-24 Clec9a was further shown to play a crucial role in antiviral immunity by cross-presentation of virus-infected dead cell material.25,26
Overview of vaccine targeting approaches
| Vaccine . | Species . | Targeting moiety . | Adjuvant . | Adjuvant- antigen linked? . | Reference . |
|---|---|---|---|---|---|
| Protein conjugate | Mouse | αDec-205 Ab | αCD40 Ab, MALP-2, | No | 16,78 |
| Pam3Cys, polyI:C, polyICLC | |||||
| LPS, R848, CpG | |||||
| CpG | Yes | 93 | |||
| αMR Ab | CpG | No | 17 | ||
| αLangerin Ab | PolyI:C, PolyICLC, αCD40 Ab | No | 60 | ||
| αDC-SIGN Ab | None | No | 99 | ||
| αDectin-1 Ab | PolyI:C | No | 18 | ||
| αClec9A Ab | PolyI:C, PolyICLC, αCD40 Ab, | No | 60,34,100 | ||
| Curdlan | |||||
| αSiglec-H Ab | CpG | No | 29,30 | ||
| Lewis-X or -B* | None | No | 101 | ||
| Tn antigen† | CpG, alum, αCD40 Ab | No | 19 | ||
| αBST-2 Ab | PolyI:C | No | 28 | ||
| Human | αDec-205 Ab | CD40 Ligand | No | 102 | |
| αMR Ab | R848, MALP-2, Loxoribine, | No | 103 | ||
| Pam3CSK4, Flagellin, LPS, | |||||
| PolyI:C, CD40 Ligand | |||||
| αDC-SIGN Ab | PolyI:C, R848 | No | 20 | ||
| αDCIR Ab | PolyI:C, LPS, CL075, CD40 | No | 21,104 | ||
| Ligand, CpG-C, Loxoribine | |||||
| αClec9A Ab | PolyI:C, R848 | No | 44,105 | ||
| Oxidized mannan‡ | None | No | |||
| Polymer particle | Mouse | αDec-205 Ab | Particle composition | Yes | 106 |
| αDec-205 Ab | PolyI:C, R848 | Yes | 91 | ||
| Human | αDC-SIGN Ab | PolyI:C, R848 | Yes | 91 | |
| Liposome | Mouse | αDec-205 Ab | IFNγ or LPS | Yes | 96 |
| Mannose‡ | Pam3CAG, Pam2CAG, | Yes | 66 | ||
| Pam2CGD | |||||
| Lewis-X or -B* | — | — | 107 | ||
| Lewis A or tri- | — | — | 108 | ||
| GlcNAc‡ | |||||
| Mannopentaose‡ | — | — | 109 | ||
| Human | αDec-205 Ab | — | — | 110 | |
| αDC-SIGN Ab | — | — | 111 | ||
| Virus | Mouse | CD40 Ligand | CD40 Ligand | Yes | 95 |
| Mutated Sindbis | — | — | 94 | ||
| Virus glycoprotein* | |||||
| Human | αDC-SIGN Ab | — | — | 112 | |
| αCD40 scFv | αCD40 scFv | No | 113 | ||
| CD40 Ligand | CD40 Ligand | Yes | 114 |
| Vaccine . | Species . | Targeting moiety . | Adjuvant . | Adjuvant- antigen linked? . | Reference . |
|---|---|---|---|---|---|
| Protein conjugate | Mouse | αDec-205 Ab | αCD40 Ab, MALP-2, | No | 16,78 |
| Pam3Cys, polyI:C, polyICLC | |||||
| LPS, R848, CpG | |||||
| CpG | Yes | 93 | |||
| αMR Ab | CpG | No | 17 | ||
| αLangerin Ab | PolyI:C, PolyICLC, αCD40 Ab | No | 60 | ||
| αDC-SIGN Ab | None | No | 99 | ||
| αDectin-1 Ab | PolyI:C | No | 18 | ||
| αClec9A Ab | PolyI:C, PolyICLC, αCD40 Ab, | No | 60,34,100 | ||
| Curdlan | |||||
| αSiglec-H Ab | CpG | No | 29,30 | ||
| Lewis-X or -B* | None | No | 101 | ||
| Tn antigen† | CpG, alum, αCD40 Ab | No | 19 | ||
| αBST-2 Ab | PolyI:C | No | 28 | ||
| Human | αDec-205 Ab | CD40 Ligand | No | 102 | |
| αMR Ab | R848, MALP-2, Loxoribine, | No | 103 | ||
| Pam3CSK4, Flagellin, LPS, | |||||
| PolyI:C, CD40 Ligand | |||||
| αDC-SIGN Ab | PolyI:C, R848 | No | 20 | ||
| αDCIR Ab | PolyI:C, LPS, CL075, CD40 | No | 21,104 | ||
| Ligand, CpG-C, Loxoribine | |||||
| αClec9A Ab | PolyI:C, R848 | No | 44,105 | ||
| Oxidized mannan‡ | None | No | |||
| Polymer particle | Mouse | αDec-205 Ab | Particle composition | Yes | 106 |
| αDec-205 Ab | PolyI:C, R848 | Yes | 91 | ||
| Human | αDC-SIGN Ab | PolyI:C, R848 | Yes | 91 | |
| Liposome | Mouse | αDec-205 Ab | IFNγ or LPS | Yes | 96 |
| Mannose‡ | Pam3CAG, Pam2CAG, | Yes | 66 | ||
| Pam2CGD | |||||
| Lewis-X or -B* | — | — | 107 | ||
| Lewis A or tri- | — | — | 108 | ||
| GlcNAc‡ | |||||
| Mannopentaose‡ | — | — | 109 | ||
| Human | αDec-205 Ab | — | — | 110 | |
| αDC-SIGN Ab | — | — | 111 | ||
| Virus | Mouse | CD40 Ligand | CD40 Ligand | Yes | 95 |
| Mutated Sindbis | — | — | 94 | ||
| Virus glycoprotein* | |||||
| Human | αDC-SIGN Ab | — | — | 112 | |
| αCD40 scFv | αCD40 scFv | No | 113 | ||
| CD40 Ligand | CD40 Ligand | Yes | 114 |
Representative targeting studies are grouped by vaccine design. In addition, information on the presence and form of adjuvant in the vaccine formulations of these studies is depicted.
Binds to DC-SIGN.
Binds to MGL.
Binds to MR.
However, Clec9a gained its popularity as a putative target because of its more restricted expression by CD8α+ DCs (and plasmacytoid [p] DCs) in the mouse. The CD8α+ DC subset is described in many studies as particularly suited and well-equipped for the cross-presentation of antigen and priming of cytotoxic T lymphocytes (CTLs).27 Therefore, this DC subset is an interesting target for the induction of cellular immunity to fight diseases where strong Th1 responses are regarded as essential. Although pDCs are also implicated in antigen presentation to CD8+ T cells upon antigen targeting to pDC surface receptors,28-30 this cell type’s most striking feature is its potential to produce vast amounts of IFN I, which is crucial for antiviral defense.31 Because monocyte-derived DCs (moDCs), CD8α– DCs, and macrophages were also reported to cross-prime antigen to CD8+ T cells, CLRs expressed by these cell types might gain interest.32,33 Although these studies demonstrate the cross-presentation potential of other cell types, there is compelling evidence for a privileged role for CD8α+ DCs to function as major inducers of potent CTL responses in the murine system.
In humans, the situation is less clear, because only minute numbers of DCs can be gained from blood or from scarce lymphoid material, which complicates research. Recently, BDCA3+ DCs were described as the putative human equivalent of mouse CD8α+ DCs. This was based on phenotypic characteristics, the expression of particular transcription factors,34-38 and the fact that they cross-present antigen to CD8+ T cells.39-44 In addition to BDCA3+ DCs, blood-derived CD1c+ DCs, CD16+ DCs, and moDCs cross-present antigen to specific T-cell clones, albeit to a lesser extent.39,41,42 A study using cells derived from human spleens confirms that cross-presentation is not restricted to BDCA3+ DCs but shared with CD1b/c+ and CD16+ DCs.45 Yet, BDCA3+ (CD141+) DCs isolated from lung, liver, or dermis, or from the migratory fraction of skin-draining lymph nodes, were found to be superior cross-presenters of soluble antigen.46 Despite the fact that they have been exploited mainly for immunostimulation, a recent study reported that BDCA3+ DCs suppressed immune responses by the constitutive production of IL-10 and the induction of regulatory T cells.47 These findings, and other studies reporting cross-presentation of various forms of antigen by pDCs48-50 and CD1a– skin-draining lymph node resident DCs,51 raise the question of whether one universal vaccine target actually exists in humans. Table 1 provides an overview of DC subsets with their respective properties and functions. This can serve as orientation for the choice of target DC subset when aiming at specific immune responses.
TLRs on the other hand represent triggers to mediate adjuvanticity in modern vaccine formulation. Their ligation results in MyD88- or TRIF-dependent signaling and, thus, in the activation of APCs. Such activation in combination with antigenic uptake allows for direct priming of CTLs. Depending on their cellular location, TLRs are specialized in the detection of either extracellular or intracellular pathogens. Intracellular TLRs, which recognize different classes of nucleic acids, are considered particularly efficient targets for well-defined synthetic vaccine adjuvants. Prominent examples are the dsRNA mimetic polyinosinic:polycytidylic acid (polyI:C) as TLR3 agonist that induces type I IFNs and inflammatory cytokines,52 the imidazoquinolines imiquimod and R848 and synthetic polyU strands, which induce signaling by TLRs 7 and/or 8,53-55 and CpG DNA constituting a TLR9 agonist.56,57 Among these adjuvants, imiquimod and polyICLC, a derivative of polyI:C, revealed therapeutic potential in vaccination approaches for cancer, allergy, and infectious diseases in clinical trials. For the design of vaccination strategies, it is important to bear in mind that there are differences in the expression of TLRs between mouse and human DCs. One example is the absence of TLR9 in many human DC subsets considered to be interesting targets for vaccination approaches (Table 1).
Initial clinical trials investigating antigen targeting to APCs, in combination with TLR ligands (TLR-Ls) as adjuvants, are in progress. One encouraging example of targeting APCs is the phase I study by Morse et al., in which targeting of the human chorionic gonadotropin-β chain to the mannose receptor, when coadministered with the growth factor granulocyte macrophage–colony-stimulating factor and the TLR-Ls R848 (resiquimod) and polyICLC, resulted in antigen-specific cellular and humoral immunity.8
Codelivery of antigens and adjuvants by suitable vaccine carriers
To induce immunity rather than tolerance, it is necessary to have an activation signal in addition to the antigen. This concept appears to also apply for studies in which antigen is targeted to surface receptors: the presence of adjuvant induces the activation of effector T cells, whereas in contrast the absence of adjuvant results in immunosuppression.58,59 Interestingly, Idoyaga et al found no difference in the targeting of antigen to different receptors on the same cell in the presence of adjuvant.60 Thus, this ratifies immunostimulatory approaches using various CLRs as mere address labels and has shifted the focus of vaccine design to the DC subsets and how to activate them. Whether such activation should be supplied by mere coadministration of a stimulus, or whether antigen and adjuvant should be physically linked to ensure codelivery to the same cell, still remains a point of discussion. A recent study using nanoparticles to deliver antigen and adjuvant showed better humoral responses upon injection of both agents in separate particles than when combined in a single particle.61 Nevertheless, many studies aiming to induce cellular responses show that physically linking the antigen and adjuvant improves T-cell responses. In addition to chemically cross-linking the components,62,63 studies also compared the concept of packaging antigen and adjuvants into vehicles, such as polymer particles,64,65 liposomes,66 viruslike particles,67 or three-dimensional scaffolds.68 A major advantage of this approach is that the vaccine carrier content is protected from possible degradation and shielded from premature undesired receptor interaction, such as nucleic acids with scavenger receptors.69,70 In Figure 1, we provide an overview of potential vaccine carriers to facilitate codelivery strategies and illustrate their advantages and possible disadvantages. We regard polymers, such as the widely applied poly(lactic-co-glycolic) acid (PLGA), as a good choice for future vaccine formulations. Polymer particles may overcome the general stability issue of liposomes and, in contrast to viruslike particles, constitute nonimmunogenic vehicles, which would allow for possible prime-boost regimens. If biodegradable, polymer particles will gradually release their content over time (days to weeks).
Pros and cons of antigen and adjuvant codelivery vehicles. Arguments for and against the use of various carrier vehicles for (targeted) codelivery of multiple vaccine components.
Pros and cons of antigen and adjuvant codelivery vehicles. Arguments for and against the use of various carrier vehicles for (targeted) codelivery of multiple vaccine components.
Improved antigen processing and presentation as a result of colocalization of antigen and stimulus in the same phagosome can explain why codelivering antigen and adjuvant improves T-cell responses.71 At the same time, this approach ensures activation of the cells that have seen the antigen, which is crucial for efficient CD8+ T-cell priming.72
To target or not to target?
Targeting of antigen to specific DC subsets reduces the required antigen dose substantially and therefore proved an attractive model for the priming of strong T-cell responses.58 Hence, it appears logical to combine the targeting and codelivery strategies to provide specifically the cell of choice with both antigen and adjuvant. In this way, vaccines become concentrated in APCs specialized for antigen (cross-) presentation and T-cell priming, which, as a consequence, could further reduce the overall vaccine dose. Conversely, several studies suggest that multiple DC subsets are required to induce optimal T-cell immunity.73,74 These and other studies also describe a dependency on type I IFN in raising efficient immune responses, which can be produced by various cell types such as pDCs, myeloid DCs, monocytes, or stromal cells.75-78 Together with the controversy over which DC subset may or may not present the best target, this argues against targeting vaccines to single APC subsets and justifies the question: “Targeting DCs—why bother?”
Targeted codelivery of antigen and adjuvant reduces the risk of adverse reactions
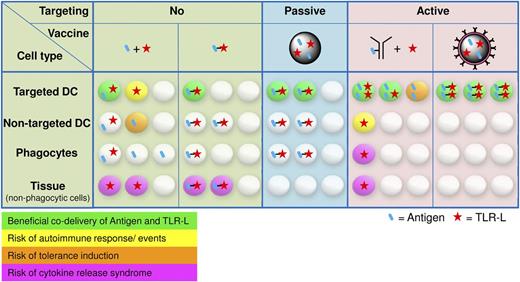
Major arguments in favor of cotargeting antigen and adjuvant lie in a more controlled vaccine application and in a reduced risk of adverse reactions, such as autoimmune responses, induction of tolerance, or unwanted systemic cytokine release (Figure 2). Depending on the frequency and route of vaccine administration, nonphagocytic tissue cells can become overstimulated when exposed to vaccine adjuvants such as TLR agonists. These overstimulated tissue cells may respond with unduly cytokine release, resulting in organ destruction.79,80 Upon intradermal vaccine administration, not only APCs could become activated by TLR agonists, but also keratinocytes, endothelial and mast cells, and fibroblasts or adipocytes, all of which were shown to express TLRs.81 Furthermore, activation of APCs through TLR stimuli in the absence of sufficient antigen could result in the induction of autoimmune responses against the self-antigens presented.82 Indeed, autoimmunity that is dormant, or which has a weak phenotype, can worsen upon stimulation with TLR agonists. This is exemplified by superficial basal cell carcinoma patients experiencing flares of previously well-controlled psoriasis upon treatment with imiquimod (R837) cream.83,84 Thus, their potential to break tolerance, which is so valuable for antitumor therapy, makes TLR agonists at the same time a potentially dangerous means. We believe it is important to administer these powerful agents in a controlled manner. The fact that classical vaccines consisting of pathogen material generally do not cause major health issues may be associated with their local administration. Even more defined adjuvants are not allowed for systemic use, such as the synthetic TLR7 agonist R837 that is currently only approved for topical use in humans, limiting its full potential. Furthermore, separate delivery of antigen and adjuvant may also result in tolerance. This is difficult to substantiate because most vaccine trials merely evaluate whether a vaccine induces the desired humoral or cellular response, not whether tolerance is induced. Adverse reactions to passive targeting using particulate vaccine carriers are unlikely (Figure 2). Here the concomitant uptake of antigen and adjuvant caused by a defined shape and size is naturally restricted to APCs. However, much less antigen will be taken up by the cell of choice compared with the actively targeted approach.
Stochastic visualization of antigen and TLR-L uptake for different vaccine-targeting approaches. The distribution of antigen and TLR-L is based on (A) their physical linkage, (B) their targeting moiety, and (C) their exposure to cells in the various tissues. Depending on the route of vaccine administration, nonphagocytic tissue cells expressing TLRs may become activated.
Stochastic visualization of antigen and TLR-L uptake for different vaccine-targeting approaches. The distribution of antigen and TLR-L is based on (A) their physical linkage, (B) their targeting moiety, and (C) their exposure to cells in the various tissues. Depending on the route of vaccine administration, nonphagocytic tissue cells expressing TLRs may become activated.
Potential targeting strategies: from passive to active targeting
Viruses are known to induce strong cytotoxic T-cell responses in the host and may map the way for successful vaccination strategies. Because the sizes of most viruses span a range from 10 to 300 nm, a possible vaccine mimetic should preferentially fall within this range. Interestingly, this is also the size that allows for lymphatic drainage, whereas larger compounds have to be actively transported to the lymph nodes by peripheral DCs.6,85,86 Whether passive or active transport is favorable also depends on which DC subsets are to be targeted, bearing in mind that a combination of both would allow reaching several subsets and possible collaboration to achieve optimal and prolonged immune responses. This also highlights the importance of the route of vaccine administration: subcutaneous injections allow for size-dependent direct drainage through the lymph; intradermal vaccine administration also relies on active transport by skin DCs to the draining lymph nodes. A less invasive method of applying vaccines into the dermis is currently being investigated in the form of (nano) patches that contain dissolving microneedles.87 The intradermal as well as the classic intramuscular vaccine routes also result in a prolonged vaccine supply and consequently a sustained priming period. Interestingly, some light was recently shed on the poorly studied presence of DC subsets in skeletal muscles, describing subsets of moDCs and both CD8α+ and CD11b+ conventional DCs, which migrate after the uptake of antigen and activation to local lymph nodes.88 Intranasal vaccine application reaches pulmonary or lung DCs, and consequently the respective draining lymph nodes. Here the CD11b– CD103+ DCs were ascribed a major role in the uptake of particulates from the airways and their presentation to CD8+ T cells.89,90 This route bears significant potential for a universal vaccine application, because inhalation of dry-powder vaccines is less invasive and overcomes the need for trained medical practitioners and cold storage problems in less developed countries. Intravenous vaccine administration results in the capture by many tissue-resident DCs in the spleen and lymph nodes but may require shielding or targeted approaches if potent immune modulators are used.91 Another way of reaching these cells is direct vaccine application into the lymph nodes (intranodal), where combined administration of antigen and slow polyI:C-releasing microparticles was shown to induce high frequencies of antigen-specific T cells.92 Unequivocally, the choice for a specific route of administration and vaccine carrier are closely intertwined and together determine how effective the passive targeting strategy will be.
Active receptor-dependent targeting of antigen and adjuvant takes vaccine targeting a step further and has recently been investigated. Although direct antibody-antigen-adjuvant conjugates proved protective in a tumor mouse model, further studies of these conjugates revealed preferential uptake mediated via the exposed antigen peptide and its CpG nucleic acid adjuvant moieties over the antibody-binding specificity.93 A refinement of this concept in which antigen and adjuvant are shielded from possible interactions to ensure specific vaccine delivery could be achieved by using vaccine carriers including viruslike particles, liposomes, and polymer particles. The principle of cotargeting antigen and adjuvant is also applicable for gene therapy and might result in a renaissance of this field. For example, Hangalapura and coworkers targeted tumor-antigen–bearing adenovirus to CD40. Although CD40 expression is not restricted to DCs, its ligation results in activation of this cell, thereby improving the antigen-specific CD8+ T-cell response in a mouse melanoma model.94 Lentiviruses are also explored as vehicles for DC targeting in vivo. Lentiviral vectors with Sendbis virus glycoprotein as a ligand for DC-SIGN induce both humoral and cellular responses as well as protection in a mouse tumor model. The absence of adjuvant in this approach argues for an intrinsic stimulatory potential of this vector.95 Nonviral approaches, such as the use of targeted liposomes by van Broekhoven et al and our use of antibody-coated polymer-based nanoparticles,91,96 can overcome the uncertainties of viral strategies. Although the former study lacks a comparison of targeted vs systemic adjuvant application, we showed that the dose of TLR agonists could be reduced 100-fold upon targeting. Importantly, undesired adjuvant effects, such as high blood cytokine levels and hypothermia, were also reduced in the low-dose targeted approach without compromising vaccine efficacy.
Although passive targeting by altering factors such as size and administration route can influence vaccine distribution to some extent, for higher specificity, vaccines must bear targeting molecules, enabling cell type–specific receptor binding. Actively targeted vaccines cannot only induce potent humoral and cellular responses at reduced antigen and adjuvant dosage, but also allow in combination with shielding strategies the use of highly potent immune modulators by avoiding the risk of adverse effects, even when applied systemically (see also Figure 3).
Pros and cons of cotargeting antigen and adjuvant to dendritic cells. Arguments for and against the concept of cotargeting antigen and adjuvant to particular dendritic cell subsets.
Pros and cons of cotargeting antigen and adjuvant to dendritic cells. Arguments for and against the concept of cotargeting antigen and adjuvant to particular dendritic cell subsets.
The immunologist’s toolbox
Vaccination is by far the greatest success within the field of immunology to date. Current immunotherapeutic approaches seek to continue this success story to treat chronic infection, cancer, and autoimmune disease by refining the classic vaccine approach. One clear trend is the substitution of actual pathogenic material with synthetic mimetics, with TLR-Ls being a good example in this respect. A second development is the shift toward more complex, but highly controlled, vaccine design. A broad spectrum of vaccine carriers harboring multiple components is being evaluated to increase vaccine efficacy and unravel the underlying immunologic mechanisms, while further control over the induced responses is gained by the addition of targeting moieties. Targeted strategies currently focus mainly on distinct APC subsets. In the near future, these could be mixed and matched to trigger different players of the immune system and to fine-tune the desired immune or tolerogenic responses (Figure 4). We envisage that the immunologist’s toolbox, which consists of submicron-sized targeted carriers harboring both antigen and immunomodulators, will teach us how various subsets may be reached and properly activated. At present, the development of complex vaccine carriers targeted to multiple receptors seems an extremely costly pharmaceutical nightmare. Therefore, in humans, passive vaccine targeting, by modulation of vaccine carrier size and shape or route of administration, or actively targeting a single receptor shared by several cell types, might at this stage represent the most promising strategy. We nevertheless feel that further development of the immunologist’s toolbox is essential to unravel the complexity of immune cell cross-talk and anticipate combinations of targeted approaches, which will ultimately allow full exploitation of the immune system for vaccination purposes.
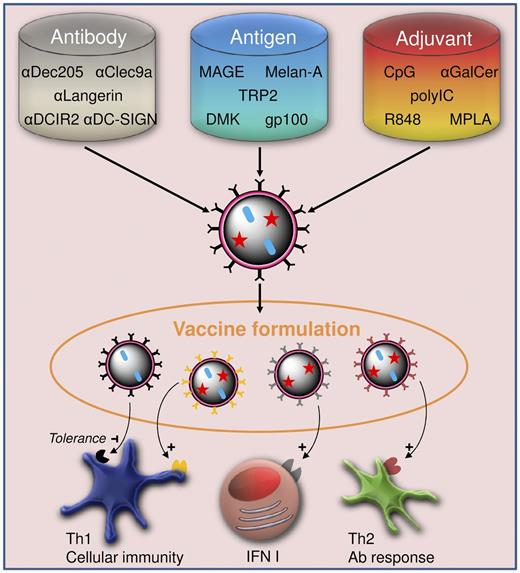
Fine-tuning vaccine formulations to tailor immune responses to diseases. Vehicle preparation from a choice of antigen, adjuvant, and targeting moieties, such as antibodies, results in versatile vaccine formulations. The combination of different vehicles in one vaccine allows not only for a controlled provision of antigen and adjuvant to the targeted cell subsets, but also for tailoring of Th1, Th2, and type I interferon responses to meet the needs for treatment of specific diseases.
Fine-tuning vaccine formulations to tailor immune responses to diseases. Vehicle preparation from a choice of antigen, adjuvant, and targeting moieties, such as antibodies, results in versatile vaccine formulations. The combination of different vehicles in one vaccine allows not only for a controlled provision of antigen and adjuvant to the targeted cell subsets, but also for tailoring of Th1, Th2, and type I interferon responses to meet the needs for treatment of specific diseases.
Acknowledgments
This work was supported by grants from the EU (ERC advanced PATHFINDER 269019) and the Dutch Cancer Society (KUN2009-4402), as well as a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, grant No. FES0908).
C.F. received the NWO Spinoza award.
Authorship
Contribution: M.K. prepared the initial draft of the manuscript. P.J.T. and C.G.F. provided further knowledge, insights, and discussions, and helped in critical review. All authors were involved in the design of the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carl G. Figdor, Department of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Center, Geert Grooteplein 26-28, 6525GA Nijmegen, the Netherlands; e-mail: c.figdor@ncmls.ru.nl