Key Points
Immunization against protamine/heparin complexes was frequently observed in patients undergoing cardiac surgery.
Platelet-activating anti–protamine-heparin antibodies are a potential risk factor for early postoperative thrombosis and thrombocytopenia.
Abstract
Protamine, which is routinely used after cardiac surgery to reverse the anticoagulant effects of heparin, is known to be immunogenic. Observing patients with an otherwise unexplained rapid decrease in platelet count directly after protamine administration, we determined the incidence and clinical relevance of protamine-reactive antibodies in patients undergoing cardiac-surgery. In vitro, these antibodies activated washed platelets in a FcγRIIa-dependent fashion. Using a nonobese diabetic/severe combined immunodeficiency mouse model, those antibodies induced thrombocytopenia only when protamine and heparin were present but not with protamine alone. Of 591 patients undergoing cardiopulmonary bypass surgery, 57 (9.6%) tested positive for anti–protamine-heparin antibodies at baseline and 154 (26.6%) tested positive at day 10. Diabetes was identified as a risk factor for the development of anti–protamine-heparin antibodies. In the majority of the patients, these antibodies were transient and titers decreased substantially after 4 months (P < .001). Seven patients had platelet-activating, anti–protamine-heparin antibodies at baseline and showed a greater and more prolonged decline in platelet counts compared with antibody-negative patients (P = .003). In addition, 2 of those patients experienced early arterial thromboembolic complications vs 9 of 584 control patients (multivariate analysis: odds ratio, 21.58; 95% confidence interval, 2.90-160.89; P = .003). Platelet-activating anti–protamine-heparin antibodies show several similarities with anti–platelet factor 4-heparin antibodies and are a potential risk factor for early postoperative thrombosis.
Introduction
Protamine, a positively charged DNA-binding protein purified from salmon sperm, is administered as protamine sulfate to neutralize the anticoagulant activity of heparin (eg, after cardiopulmonary bypass surgery)1-5 and as a stabilizer of insulin.6 Protamine is immunogenic, and anti-protamine antibodies are found in patients treated with insulin.6,7 We found anti–protamine-heparin antibodies in patients undergoing cardiac surgery,8,9 and Chudasama et al showed high immunogenicity of protamine-heparin complexes in a mouse model.10 Some of these features resemble immunogenicity of complexes between heparin and positively charged platelet factor 4 (PF4), which are well known to induce the causative antibodies of heparin-induced thrombocytopenia, a prothrombotic adverse drug reaction.5,11
After observing patients with acute thrombocytopenia, which was more pronounced than typically expected after cardiopulmonary bypass surgery because of dilution and consumption, we systematically addressed the presence and potential clinical relevance of anti–protamine-heparin antibodies in patients undergoing cardiopulmonary bypass surgery, and characterized the interaction of these antibodies with platelets. We found that platelet-activating anti–protamine-heparin antibodies, present at the time of protamine application, are associated with more pronounced thrombocytopenia and may be a risk factor for early thromboembolic complications in patients undergoing cardiac surgery.
Materials and methods
Patients and sera
The index patient raising suspicion for protamine-induced complications had been referred for laboratory assessment of heparin-induced thrombocytopenia. To define the frequency of anti–protamine-heparin antibodies at the time of cardiac surgery, and the formation of anti–protamine-heparin antibodies after cardiopulmonary bypass, we investigated serum samples of a previously reported cohort of 591 patients undergoing cardiopulmonary bypass surgery.12 Samples were obtained preoperatively (day 0), day 6 and day 10 postoperatively, and from a subgroup 120 days postoperatively, and were stored at −80°C until use. All patients had received protamine to neutralize heparin given during surgery. All patients had been screened for PF4/heparin antibodies. All thromboembolic complications were objectively documented at diagnosis and had already been adjudicated in the aforementioned study, and only then we assessed the patients in a blinded fashion for protamine-reactive antibodies.
Clinical diagnoses were identified according to the patient hospital chart information. Diabetes was defined as either use of hyperglycemic agents or other data according to outpatient records. Hypertension was defined as a systolic blood pressure of more than 140 mm Hg, a diastolic blood pressure of more than 90 mm Hg, or use of antihypertensive therapy at the time of hospital admission. Previous myocardial infarction was defined as either self-reported history or electrocardiographic evidence at hospital admission. Congestive heart failure (CHF) was defined according to outpatient reports or current manifestations suggestive of CHF and impaired heart ejection fraction on echocardiography. Peripheral vascular disease was defined as self-reported history or other data according to outpatient reports.
Serological studies
In vitro, we assessed anti-protamine and anti–protamine-heparin IgG antibodies by enzyme immunoassay (EIA) using microtiter plates coated with protamine sulfate (20 µg/mL; Sigma-Aldrich, Munich, Germany) in a phosphate buffer (pH 7.5) or with protamine-heparin complexes (a mixture of 20 µg/mL of protamine sulfate with an excess of unfractionated heparin). In both cases, plates were saturated with goat serum. Bound antibodies were detected by goat anti-human IgG (cutoff value, 0.50; optical density [OD], 450 nm).
As a “functional” (platelet activation) assay, we used the heparin-induced platelet activation assay (HIPA)13 with minor modifications. Serum was incubated with washed platelets of 4 donors (tested individually) under 6 test conditions: (1) buffer, (2) protamine (2 µg/mL), (3) heparin (0.2 IU/mL), (4) protamine (2 µg/mL) and heparin (0.2 IU/mL), (5) protamine (2 µg/mL) and heparin (100 IU/mL), and (6) protamine (2 µg/mL) and heparin (0.2 IU/mL) and the FcγIIa receptor-blocking monoclonal antibody IV.3 (10 µg/mL) (final concentrations). Platelet aggregation with at least 2 platelet donors’ suspensions in the presence of protamine (or protamine and 0.2 IU/mL of heparin), but not buffer or heparin alone, was deemed positive. Laboratory personnel were blinded to the patients’ clinical data. Serial samples from individual patients were assessed in parallel to avoid interassay variation.
Anti–PF4-heparin antibodies were investigated by an in-house PF4-heparin assay14 and the HIPA test.13 The index patient was assessed by two EIAs for PF4-heparin antibodies: (1) Asserachrom HPIA (Diagostica Stago, Asnières, France) and (2) GTI-IgG (Gen-Probe GTI Diagnostics, Brookfield, WI), and an EIA that is also able to detect anti-IL8 and anti-NAP2 antibodies,15 and by HIPA.
Animal model
We assessed the biologic effects of anti–protamine-heparin antibodies in a nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse model.16,17 Human platelets supplemented with heparin (0.6 IU/mL), protamine (6 µg/mL), heparin (0.6 IU/mL) and protamine (6 µg/mL), or buffer were injected into NOD/SCID mice (The Jackson Laboratory, Bar Harbor, Maine). After 30 minutes (baseline), the IgG fraction of anti–protamine-heparin antibody-positive sera (or controls) was intraperitoneally injected. Survival of human platelets in the mouse circulation and their activation status were assessed (platelet count; CD62p expression) after 60, 120, and 300 minutes. Platelet activation dependency on platelet FcγIIa receptors was investigated using IV.3 F(ab)′2-fragments.
Structural studies
Changes in the secondary structure of protamine on interaction with heparin were studied by circular dichroism spectroscopy in a 5-mm cuvette (Hellma, Müllheim, Germany) using far UV circular dichroism spectra. Wavelength between 185 and 260 nm were recorded using a Chirascan circular dichroism spectrometer (Applied Photophysics, Leatherhead, UK). Protamine (60 µg/mL) was incubated with increasing heparin concentrations at 20°C. Spectra of protamine and protamine-heparin complexes were corrected for baselines, path length, and concentrations to obtain wavelength-dependent mean residue δ ε values of the protamine–heparin complexes.
Statistical analysis
Statistical analyses were performed using GraphPad prism 5. Nonparametric tests were used when data failed to follow a normal distribution. Platelet counts and early postsurgery thrombotic complications were compared in relationship to antibody status. Analysis-of-covariance models to assess the association between antibody status and thromboembolic complications or platelet count were performed by multiple linear regression analysis using SPSS version 20 software (SPSS, Chicago, IL). Models were adjusted for the presence diabetes, hypertension, previous myocardial infarction, CHF, and peripheral vascular disease. Group comparison was performed using the Wilcoxon rank-sum test, and the Fisher exact test with categorical variables. All analyses were 2-tailed; a P value of < .05 was assumed to represent statistical significance.
Ethics
This prospective study has been approved by the ethical committee of the Ernst-Moritz-Arndt University; all patients gave informed consent in accordance with the Declaration of Helsinki. The animal studies were approved by the local authorities.
Results
Index patient
A 50-year-old man underwent mitral valve replacement/aortic valve reconstruction with heparin anticoagulation for cardiopulmonary bypass; the protamine platelet count after protamine administration decreased abruptly from 100 × 109/L to 26 × 109/L. Thereafter, platelet counts increased to 88 × 109/L (day 6). As cardiac function did not recover, the patient was scheduled for a cardiac-assist device placement. Protamine was given again after the cardiopulmonary bypass procedure, and the platelet count again decreased abruptly from 80 × 109/L to 31 × 109/L. Heparin was replaced by argatroban because of suspected heparin-induced thrombocytopenia, with platelet count recovery. However, on postoperative day 7, the patient tested negative for anti–PF4-heparin antibodies in the serum (2 immunoassays and a heparin-induced platelet activation test) and anti-IL8 and anti-NAP2 antibodies (1 immunoassay and 1 functional assay). However, he tested strongly positive for the presence of protamine and heparin on the anti–protamine-heparin immunoassay and functional assay (a preoperative blood sample was not available for testing).
Anti-protamine and anti–protamine-heparin antibodies in 591 patients undergoing cardiac surgery
In this cohort study, 591 patients were enrolled, of whom 14 (2.4%) tested positive for anti-protamine IgG antibodies (only protamine-coated) before surgery (mean OD, 0.96 ± 0.55). By day 6, an additional 16 (2.8%) of 578 patients seroconverted to an OD of more than 0.5 (mean OD, 1.13 ± 0.61); by day 10, an additional 19 (3.3%) of 578 patients had seroconverted (mean OD, 1.46 ± 0.76) (Figure 1). Three patients no longer had seropositivity (n = 1 until day 6; n = 2 until day 10). In total, 46 (8.0%) of 578 patients tested positive for anti-protamine IgG at day 10 postsurgery.
Prevalence of anti-protamine and anti–protamine-heparin antibodies in patients undergoing cardiac surgery at different time points. Binding of IgG to either protamine (left) or protamine-heparin complexes (right panel) was assessed by EIA in patients undergoing cardiopulmonary bypass (n = 591) before surgery (day 0) and at day 6 and day 10 after surgery. In a subgroup of 161 patients, a follow-up sample was available more than 120 days after surgery.
Prevalence of anti-protamine and anti–protamine-heparin antibodies in patients undergoing cardiac surgery at different time points. Binding of IgG to either protamine (left) or protamine-heparin complexes (right panel) was assessed by EIA in patients undergoing cardiopulmonary bypass (n = 591) before surgery (day 0) and at day 6 and day 10 after surgery. In a subgroup of 161 patients, a follow-up sample was available more than 120 days after surgery.
However, an even greater number of patients tested positive for anti–protamine-heparin IgG antibodies (protamine-heparin complexes coated): 57 (9.6%) of 591 tested positive at baseline (mean OD, 1.15 ± 0.55), 52 (9.0%) of 578 seroconverted (mean OD, 1.35 ± 0.74) at day 6, and 154 patients (26.6%) tested positive (mean OD, 1.47 ± 0.78) by day 10 (Figure 1). Twenty patients no longer seropositivity (n = 8 until day 6; n = 12 until day 10).
In the functional assay (Figure 2), 24 (15.6%) of the anti–protamine-heparin IgG-positive sera induced platelet activation in the presence of protamine. The reaction was enhanced (shortened lag time) when 0.2 IU/mL of heparin were added (median, 25 minutes [range, 5-40 minutes] vs 14 minutes [range, 5-35 minutes]; P = .047). Platelet activation was inhibited by 100 IU/mL of heparin and by the FcγIIa receptor-blocking IV.3 antibody. None of the sera reacted in the presence of buffer, but 5 sera reacted weakly in the presence of heparin. Interestingly, all but 2 of the platelet-activating sera showed increased binding to protamine-heparin (mean OD, 2.08 ± 0.74) compared with protamine alone (mean OD, 1.88 ± 0.96; P = .025).
Serological characterization of anti–protamine-heparin antibodies: anti–protamine-heparin antibodies are capable of platelet activation in a heparin-dependent manner via cross-linking FcγIIa receptors. Heat-inactivated sera from patients with anti–protamine-heparin antibodies were incubated with washed platelets in the presence of buffer, heparin (0.2 IU/mL), and protamine (2 µg/mL) with or without heparin (0.2 IU/mL). Inhibition studies were performed with protamine plus high heparin (100 IU/mL) or protamine (2 µg/mL) plus heparin (0.2 IU/mL) in the presence of FcγIIa receptor-blocking antibody (IV.3). Platelet aggregation was determined by change in turbidity of the suspension and assessed every 5 minutes, as described.13
Serological characterization of anti–protamine-heparin antibodies: anti–protamine-heparin antibodies are capable of platelet activation in a heparin-dependent manner via cross-linking FcγIIa receptors. Heat-inactivated sera from patients with anti–protamine-heparin antibodies were incubated with washed platelets in the presence of buffer, heparin (0.2 IU/mL), and protamine (2 µg/mL) with or without heparin (0.2 IU/mL). Inhibition studies were performed with protamine plus high heparin (100 IU/mL) or protamine (2 µg/mL) plus heparin (0.2 IU/mL) in the presence of FcγIIa receptor-blocking antibody (IV.3). Platelet aggregation was determined by change in turbidity of the suspension and assessed every 5 minutes, as described.13
Only 1 of 24 sera that activated platelets in the presence of protamine-heparin also showed the typical pattern found in heparin-induced thrombocytopenia,18 that is, activation of platelets in the presence of 0.2 IU/mL of heparin within 30 minutes (indicated by the open circle in Figure 2). Of the 154 patients with anti–protamine-heparin IgG antibodies, 97 (63.0%) also tested positive for anti–PF4-heparin IgG.
Association of platelet-activating anti–protamine-heparin antibodies with postoperative outcomes
In patients with IgG antibodies against protamine-heparin, a history of diabetes was identified as a risk factor for preoperative seropositivity (62.0% vs 36.5%; P < .001). Unfortunately, we did not have available the specific type of treatment the patients had received before hospital admission (insulin or oral hypoglycemic agents). No significant difference was observed regarding a history of hypertension (100% vs 90.4%; P = .221), previous myocardial infarction (6% vs 12.4%; P = .346), or peripheral vascular disease (14.0% vs 17.4%; P = .635) (Table 1).
Demographics of 591 patients who received protamine during cardiopulmonary bypass surgery
| Patient characteristics . | Total no. of patients: 591 (100%) . | Status of protamine-heparin IgG-antibodies at baseline . | P value . | ||
|---|---|---|---|---|---|
| No antibodies (n = 534; 90.4%) . | Non–platelet-activating antibodies (n = 50; 8.4%) . | Platelet-activating antibodies (n = 7; 1.2%) . | |||
| Age (y) | 67.5 ± 9.5 | 67.5 ± 9.7 | 67.6 ± 7.6 | 72.3 ± 8.7 | .414 |
| Female sex (%) | 162 (27.4) | 145 (27.2) | 16 (32.0) | 1 (14.3) | .688 |
| Body mass index | 29.0 ± 4.6 | 28.9 ± 4.6 | 30.3 ± 4.3 | 28.9 ± 4.1 | .281 |
| Diabetes (%) | 233 (39.4) | 195 (36.5) | 31 (62.0) | 7 (100) | < .001 |
| Hypertension (%) | 539 (91.2) | 483 (90.4) | 50 (100.0) | 6 (85.7) | .221 |
| Previous myocardial infarction (%) | 70 (11.8) | 66 (12.4) | 3 (6.0) | 1 (14.3) | .346 |
| Congestive heart failure (%) | 5 (0.8) | 2 (0.4) | 3 (6.0) | 0 (0) | .001 |
| Peripheral vascular disease (%) | 102 (17.3) | 93 (17.4) | 7 (14.0) | 2 (28.6) | .635 |
| Patient characteristics . | Total no. of patients: 591 (100%) . | Status of protamine-heparin IgG-antibodies at baseline . | P value . | ||
|---|---|---|---|---|---|
| No antibodies (n = 534; 90.4%) . | Non–platelet-activating antibodies (n = 50; 8.4%) . | Platelet-activating antibodies (n = 7; 1.2%) . | |||
| Age (y) | 67.5 ± 9.5 | 67.5 ± 9.7 | 67.6 ± 7.6 | 72.3 ± 8.7 | .414 |
| Female sex (%) | 162 (27.4) | 145 (27.2) | 16 (32.0) | 1 (14.3) | .688 |
| Body mass index | 29.0 ± 4.6 | 28.9 ± 4.6 | 30.3 ± 4.3 | 28.9 ± 4.1 | .281 |
| Diabetes (%) | 233 (39.4) | 195 (36.5) | 31 (62.0) | 7 (100) | < .001 |
| Hypertension (%) | 539 (91.2) | 483 (90.4) | 50 (100.0) | 6 (85.7) | .221 |
| Previous myocardial infarction (%) | 70 (11.8) | 66 (12.4) | 3 (6.0) | 1 (14.3) | .346 |
| Congestive heart failure (%) | 5 (0.8) | 2 (0.4) | 3 (6.0) | 0 (0) | .001 |
| Peripheral vascular disease (%) | 102 (17.3) | 93 (17.4) | 7 (14.0) | 2 (28.6) | .635 |
As protamine is given near the end of surgery, patients with platelet-activating protamine-reactive IgG antibodies present before surgery might be at higher risk for adverse outcomes. Indeed, the 7 patients with platelet-activating, anti–protamine-heparin antibodies before surgery showed a more pronounced and prolonged decrease in platelet counts compared with the 50 patients with non–platelet-activating anti–protamine-heparin antibodies (Figure 3). Of note, the platelet count course in patients with nonactivating anti–protamine-heparin antibodies did not differ significantly from that in antibody-negative patients (Figure 3). Analysis of covariates and adjustment of the risk to the presence of diabetes, hypertension, previous myocardial infarction, and peripheral vascular disease showed that patients with platelet-activating anti–protamine-heparin antibodies had significantly lower postoperative platelet counts than those with nonactivating anti–protamine-heparin antibodies and those testing negative for such antibodies (P = .004; P = .005, respectively).
Platelet counts of patients after cardiopulmonary bypass. Patients were grouped depending on the presence of anti–protamine-heparin antibodies (absent, group A [n = 534]) and the capability of these antibodies to activate platelets in vitro (non–platelet-activating anti–protamine-heparin antibodies, group B [n = 50]; platelet-activating anti–protamine-heparin antibodies, group C [n = 7]). The means of platelet counts at the corresponding day after surgery are plotted. The insert table shows the P values comparing the different platelet count curves with each other.
Platelet counts of patients after cardiopulmonary bypass. Patients were grouped depending on the presence of anti–protamine-heparin antibodies (absent, group A [n = 534]) and the capability of these antibodies to activate platelets in vitro (non–platelet-activating anti–protamine-heparin antibodies, group B [n = 50]; platelet-activating anti–protamine-heparin antibodies, group C [n = 7]). The means of platelet counts at the corresponding day after surgery are plotted. The insert table shows the P values comparing the different platelet count curves with each other.
The most important clinical finding is that 7 patients with platelet-activating anti–protamine-heparin antibodies at baseline had an increased risk for early thromboembolic complications. In 2 (28.6%) of these 7 patients, arterial occlusions developed (symptomatic early coronary bypass occlusion in 1 patient, diagnosed at day 2; and,stroke in the other patient, diagnosed at day 3), compared with 9 thromboembolic events occurring by day 7 in the remaining 584 (1.5%) of 591 patients (odds ratio, 25.6; 95% confidence interval, 4.37-149.59; P = .006). After adjustment for the presence of diabetes, arterial hypertension, previous myocardial infraction, CHF, and peripheral vascular disease at baseline by use of logistic regression analysis, platelet-activating protamine-heparin antibodies remained an independent risk factor for early thromboembolic complications (odds ratio, 21.58; 95% confidence interval, 2.90-160.89; P = .003). In all 11 patients with thromboembolic complications after surgery, heparin-induced thrombocytopenia had been ruled out by a negative test result for platelet-activating anti–PF4-heparin antibodies (HIPA), although 2 patients tested weakly positive for PF4-heparin IgG antibodies at day 6 (OD, 0.53; and OD, 0.82). These 2 patients had no platelet-activating protamine-heparin antibodies. Neither mortality nor hospital length of stay differed between the groups.
Persistence of anti–protamine-heparin antibodies
Of the 161 patients in our long-term follow-up study, 43 (26.7%) tested positive for anti–protamine-heparin antibodies at day 10, but only 18 (11.2%) continued to test positive on EIA after more than 120 days of follow-up (P < .001). These 18 patients also had considerably decreased OD (P < .001; Figure 1); of these, 12 (66%) had diabetes.
In vivo characterization of anti–protamine-heparin antibodies (animal model)
In the NOD/SCID mouse model (Figure 4), the addition of protamine and heparin together with anti–protamine-heparin IgG strongly decreased platelet survival rate to 23% (range, 20%-28%), whereas destruction of human platelets was only minimally enhanced by anti–protamine-heparin IgG in the presence of protamine alone (platelet survival rate, 77%; range, 71%-80%). Platelet destruction was largely inhibited by FcγIIa-inhibiting IV.3 F(ab)′2-fragments (platelet survival rate, 65%; range, 45%-70%). No enhanced platelet clearance was observed by the administration of IgG from healthy donors in the presence of protamine and heparin (platelet survival rate, 76%; range, 72%-80%) (Figure 4). Platelet counts were compared at 330 minutes after injection of anti–protamine-heparin IgG.
Anti–protamine-heparin antibodies cause platelet activation and destruction in vivo in a heparin-dependent manner via cross-linking the FcγIIa receptor. Human platelets supplemented with protamine, or protamine-heparin, or protamine-heparin and F(ab)´2 fragments of the FcRγIIa-inhibiting monoclonal antibody IV.3, or buffer were injected retro-orbitally into NOD/SCID mice. After 30 minutes (baseline), the number of circulating human platelets was estimated (100%), and the IgG fractions of patient sera containing anti–protamine-heparin antibodies (or the IgG fraction from healthy control participants) were injected intraperitoneally. The percentage survival of human platelets was measured after 60, 120, and 300 minutes. Results are shown as a median (range) of experiments performed with anti–protamine-heparin antibodies obtained from 7 patients after CPB (A). Expression of the platelet activation marker human CD62p was estimated at 30, 60, and 120 minutes after platelet injection (B). The control IgG fraction did not cause platelet elimination (A, open circles and dashed line) and minor expression of CD62p (median fluorescence intensity, 40; range, 35-46) at 120 minutes after platelet injection in the presence of protamine-heparin (not shown).
Anti–protamine-heparin antibodies cause platelet activation and destruction in vivo in a heparin-dependent manner via cross-linking the FcγIIa receptor. Human platelets supplemented with protamine, or protamine-heparin, or protamine-heparin and F(ab)´2 fragments of the FcRγIIa-inhibiting monoclonal antibody IV.3, or buffer were injected retro-orbitally into NOD/SCID mice. After 30 minutes (baseline), the number of circulating human platelets was estimated (100%), and the IgG fractions of patient sera containing anti–protamine-heparin antibodies (or the IgG fraction from healthy control participants) were injected intraperitoneally. The percentage survival of human platelets was measured after 60, 120, and 300 minutes. Results are shown as a median (range) of experiments performed with anti–protamine-heparin antibodies obtained from 7 patients after CPB (A). Expression of the platelet activation marker human CD62p was estimated at 30, 60, and 120 minutes after platelet injection (B). The control IgG fraction did not cause platelet elimination (A, open circles and dashed line) and minor expression of CD62p (median fluorescence intensity, 40; range, 35-46) at 120 minutes after platelet injection in the presence of protamine-heparin (not shown).
Decreased platelet survival rate by anti–protamine-heparin antibodies was associated with platelet activation (Figure 4, right panel). The platelet activation marker human CD62p at 150 minutes was much higher expressed in the presence of patient IgG and protamine and heparin (median fluorescence intensity, 117; range, 100-140; 84% of human platelets were positive) compared with protamine alone (median fluorescence intensity, 48; range, 45-68, P = .028; 32% of human platelets were positive). Platelet activation by patient IgG was inhibited by IV.3 F(ab)′2-fragments (median fluorescence intensity, 35; range, 30-45; P = .029; 43% of human platelets were positive). In the presence of buffer and anti–protamine-heparin IgG, huCD62p expression was minimal (median fluorescence intensity, 25; range, 18-28; 12% of human platelets were positive). Control IgG did not increase CD62p expression on circulating platelets either in the presence of buffer (median fluorescence intensity, 34; range, 30-45) or protamine alone (median fluorescence intensity, 36.5; range, 30-42), or pro-tamine and heparin (median fluorescence intensity, 40; range, 35-46).
Characterization of protamine–heparin complexes
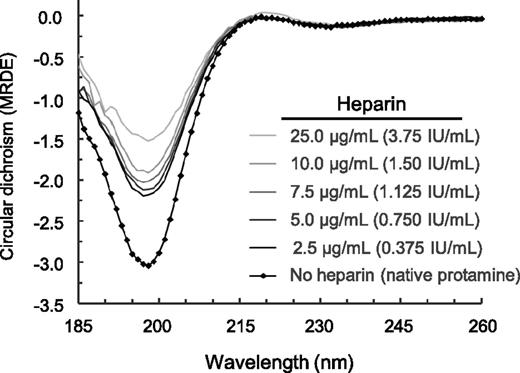
In circular dichroism spectroscopy, the spectrum of protamine showed a negative band at 198 nm, which is usually attributed to the presence of random coil structures (Figure 5). Increasing heparin concentrations decreased the ellipticity values, indicating a decrease in the respective secondary structure content on a qualitative level.
Changes in the secondary structure of protamine upon interaction with heparin. The complex formation was carried out at 20°C directly within the circular dichroism cuvette and was recorded using far UV circular dichroism spectra (185-260 nm) with a Chirascan circular dichroism spectrometer. The spectra of protamine and of protamine-heparin complexes were corrected for the baselines, path length, and concentration to obtain the wavelength-dependent mean residue δ ε values of the protamine-heparin complexes. Note that increasing heparin concentrations decreased the ellipticity values, indicating a decrease in the respective secondary structure content on a qualitative level.
Changes in the secondary structure of protamine upon interaction with heparin. The complex formation was carried out at 20°C directly within the circular dichroism cuvette and was recorded using far UV circular dichroism spectra (185-260 nm) with a Chirascan circular dichroism spectrometer. The spectra of protamine and of protamine-heparin complexes were corrected for the baselines, path length, and concentration to obtain the wavelength-dependent mean residue δ ε values of the protamine-heparin complexes. Note that increasing heparin concentrations decreased the ellipticity values, indicating a decrease in the respective secondary structure content on a qualitative level.
Discussion
This study shows that the preoperative presence of platelet-activating anti–protamine-heparin antibodies in cardiac surgery using heparin anticoagulation during cardiopulmonary bypass with protamine neutralization is associated with early and prolonged postoperative thrombocytopenia and indicates that these antibodies may also be associated with an increased risk for arterial thrombosis. This finding bears several similarities to heparin-induced thrombocytopenia: both disorders are characterized by IgG class antibodies that activate platelets through FcγIIa receptors; both are associated with thrombocytopenia; and both heparin and protamine administration in association with cardiac surgery are associated with very high frequencies (25%-50%) of subsequent, but transient, formation of anti–PF4-heparin and anti–protamine-heparin antibodies, respectively. However, a major clinical difference exists between protamine-induced thrombocytopenia and heparin-induced thrombocytopenia: whereas protamine-induced thrombocytopenia seems to be associated with early-onset thrombocytopenia and, potentially, with early thrombotic events (as a consequence of antibodies already detectable at the time of surgery), heparin-induced thrombocytopenia more commonly causes thrombocytopenia and thrombosis at 1 to 2 weeks after surgery because of newly formed antibodies that become detectable beginning ∼5 days after surgery.19,20
Although we found that 26.6% of patients tested positive for anti–protamine-heparin antibodies at day 10 after cardiac surgery, which is similar to findings reported by Lee et al21 and Pouplard et al,22 only a small subset of these antibodies seem to be clinically relevant. As with heparin-induced thrombocytopenia, anti–protamine-heparin antibodies of IgG class with platelet-activating properties evince greater pathogenicity than do non–platelet-activating anti–protamine-heparin antibodies, particularly when present before surgery. The preoperative presence of these antibodies is associated with a more pronounced decrease in postsurgery platelet counts and might even be associated with an increased risk for new arterial thrombotic complications. In our study, 7 patients (1.2%) had these platelet-activating antibodies present before cardiac surgery, of whom early symptomatic bypass graft reocclusion developed in the first patient (day 2) and the other patient experienced a thrombotic stroke (confirmed at day 3). Although such findings may indicate that these antibodies are a risk factor for early thrombotic complications after cardiac surgery, our study does not establish a definite association because of the overall small number of patients affected. In the studies of Lee et al21 and Pouplard et al,22 the protamine-heparin IgG antibodies were not associated with an increased risk for new thrombosis. However, in these studies, the analyses were not stratified for platelet-activating antibodies at baseline. Thus, the clinical effects of the platelet-activating antibodies might have been obscured by the presence of many non–platelet-activating protamine-heparin antibodies, a phenomenon also well known in heparin-induced thrombocytopenia.
An increased risk for early postoperative thrombotic complications would present a contrast to the clinical picture of heparin-induced thrombocytopenia after cardiac surgery. To date, immediate and very early thrombotic complications attributable to the preoperative presence of platelet-activating anti–PF4-heparin antibodies has not been reported, perhaps because of a protective anticoagulant effect of the high amounts of heparin given at cardiac surgery. In contrast, for patients with anti–protamine-heparin antibodies, the platelet-activating effects of these antibodies coincide with an abrupt neutralization of the anticoagulant effects of heparin by administration of protamine.
Our observation of a prolonged decrease in platelet counts in patients with platelet-activating anti–protamine-heparin IgG antibodies raises more questions. Protamine has a short half-life (∼5 minutes).23,24 As protamine is not a circulating endogenous protein like PF4, it is unlikely that a mechanism similar to the one observed in delayed-onset HIT25 (ie, cross-reactivity of PF4-heparin antibodies with PF4 bound to glycosaminoglykans26 ) is also the cause for prolonged thrombocytopenia in patients with protamine-heparin antibodies. It is also unlikely that the antibodies cross-react with epitopes of proteins other than protamine. However, protamine-heparin complexes circulate much longer than protamine alone, as shown by a rebound of the anticoagulant activity of heparin frequently seen several hours after protamine application.27 Also, protamine attached to platelet surfaces may circulate longer or is even taken up into platelet α granules such as abciximab and is later reexposed.28 Another possibility to explain this observation is that protamine-heparin-antibody complexes bind to megakaryocytes and thereby impair megakaryocytopoiesis, as shown for antibodies dependent on the glycoprotein IIb-IIIa antagonist, eptifibatide.29 These theoretical considerations require further study.
In view of the high immunization rate and a prevalence of ∼4% of platelet-activating anti–protamine-heparin antibodies at day 10 after surgery, the transience of anti–protamine-heparin IgG antibodies is an important finding of our study. Most patients who tested strongly positive at day 10 after surgery tested negative in the follow-up sample taken at more than 120 days after surgery, and the remaining positive samples had a lower OD (Figure 1). Thus, if the patient requires a second cardiac procedure after several months or years, the anti–protamine-heparin antibodies will have likely disappeared, allowing safe subsequent exposure to protamine. The transience of anti–protamine-heparin antibodies resembles that reported for antibodies implicated in heparin-induced thrombocytopenia.30 However, if there is subsequent exposure to protamine in patients who still have circulating platelet-activating anti–protamine-heparin antibodies, a risk for thrombotic complications might still be present.
We also provide further insights into the pathogenesis of thrombocytopenia and thrombotic complications induced by anti–protamine-heparin antibodies. These antibodies cause intravascular platelet activation via the platelet FcγIIa receptor, both in vitro and in vivo (mouse model). This mechanism also resembles the well-characterized platelet activation pathway induced by anti–PF4-heparin IgG. Of potential relevance for diabetic patients requiring (protamine-containing) insulin products, intravascular platelet activation was only induced by anti–protamine-heparin antibodies, and only if both protamine and heparin were given together. This is also fully consistent with the observed risk for early arterial thrombosis in patients with platelet-activating antibodies at the time of combined heparin and protamine exposure. Cardiopulmonary bypass itself has prothrombotic and hypercoagulable effects that are likely enhanced by concomitant anti–protamine-heparin antibody-mediated platelet activation, thereby increasing the overall risk for acute arterial thrombosis.
Protamine is purified from salmon sperm, and immunization against protamine can occur in patients with fish allergy1,31,32 and in diabetic patients treated with protamine-containing insulin preparations.6,33 In addition, men who have undergone vasectomy develop antibodies against protamine.7,8,31,34 A limitation of our study was that that we did not have available the types of antidiabetic treatment the patients received before hospital admission (insulin or oral hypoglycemic agents), and whether the patients had undergone vasectomy. Therefore, we can only describe the association of diabetes with an increased risk for development of these antibodies. Nevertheless, it is tempting to hypothesize that protamine in insulin preparations may enhance the risk for formation of anti–protamine-heparin antibodies, but the anti-protamine antibodies seem to differ from those against protamine-heparin complexes. Only a subset of antibodies reacting with protamine-heparin complexes also bound to protamine alone, and in our mouse model, the antibodies required both protamine and heparin to cause platelet activation and thrombocytopenia. Protamine forms immunogenic macromolecular complexes with heparin,10 and we show by circular dichroism spectroscopy that protamine changes its conformation when complexed with heparin. Thus, heparin binding likely induces neoepitopes on protamine. Despite the similarities between the immune responses to protamine-heparin and PF4-heparin, the epitope(s) recognized by platelet-activating anti–protamine-heparin antibodies seem to differ from those recognized by platelet-activating anti–PF4-heparin antibodies: only one of the activating anti–protamine-heparin antibodies caused platelet activation in the presence of heparin alone, which would be a typical reaction pattern for PF4–heparin-reactive antibodies.
We have recently shown that anti–PF4-heparin antibodies potentially resemble an ancient bacterial host defense mechanism, by which a danger signal epitope is expressed when the positively charged protein PF4 binds to bacterial polyanions or heparin.35-37 Protamine seems to be another example of such a mechanism, which may indicate that this type of rapid, but transient, immune reaction toward conformationally altered proteins might be more common than anticipated.
Although our study, together with the recent observations of others (Lee et al21 and Pouplard et al22 ), shows that anti–protamine-heparin antibodies are frequent and are potentially clinically relevant, additional multicenter prospective studies are required to further define the prevalence and clinical consequences of anti–protamine-heparin antibodies. Our study provided the laboratory tools to identify these patients, to differentiate among the various types of anti-protamine antibodies (particularly the pathogenic platelet-activating anti–protamine-heparin antibodies), and to obtain a suitable animal model to further study the pathogenesis of such antibodies.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge the excellent technical support of Jessica Bagemühl, Ulrike Strobel, Annika Krautwurst, Olivia Heidecke, and Astrid Giptner.
This study was supported by the German Federal Ministry for Education and Research (CAN04/006, ZIK-HIKE FKZ 03Z2CN11, 03Z2CN12) and the “Forschungsverbund Molekulare Medizin” of the Ernst-Moritz-Arndt-University Greifswald.
H.Z. is a PhD candidate, and this study is submitted in partial fulfillment of the requirement for a PhD.
Authorship
Contribution: T.B., U.J.S., and A.G. designed the study; M.D., S.B., H.Z., J.A., and T.B. performed the experiments; S.P., S.S., J.A., T.B., and H.Z. collected the data; H.Z., T.K., A.G., T.E.W., and T.B. analyzed and interpreted the data; T.B., S.P., S.S., T.E.W., and A.G. interpreted the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Greinacher, Institute for Immunology und Transfusion Medicine, Ernst-Moritz-Arndt-University Greifswald, Sauerbruchstrasse, 17475 Greifswald, Germany; e-mail: greinach@uni-greifswald.de.



![Figure 3. Platelet counts of patients after cardiopulmonary bypass. Patients were grouped depending on the presence of anti–protamine-heparin antibodies (absent, group A [n = 534]) and the capability of these antibodies to activate platelets in vitro (non–platelet-activating anti–protamine-heparin antibodies, group B [n = 50]; platelet-activating anti–protamine-heparin antibodies, group C [n = 7]). The means of platelet counts at the corresponding day after surgery are plotted. The insert table shows the P values comparing the different platelet count curves with each other.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/15/10.1182_blood-2012-10-460691/4/m_2821f3.jpeg?Expires=1767797077&Signature=ZtwbEdKq2AftkFY-jNbgHREfyxqAbJYy113fz7sNMb72GTBHG~mFnf~HDp0zSMOmbpNPFlgEZCJ1lPLDGzc4T-1qWgAgInqsBkLmewq3ebDkS-8Vcfnk6UabyqOaiK611j7pPwYmATQrBAgsxWPGNn5ppQay7uXiJU6~QfsIm1Mb~0TG8CC~w6G7eFXbIME7VeIUVKYC3NVuy-27RHqo7n9oQ9Efzm43h-Qy3eOVpADtkKwEtcD8cQmMQLD1YfX3KNGgqYpziO4eZkJtrFrLQ7-yfM4wbCAwCivsfXQtjczmF7NaJWES1~WGgyPCS8gvddNI-2V4kxW5sS~1BbjZPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)