In this issue of Blood, Corselli et al1 purify CD146+ pericytes from human adipose or fetal bone marrow and demonstrate that these cells are capable of supporting the self-renewal and proliferation of transplantable human cord blood hematopoietic stem cells (HSCs).
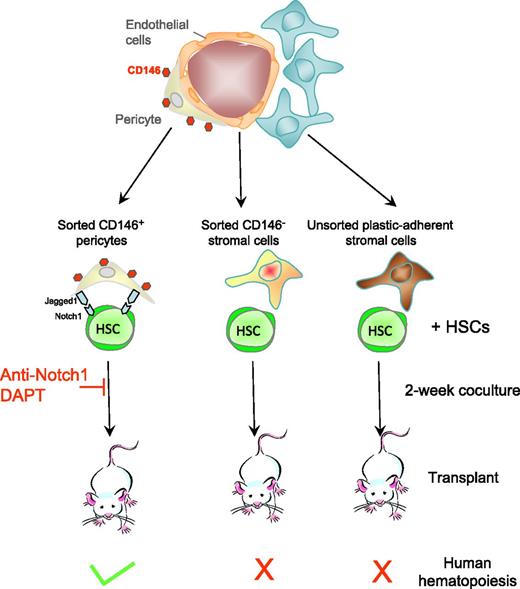
Human adipose or fetal bone marrow. CD146+ pericytes from human adipose and fetal bone marrow support human HSCs in vitro. CD146+ pericytes that ensheath and nurture blood vessels in adipose and fetal bone marrow are sorted as well as CD146− stromal cells. Sorted cells, as well as unfractionated plastic-adherent mesenchymal stromal cells, from these tissues are cocultured with human cord blood CD34+ hematopoietic stem and progenitor cells for 2 weeks, and then transplanted into immunodeficient mice. Only cocultures with CD146+ pericytes generate human HSCs capable of reconstituting human hematopoiesis in mice with long-term self-renewal in serial transplantations. The HSC-supportive effect of pericytes is mediated in part by Jagged expressed on pericytes and Notch1 expressed on HSCs as anti-Notch1 antibodies and the γ-secretase inhibitor DAPT block the supportive effect.
Human adipose or fetal bone marrow. CD146+ pericytes from human adipose and fetal bone marrow support human HSCs in vitro. CD146+ pericytes that ensheath and nurture blood vessels in adipose and fetal bone marrow are sorted as well as CD146− stromal cells. Sorted cells, as well as unfractionated plastic-adherent mesenchymal stromal cells, from these tissues are cocultured with human cord blood CD34+ hematopoietic stem and progenitor cells for 2 weeks, and then transplanted into immunodeficient mice. Only cocultures with CD146+ pericytes generate human HSCs capable of reconstituting human hematopoiesis in mice with long-term self-renewal in serial transplantations. The HSC-supportive effect of pericytes is mediated in part by Jagged expressed on pericytes and Notch1 expressed on HSCs as anti-Notch1 antibodies and the γ-secretase inhibitor DAPT block the supportive effect.
Cord blood is an easily accessible source of HSCs for transplantations. However, the absolute number of HSCs per cord blood unit is generally too low to ensure rapid engraftment and immune reconstitution in adults after transplantation. The ability to expand cord blood ex vivo in a bioreactor would overcome this limitation; however, to date, this has proven difficult to achieve. The main reason for the difficulty? Once isolated from supportive niches in the bone marrow, HSC proliferation is accompanied by rapid differentiation into lineage-committed progenitors that have lost their long-term self-renewal potential and ability to regenerate the whole hematopoietic system after transplantation. Therefore, ex vivo expansion of genuine long-term reconstituting HSCs requires the prior identification of the missing factors from the niche, which is much harder than it sounds because (1) the bone marrow is encased in a hard, mineralized bone and is, consequently, not easy to access and process for cellular and molecular studies and (2) there is a lack of molecular markers for HSC-supportive niche cells.
The first marker identified was STRO-1, an antibody that binds to pericytes that wrap around the vasculature in human bone marrow and dental pulp.2,3 STRO-1+ pericytes can differentiate into osteoblasts and adipocytes and, most importantly, can support the production of human colony forming unit–mix for a few weeks in cocultures.2,3 However, STRO-1 has major shortcomings that prevented its use in clinical applications and further functional characterization of STRO-1+ pericytes. Indeed, STRO-1 (1) identifies a glycosylation motif and (2) is an immunoglobulin M that loses its binding properties upon purification or biochemical modifications (such as covalent binding to a fluorophore, a magnetic bead or biotin). The next breakthrough came from the realization that CD146, also called melanoma cell adhesion molecule or MUC18, is expressed at the surface of pericytes from many different human tissues such as bone marrow, adipose, pancreas, and placenta.3-5 CD146+ human pericytes have myogenic and osteogenic potential and can form ectopic bones containing an ectopic bone marrow when transplanted subcutaneously in immunodeficient mice.4 Although these findings are suggestive that CD146+ pericytes could support hematopoiesis, this has never been experimentally tested.
In this article,1 Corselli et al first demonstrate that CD146+ pericytes from human adult adipose and fetal bone marrow express molecules that are well-known markers of HSC-supportive pericytes in the mouse bone marrow. In cocultures with cord blood lineage-negative CD34+ HSCs, sorted CD146+ pericytes from these 2 tissues are much better at maintaining production of human leukocytes and CD34+ hematopoietic stem and progenitor cells than CD146− stromal cells or unfractionated plastic-adherent mesenchymal stromal cells (see figure). Most importantly, sorted CD146+ pericytes could maintain human HSCs that engraft immunodeficient mice and serially transplant, the hallmarks of long-term reconstituting human HSCs. In sharp contrast, CD146− and unfractionated bone marrow mesenchymal stromal cells could not.
Another important point of the Corselli article is that human HSCs did not require the addition of exogenous recombinant cytokines to be maintained by CD146+ pericytes for 2 weeks. Therefore, these pericytes provide the necessary factors for HSCs to survive, proliferate, and generate differentiated leukocytes while maintaining a pool of undifferentiated reconstituting HSCs. Finally, the supportive effect of CD146+ pericytes required cell-cell contact and was mediated in part by the transmembrane Notch ligand Jagged-1, abundantly expressed at the surface of these pericytes, interacting with its receptor Notch1 expressed on HSCs. Indeed, anti-Notch1 antibodies and DAPT (N-[(3,5-Difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester), an inhibitor of γ-secretase that is necessary to Notch signaling, both inhibited HSC support by sorted pericytes. These results provide compelling evidence that CD146+ from adult and fetal tissues support and maintain human HSCs in vitro. These conclusions are congruent with observations in the mouse also showing that pericytes are necessary to maintain HSCs in the mouse bone marrow in vivo. Indeed, conditional deletion of the Kitl gene, which encodes the ligand of the tyrosine kinase c-kit, in pericytes,6 or ablation of nestin-positive pericytes7 compromise the maintenance of HSCs in the mouse bone marrow.
However, a number of questions remain to be further investigated. The authors did not determine whether CD146+ pericytes could support the actual ex vivo expansion of human reconstituting HSCs by quantifying content in reconstituting HSCs before and after coculture. It would also be interesting to evaluate the supportive effect of pericytes combined with bone marrow endothelial cells. Indeed, studies in the mouse clearly indicate that pericytes are not the sole HSC-supportive stromal cells in the bone marrow as endothelial cells are also essential to maintain HSC in their niche via expression of Kit ligand6 or regulate their proliferation via E-selectin.8 Similar to pericytes, human umbilical vein endothelial cells transformed with an Akt kinase activating adenoviral gene are able to expand transplantable human HSCs.9 Therefore by analogy with mouse bone marrow niches, in which both endothelial cells and pericytes act in concert to maintain HSCs, cultures with a combination of human pericytes and endothelial cells may support human HSC ex vivo expansion longer or more efficiently. Finally, there is some irony in the findings of this article as HSC-supportive pericytes were isolated from adipose collected from liposuctions. While adipocytes seem to be detrimental to HSCs,10 the pericytes that maintain the vasculature feeding adipocytes are beneficial to HSCs once separated from the fat. One of the most abundant and wasteful human tissues in the developed world could finally be put to good use to recipients of HSC transplantations.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal