Key Points
Our data support an important role for the KLF1-BCL11A axis in erythroid maturation and hemoglobin switching.
In adults, gamma-globin levels decline in Bcl11a and Klf1::Bcl11a mutants, suggesting an additional layer of gamma-globin silencing.
Abstract
B-cell lymphoma 11A (BCL11A) downregulation in human primary adult erythroid progenitors results in elevated expression of fetal γ-globin. Recent reports showed that BCL11A expression is activated by KLF1, leading to γ-globin repression. To study regulation of erythropoiesis and globin expression by KLF1 and BCL11A in an in vivo model, we used mice carrying a human β-globin locus transgene with combinations of Klf1 knockout, Bcl11a floxed, and EpoRCre knockin alleles. We found a higher percentage of reticulocytes in adult Klf1wt/ko mice and a mild compensated anemia in Bcl11acko/cko mice. These phenotypes were more pronounced in compound Klf1wt/ko::Bcl11acko/cko mice. Analysis of Klf1wt/ko, Bcl11acko/cko, and Klf1wt/ko::Bcl11acko/cko mutant embryos demonstrated increased expression of mouse embryonic globins during fetal development. Expression of human γ-globin remained high in Bcl11acko/cko embryos during fetal development, and this was further augmented in Klf1wt/ko::Bcl11acko/cko embryos. After birth, expression of human γ-globin and mouse embryonic globins decreased in Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice, but the levels remained much higher than those observed in control animals. Collectively, our data support an important role for the KLF1-BCL11A axis in erythroid maturation and developmental regulation of globin expression.
Introduction
Sickle cell anemia (SCA) and β-thalassemia are the most common monogenic disorders in the human population, with an estimated 300,000 seriously affected children born annually.1 SCA is caused by expression of a pathological β-globin missense mutant (Glu6Val), while β-globin insufficiency underlies β-thalassemia. The symptoms of these diseases are ameliorated by high levels of γ-globin, a β-like globin that is expressed at the fetal stages of human development. Fetal hemoglobin (HbF; α2γ2) is the dominant hemoglobin during fetal liver erythropoiesis. After birth, when the site of erythropoiesis shifts to the bone marrow, γ-globin is gradually replaced by β-globin, and adult hemoglobin (HbA; α2β2) becomes the major hemoglobin. In adults, HbF levels normally decrease to less than 1%.2 For this reason, newborn patients with β-thalassemia or SCA will start manifesting disease symptoms during the first year of life. Since HbF can substitute for HbA in adults, HbF induction would alleviate the symptoms of β-thalassemia and SCA. Currently, hydroxyurea is used in the clinic with considerable success, elevating HbF levels in 40% to 50% of patients with hemoglobin-related disorders.3,4 The variable response of individual patients and the mechanisms by which hydroxyurea induces HbF are poorly understood. Safe pharmacologic reactivation of γ-globin expression therefore remains a very attractive approach.2 Thus, understanding the molecular details of γ- to β-globin switching remains an important goal, because this will reveal novel targets for development of more specific therapeutic intervention.
To study the mechanisms underlying globin switching, human β-globin locus transgenic mice have been established as models for developmental regulation of human β-like globin gene expression.2 The human β-globin locus contains five developmentally regulated globin genes in the order 5′-ε (embryonic) -Gγ-Aγ (fetal) -δ-β (adult) -3′, while the mouse β-globin locus harbors four genes in the order 5′-εy-βh1 (embryonic) -βmaj-βmin (fetal/adult) -3′.2 The α-globin loci in humans and mice contain an embryonic ζ-globin and two fetal/adult α-globin genes.5 In mice, εy, βh1, and ζ are embryonic globins expressed in primitive erythrocytes. Their expression is silenced in definitive erythrocytes, which express α- and β-globins at the fetal and adult stages of development.6 In human β-globin locus transgenic mice, the γ-globin genes behave like embryonic/early fetal genes. Switching to β-globin expression takes place between embryonic day 12 (E12) and E14,7 when the fetal liver is the major site of erythropoiesis. It has recently been demonstrated that this difference in developmental timing of globin switching is linked to alterations in the expression of the B-cell lymphoma 11A (BCL11A) repressor protein, creating a trans-acting environment in the mouse fetal liver that is nonpermissive for γ-globin expression.8
In humans, genome-wide association studies revealed a strong correlation of HbF levels with several single nucleotide polymorphisms located in the BCL11A gene.9,10 Subsequently, BCL11A was reported as a critical mediator of γ-globin silencing in the adult.8,11 It functions as a repressor through binding to cis-regulatory elements in the β-globin locus, and it interacts with the NuRD repressor complex and the GATA1 and FOG1 transcription factors. It binds to the third hypersensitivity site (5′HS3) in the β-globin locus control region and a region downstream of the Aγ-globin gene. BCL11A downregulation in sorted and expanded CD34+ human hematopoietic progenitor cells elevates γ-globin expression. BCL11A depletion does not affect the expression of well-known transcription factors regulating erythropoiesis such as KLF1 (previously known as EKLF12 ), NF-E2, GATA1, and FOG1.11 In contrast, it was recently shown that KLF1 activates BCL11A expression.13,14 KLF1 has a critical role in erythroid development, and in mice, its expression increases threefold upon the transition from primitive to definitive erythropoiesis.15 Analysis of Klf1 knockout embryos revealed lethality at E14.5 due to anemia caused by disrupted fetal liver erythropoiesis.16,17 Remarkably, KLF1 is absolutely required for activation of β-globin expression, while the α-like and embryonic β-like genes are still highly expressed in Klf1 knockout embryos.16,17 Furthermore, Klf1 knockout embryos carrying a human β-globin locus transgene fail to activate the human β-globin gene, while the γ-globin genes are fully expressed.18,19
Mutations in human KLF1 are associated with a spectrum of phenotypes, such as the In(Lu) blood group,20 zinc protoporphyria,21 increased HbA2,22 congenital dyserythropoietic anemia (CDA),23 and hereditary persistence fetal hemoglobin (HPFH).13 Analysis of the HPFH phenotype has led to the proposal that KLF1 has a dual role in γ-globin suppression through its preferential activation of the β-globin gene and as a key activator of expression of the BCL11A repressor protein.13,14 Here, we used mice carrying a human β-globin locus transgene (PAC8.1)7 and crossed them with mice carrying Klf1 knockout,16 Bcl11a floxed, and EpoRCre knockin24 alleles to interrogate the impact of these two key molecules, KLF1 and BCL11A, on erythropoiesis and globin gene regulation.
Materials and methods
Mice
All animal studies were approved by the Erasmus Medical Center Animal Ethics Committee. Transgenic mouse strains used were human β-globin locus transgenic line PAC8.17 ; Klf1 knockout allele16 ; knockin of Cre recombinase in the Epo receptor locus (EpoRCre)24 and Bcl11a floxed alleles (supplemental Figure 1A). Genotyping was performed by polymerase chain reaction (PCR) using DNA isolated from tail snips with the primers listed in supplementary Table 1. Embryos were collected at E14.5 and E18.5; head DNA was used for genotyping by PCR. Inactivation of the Bcl11a gene was analyzed by using PCR to detect Cre-mediated recombination at the Bcl11a locus. To induce stress erythropoiesis, mice were injected subcutaneously with 0.4% (weight to volume ratio [w/v]) phenylhydrazine (Sigma-Aldrich, St. Louis, MO) in saline (12 μL/g body weight) for 2 consecutive days (days 1 and 2). Mice were collected at day 5 for analysis.
Blood analysis
Peripheral blood was collected from the mandibular vein of >10-week-old mice, and standard blood parameters were measured by using an automated hematologic analyzer (Scil Vet ABC, Viernheim, Germany). Blood smears were stained with May-Grünwald-Giemsa and scored double-blinded by K.v.L. Cytospins of peripheral blood were stained with a combination of neutral benzidine and histologic dyes.25
Statistical tests
Statistical analysis of blood parameters was performed by using analysis of variance with Bonferroni correction; flow cytometry data and globin expression results were analyzed by using Mann-Whitney tests. All statistical tests were implemented by Stata 11.1 software (StataCorp, College Station, TX). Excel 2003 (Microsoft, Redmond, WA) was used to draw the graphs. Values plus or minus standard error of the mean are displayed in the figures.
RNA isolation, S1 nuclease protection assays, and QRT-PCR analyses
RNA was isolated from mouse erythroid tissues by using TRI reagent (Sigma-Aldrich). For each S1 nuclease protection assay, 2.5 μg of RNA was used as described.13 Synthesis of complementary DNA and quantitative real-time PCR (QRT-PCR) were performed as described.26 Expression of different globin genes was measured using the control (ct) values; ct values obtained for α-globin expression were used for normalization. The oligonucleotides used for QRT-PCR are listed in supplementary Table 1.
Protein extraction and western blotting
Whole cell lysates were prepared by using radio-immunoprecipitation assay buffer.26 To visualize γ-globin expression at the protein level, whole-cell lysates of ∼3 × 105 red cells were loaded on 12.5% sodium dodecyl sulfate-polyacrylamide gels for electrophoresis, and the gels were transferred to nitrocellulose membranes and probed with γ-globin antibody (catalogue no. sc-21756; Santa Cruz Biotechnology, Santa Cruz, CA). Staining for actin served as loading control (catalogue no. sc-1616; Santa Cruz Biotechnology).
Flow cytometry analysis
Whole blood and single-cell suspensions collected from bone marrow and spleen were washed twice with phosphate-buffered saline and then resuspended in phosphate-buffered saline containing 1% (w/v) bovine serum albumin and 1-mM EDTA. Approximately 106 cells were incubated for 30 minutes with CD71-fluorescein isothiocyanate (CD71-FITC) (553266; BD Biosciences, San Jose, CA) and Ter119-PE (553673; BD Biosciences) antibodies (diluted 1:200) and DRAQ5 (diluted 1:500; Biostatus, Shepshed, UK) in a final volume of 100 μL. The cells were washed, and live cells were distinguished negatively by 7-aminoactinomycin D staining (A1310; Invitrogen, Carlsbad, CA). Cells were measured on a FACScan instrument (BD Biosciences), and data were analyzed with FlowJo software v7.6 (Tree Star, Stanford, CA). Bone marrow cells were sorted by using Ter119 antibody and MACS separation columns according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany).
Erythropoietin assays
Whole blood was centrifuged at 4 krpm for 10 minutes, and sera were collected and stored at −20°C. Erythropoietin concentrations were measured by using an enzyme-linked immunosorbent assay (mouse/rat Epo immunoassay, Quantikine; R&D Systems, Minneapolis, MN).
Immunohistochemistry
Immunohistochemistry for γ-globin expression was performed as described.27
Results
Generation of compound transgenic animals
The role of KLF1 in erythropoiesis has been widely studied,12,13 but much less is known about the function of BCL11A in red cell development. The role of KLF1 and BCL11A in the switching of fetal to adult β-like globin gene expression is of particular interest.13,14 Since mice do not have a fetal β-like globin gene, we used animals carrying a human β-globin locus transgene (line PAC8.17 ) to investigate this. In these animals, the human γ-globin genes are expressed at the embryonic and early fetal stages; the switch from γ- to β-globin expression takes place between E12.5 and E14.5 when the fetal liver is the major site of erythropoiesis.6 We crossed the PAC8.1 mice with mice carrying a knockout allele of Klf116 and a floxed allele of Bcl11a (supplemental Figure 1A-B). To obtain erythroid-specific ablation of BCL11A expression, we used a knockin allele of Cre recombinase in the erythropoietin receptor locus (EpoRCre).24 We studied mice with four genotypes: (1) PAC8.1::Bcl11afl/fl; (2) PAC8.1::Klf1ko/wt::Bcl11afl/fl; (3) PAC8.1::Bcl11afl/fl::EpoRCre/wt; and (4) PAC8.1::Klf1ko/wt::Bcl11afl/fl::EpoRCre/wt. For reasons of clarity, we will refer to these mice as (1) control; (2) Klf1wt/ko; (3) Bcl11acko/cko and (4) Klf1wt/ko::Bcl11acko/cko. To ascertain erythroid-specificity of recombination, DNA was isolated from bone marrow, spleen, and cells from control and Bcl11acko/cko mice. PCR amplification demonstrated Cre-mediated recombination at the Bcl11a locus in Bcl11acko/cko bone marrow and spleen DNA only; the PCR products were sequenced to confirm their identity (supplemental Figure 1C-D). Expression levels of BCL11A in Ter119-MACS–selected adult bone marrow cells of Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice were decreased to less than 30% of those observed in the control mice, in agreement with previous reports,24,28 while a reduction to ∼60% was observed in samples from Klf1wt/ko mice, consistent with the notion that expression of BCL11A is activated by KLF113,14 (supplemental Figure 1E). Mice with ubiquitous BCL11A deficiency die prenatally from unknown causes.29 In contrast, erythroid-specific ablation of BCL11A did not affect viability because we obtained Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko animals at the expected Mendelian ratios (supplemental Table 2). These animals appeared healthy, were fertile, and displayed no gross morphologic abnormalities. Therefore, we first analyzed standard hematologic parameters of adult mice.
Hematologic parameters of compound mutant mice
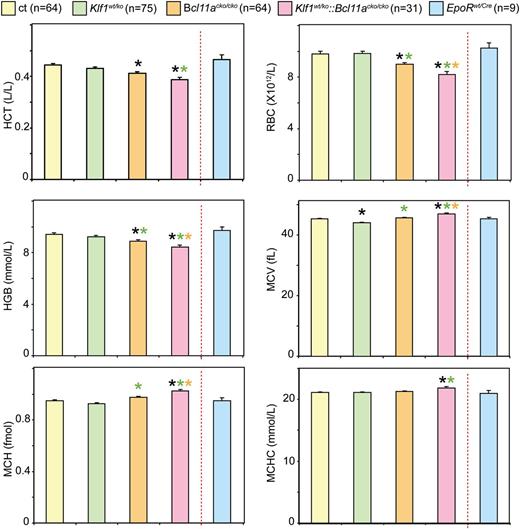
To investigate the impact of BCL11A deficiency, either with or without Klf1 mutation, on adult erythropoiesis, we determined standard hematologic parameters of the mutant mice (Figure 1). With the exception of a small reduction in mean corpuscular volume (MCV), parameters of Klf1wt/ko animals were similar to those observed in the control animals. Bcl11acko/cko animals displayed a small but significant reduction of hematocrit, red blood cell values, and hemoglobin (Hb) values. The reductions in these values were more pronounced in the Klf1wt/ko::Bcl11acko/cko animals. In addition, Klf1wt/ko::Bcl11acko/cko mice displayed small but significantly increased values for MCV, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration. We note that the observed differences were unrelated to the gender of the mice (P > .05 in all cases; data not shown). Collectively, these results indicate that Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice display a mild anemia.
Analysis of hematologic parameters. Hematologic parameter analysis revealed a mild compensated anemia in Bcl11acko/cko and Klf1wt/ko::Bcl11acko/ckomice.*P < .05 between mutant and control groups; P < .05 between mutant groups is indicated by a color-matched asterisk. HCT, hematocrit; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RBC, red blood cell count.
Analysis of hematologic parameters. Hematologic parameter analysis revealed a mild compensated anemia in Bcl11acko/cko and Klf1wt/ko::Bcl11acko/ckomice.*P < .05 between mutant and control groups; P < .05 between mutant groups is indicated by a color-matched asterisk. HCT, hematocrit; HGB, hemoglobin; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RBC, red blood cell count.
Klf1wt/ko and Klf1wt/ko::Bcl11acko/cko mice display reticulocytosis
Blood smears stained with May-Grünwald-Giemsa stain indicated that the percentage of polychromatic erythrocytes was higher in blood from Klf1wt/ko mice and further increased in the blood of Klf1wt/ko::Bcl11acko/cko mice. Furthermore, this analysis suggested that there were more erythrocytes containing nuclear remnants (Howell-Jolly bodies) in the blood of Klf1wt/ko::Bcl11acko/cko mice, compared with that of control, Klf1wt/ko, or Bcl11acko/cko mice (data not shown). Because these results indicated that the KLF1-BCL11A axis plays a role in erythroid maturation, we investigated this in more detail by using flow cytometry.
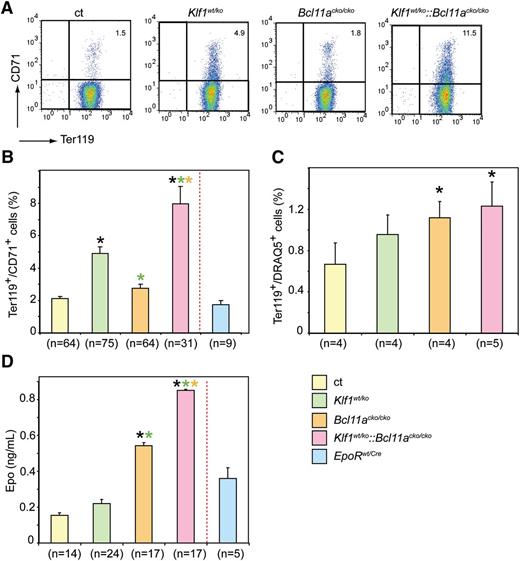
Reticulocytes are usually measured by flow cytometry analysis of the RNA content of RBCs.30 The transferrin receptor (CD71) is expressed by erythroid precursor cells committed to differentiation. Its expression peaks at the basophilic erythroblast stage and gradually decreases during terminal differentiation and maturation. Although the CD71 molecule is absent on mature erythrocytes, it is still present on the surface of circulating immature erythrocytes.31 Indeed, we found that CD71 staining correlates well with Thiazole Orange staining for RNA-containing reticulocytes (data not shown). Reticulocyte counts are higher in young mice and stabilize after 6 weeks of age.32 We therefore included only the analysis of CD71 expression in mice >10 weeks old as marker for reticulocytes and of Ter119 as a marker for all erythroid cells (Figure 2A). We found increased CD71 expression in all mutant animals. This increase was moderate but significant in Klf1wt/ko mice (mean ± sem 4.9 ± 0.40 compared with 2.1 ± 0.12 in control and 2.8 ± 0.23 in Bcl11acko/cko mice). We observed a more dramatic increase in CD71 expression in Klf1wt/ko::Bcl11acko/cko mice (8.0 ± 1.10); this difference was statistically significant compared with differences in all other groups (Figure 2B). In addition, staining with Ter119 and DRAQ5, which is a vital DNA stain, revealed an increased percentage of DRAQ5-positive erythrocytes in all mutants; this was significant in the Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice (1.2 ± 0.16 and 1.2 ± 0.23, respectively, compared with 0.67 ± 0.2 in the controls; Figure 2C).
Flow cytometry of peripheral blood and serum erythropoietin levels. (A) Examples of flow cytometry using CD71 and Ter119 staining of peripheral blood cells. (B) Higher percentage of CD71-positive cells in mutant mice with the most pronounced effect in Klf1wt/ko::Bcl11acko/cko mice. (C) Increased percentage of DRAQ5-positive cells in peripheral blood of mutant mice. (D) Compared with the controls, serum erythropoietin levels were significantly higher in Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice. *P < .05 between mutant and control groups; P < .05 between mutant groups is indicated by a color-matched asterisk. Number of mice is depicted for each experiment separately.
Flow cytometry of peripheral blood and serum erythropoietin levels. (A) Examples of flow cytometry using CD71 and Ter119 staining of peripheral blood cells. (B) Higher percentage of CD71-positive cells in mutant mice with the most pronounced effect in Klf1wt/ko::Bcl11acko/cko mice. (C) Increased percentage of DRAQ5-positive cells in peripheral blood of mutant mice. (D) Compared with the controls, serum erythropoietin levels were significantly higher in Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice. *P < .05 between mutant and control groups; P < .05 between mutant groups is indicated by a color-matched asterisk. Number of mice is depicted for each experiment separately.
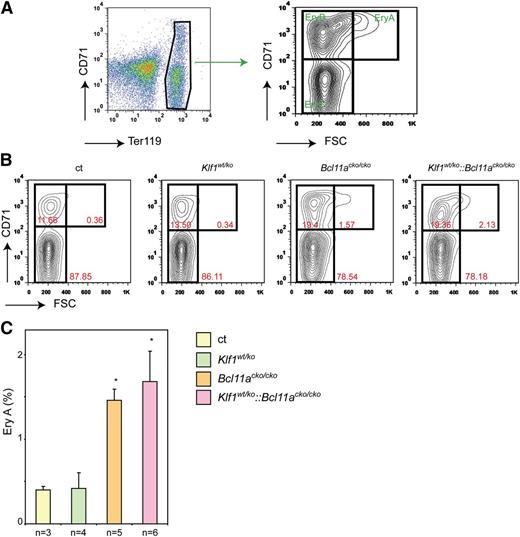
The increase in CD71- and DRAQ5-positive cell expression suggests reticulocytosis that is usually observed in response to anemic stress. Hypoxia stimulates erythropoiesis, thereby increasing the number of reticulocytes released into the circulation. We therefore determined the serum levels of erythropoietin (Epo), the major growth factor driving erythroid expansion. Despite the higher percentage of CD71-positive erythroid cells in peripheral blood, Epo levels in Klf1wt/ko mice were similar to those observed in control mice (Figure 2D). This suggests that the increased percentage of CD71-positive cells is not due to compensated anemia but more likely reflects prolonged erythroid maturation. In contrast, Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice displayed increased Epo levels (Figure 2D), suggesting that they were under anemic stress. In mice, compensatory erythropoiesis (or stress erythropoiesis) occurs exclusively in the spleen. If the increased CD71 expression and Epo levels indicate stress erythropoiesis, we would expect an increased number of spleen erythroblasts. In mutant animals, spleen size and cellularity were not significantly different from those observed in the control animals, indicating that stress erythropoiesis had not been fully activated to compensate for the minor anemic condition. To assess stress erythropoiesis at the cellular level, we investigated the erythroid compartment in the spleen by flow cytometry using the criteria described by Socolovsky et al.33 The gating strategy is depicted in Figure 3A. We observed that the EryA and EryB populations were significantly increased in the Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice (Figure 3B-C), but in Klf1wt/ko animals, these populations were similar to those observed in the controls. This supports the notion that a low stress erythropoiesis response has been activated in Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice, but not in Klf1wt/ko animals.
Flow cytometry analysis of stress erythropoiesis in the spleen. (A) Gating strategy of Ter119 and CD71-stained splenocytes to define the EryA, EryB, and EryC populations.33 The most immature cells are in the EryA population (CD71+, forward scatter (FSC)high). (B) Examples of flow cytometry plots with percentages of EryA, EryB, and EryC populations indicated. (C) EryA populations in the spleens of mice with the four different genotypes. *P < .05 between mutant and control groups.
Flow cytometry analysis of stress erythropoiesis in the spleen. (A) Gating strategy of Ter119 and CD71-stained splenocytes to define the EryA, EryB, and EryC populations.33 The most immature cells are in the EryA population (CD71+, forward scatter (FSC)high). (B) Examples of flow cytometry plots with percentages of EryA, EryB, and EryC populations indicated. (C) EryA populations in the spleens of mice with the four different genotypes. *P < .05 between mutant and control groups.
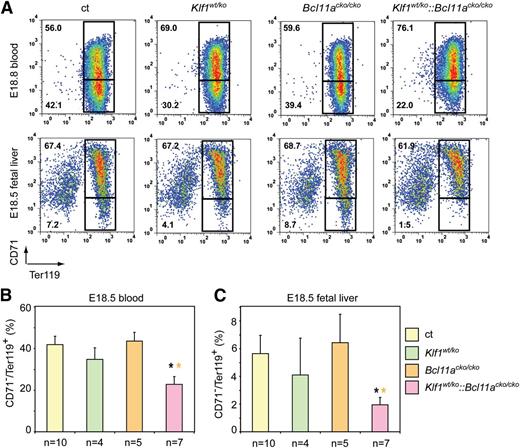
To extend these observations, we analyzed embryonic blood and fetal livers at E18.5, just prior to birth. At this stage, erythropoiesis is highly active to supply the demand of the rapidly growing embryo for more oxygen-carrying capacity. Flow cytometry analysis of E18.5 blood revealed no difference in the CD71+Ter119+ population in peripheral blood of Bcl11acko/cko embryos (55.0 ± 4.2 vs 56.7 ± 4.1 in the controls). This percentage was increased in Klf1wt/ko blood samples (64.2 ± 5.8 vs 56.7 ± 4.1 in the controls) and was highest in blood from Klf1wt/ko::Bcl11acko/cko embryos (75.6 ± 4.1 vs 56.7 ± 4.1 in the controls) (Figure 4A-B). Similar results were obtained following FACS analysis of E18.5 fetal liver cells (Figure 4A,C). Consequently, the percentage of mature CD71−/Ter119+ cells in fetal liver and peripheral blood of E18.5 Klf1wt/ko::Bcl11acko/cko embryos was significantly lower than that observed in Klf1wt/ko, Bcl11acko/cko, and control embryos (Figure 4B-C).
Flow cytometry analysis of blood and fetal liver cells at E18.5. (A) Gating strategy of Ter119 and CD71-stained blood and fetal liver cells of E18.5 embryos. Mature erythrocytes are in the lower quadrant (CD71−Ter119+). (B) The percentage of CD71−Ter119+ cells in E18.5 blood. (C) The percentage of CD71−Ter119+ cells in E18.5 fetal livers. *P < .05 between mutant and control groups.
Flow cytometry analysis of blood and fetal liver cells at E18.5. (A) Gating strategy of Ter119 and CD71-stained blood and fetal liver cells of E18.5 embryos. Mature erythrocytes are in the lower quadrant (CD71−Ter119+). (B) The percentage of CD71−Ter119+ cells in E18.5 blood. (C) The percentage of CD71−Ter119+ cells in E18.5 fetal livers. *P < .05 between mutant and control groups.
Collectively, these results suggest that haploinsufficiency for KLF1 prolongs reticulocyte maturation and that this phenotype is further exacerbated in combination with BCL11A deficiency. Despite this, the impact on erythropoiesis is modest, and none of the compound mutant mice suffer from overt anemia, even at prenatal stages when the demand for erythroid expansion is high. Furthermore, induction of anemia by phenylhydrazine treatment demonstrated that animals of all four genotypes were able to mount a normal stress response, as judged by the increase in spleen weight and the fraction of spleen cells in the EryA population (supplemental Figure 2A-B). Finally, we note that deformability of adult erythrocytes from mutant animals was similar to that observed in the controls, consistent with the normal morphology of the erythrocytes on cytospins and indicating the absence of structural red cell abnormalities (data not shown).
Regulation of γ-globin expression during fetal development
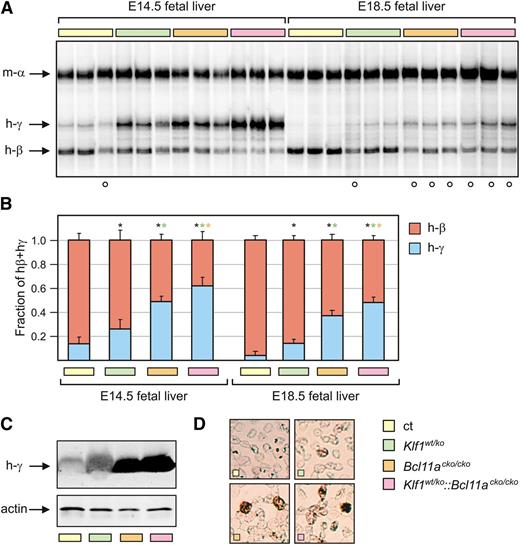
Previously, it has been demonstrated that KLF1 preferentially activates the adult β-globin gene.16,17 By using mice carrying a human β-globin locus transgene, it was shown that switching from γ- to β-globin expression was delayed in a Klf1wt/ko background.19 In addition, it was recently found that KLF1 is a direct activator of BCL11A expression.13,14 To assess the impact of the KLF1-BCL11A axis on expression of the β-like globin genes, we determined globin expression at different developmental stages in erythroid cells derived from mice of all four genotypes. Since γ-globin expression is silenced at approximately E13.5 in PAC8.1 mice,7 we assessed globin expression at E14.5 and at E18.5, the latest possible time before birth. We used total RNA extracted from fetal liver samples for globin expression analysis. The expression of γ- and β-globin messenger RNA was determined directly with S1 nuclease protection assays,13,27 and the γ/(γ + β) ratios were calculated from data obtained with QRT-PCR assays.13 Compared with Bcl11acko/cko embryos, which express high levels of γ-globin,8 Klf1wt/ko embryos displayed lower levels of γ-globin expression but these levels were still significantly higher than those observed in the control samples (Figure 5A-B), consistent with previous results.19 Notably, the highest γ-globin messenger RNA expression was observed in Klf1wt/ko::Bcl11acko/cko embryos. In addition, western blot analysis revealed higher levels of γ-globin protein in E18.5 blood samples from Klf1wt/ko::Bcl11acko/cko embryos compared with those observed in Bcl11acko/cko embryos (Figure 5C), while for both genotypes, these levels were much higher than in the control samples. Consistent with previously reported data,19 γ-globin expression was low in Klf1wt/ko E18.5 erythroid cells, although it was still significantly higher than in the control samples. Finally, to determine whether the increased γ-globin expression had a pancellular or heterocellular distribution, we performed immunohistochemistry on cytospins of E18.5 blood. The number of cells staining positive for γ-globin expression correlated well with the γ-globin levels determined by the S1 nuclease protection and QRT-PCR assays (Figure 5D). This is consistent with a pancellular distribution of γ-globin. Collectively, these data emphasize the dominant role of BCL11A in γ-globin silencing during prenatal development in mice.8 Furthermore, the observation that γ-globin levels are highest in Klf1wt/ko::Bcl11acko/cko embryos lends support to the proposed role of the KLF1-BCL11A axis in this process.13,29
Analysis of γ-globin expression at E14.5 and E18.5. (A) S1 nuclease protection assay on E14.5 and E18.5 fetal liver RNA, detecting human γ-globin (h-γ) and human β-globin (hβ) messenger RNA (mRNA). Mouse α-globin was used as a loading control. (B) QRT-PCR analysis of human globin expression. γ/γ+β ratios are significantly higher in all mutant mice compared with the controls (n = 3 to 5 for each genotype and each time point). (C) Western blot on E18.5 blood samples detecting expression of γ-globin protein. Actin was used as a loading control. (D) Immunohistochemistry of γ-globin expression (brown) in E18.5 blood. The white circles in (A) denote embryos heterozygous for the human β-globin locus transgene. *P < .05 between mutant and control groups; P < .05 between mutant groups is indicated by a color-matched asterisk.
Analysis of γ-globin expression at E14.5 and E18.5. (A) S1 nuclease protection assay on E14.5 and E18.5 fetal liver RNA, detecting human γ-globin (h-γ) and human β-globin (hβ) messenger RNA (mRNA). Mouse α-globin was used as a loading control. (B) QRT-PCR analysis of human globin expression. γ/γ+β ratios are significantly higher in all mutant mice compared with the controls (n = 3 to 5 for each genotype and each time point). (C) Western blot on E18.5 blood samples detecting expression of γ-globin protein. Actin was used as a loading control. (D) Immunohistochemistry of γ-globin expression (brown) in E18.5 blood. The white circles in (A) denote embryos heterozygous for the human β-globin locus transgene. *P < .05 between mutant and control groups; P < .05 between mutant groups is indicated by a color-matched asterisk.
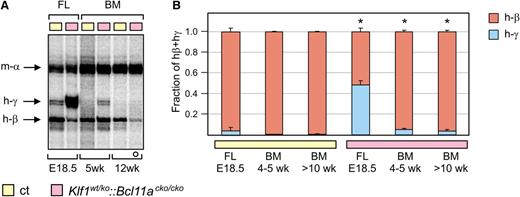
Next, we investigated γ-globin expression postnatally in young (4- to 5-week-old) and adult mice (>10 weeks old) by S1 nuclease protection and QRT-PCR analysis of bone marrow RNA. Although γ-globin expression decreased after birth, it remained significantly higher in young and adult Klf1wt/ko::Bcl11acko/cko mice compared with control mice (Figure 6A-B). In adult mutant Klf1wt/ko, Bcl11acko/cko, and Klf1wt/ko::Bcl11acko/cko mice, the levels of γ-globin increased in response to phenylhydrazine-induced anemia; such an increase was not observed in the control animals (supplemental Figure 2C). Collectively, the decline in γ-globin expression after birth suggests that additional silencing mechanisms exist that prevent high-level γ-globin expression in adult Klf1wt/ko::Bcl11acko/cko mice.
Expression of γ-globin in postnatal compound mutant mice. (A) S1 nuclease protection assay on bone marrow RNA from 5-week-old and 12-week-old mice, detecting human γ- and β-globin mRNA. Mouse α-globin was used as a loading control. For comparison, fetal liver (FL) RNA from E18.5 embryos was used. Note that the probe detecting γ-globin had a 10-fold higher specific activity than the one used in Figure 5. (B) QRT-PCR analysis of human globin expression (ct, n = 3 and Klf1wt/ko::Bcl11acko/cko, n = 4 for each time point). The white circle in (A) denotes an animal heterozygous for the human β-globin locus transgene. *P < .05 between age-matched mutant and control groups. BM, bone marrow.
Expression of γ-globin in postnatal compound mutant mice. (A) S1 nuclease protection assay on bone marrow RNA from 5-week-old and 12-week-old mice, detecting human γ- and β-globin mRNA. Mouse α-globin was used as a loading control. For comparison, fetal liver (FL) RNA from E18.5 embryos was used. Note that the probe detecting γ-globin had a 10-fold higher specific activity than the one used in Figure 5. (B) QRT-PCR analysis of human globin expression (ct, n = 3 and Klf1wt/ko::Bcl11acko/cko, n = 4 for each time point). The white circle in (A) denotes an animal heterozygous for the human β-globin locus transgene. *P < .05 between age-matched mutant and control groups. BM, bone marrow.
Dynamics of mouse globin expression
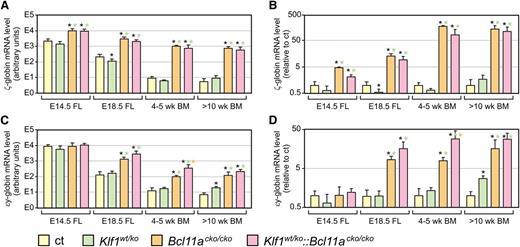
The decline in γ-globin expression in adult Klf1wt/ko::Bcl11acko/cko mice raised the question whether the endogenous mouse embryonic globin genes would display a similar expression pattern. We therefore investigated the expression of mouse ζ- and εy-globins at different developmental stages by QRT-PCR. Compared with the controls, the expression of embryonic ζ- and εy-globin was significantly higher in E14.5 and E18.5 fetal liver of Bcl11acko/cko embryos, in agreement with previous reports.8 In addition, we observed similarly increased expression in Klf1wt/ko::Bcl11acko/cko fetal livers (Figure 7). Akin to the observations on γ-globin expression, we found that the absolute amount of expression of ζ- and εy-globin declined after birth in Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice (Figure 7A,C; note logarithmic scales). In relative terms, the fold-change in ζ- and εy-globin expression was most pronounced in Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko mice compared with the age-matched control mice (Figure 7B,D; note logarithmic scales). In adult animals, this reached 150- to 200-fold increase for ζ-globin, and 30- to 50-fold increase for εy-globin. For εy-globin, the fold-change tended to be highest in Klf1wt/ko::Bcl11acko/cko mice, but this was statistically significant only in 4- to 5-week-old animals (Figure 7C-D). In addition, we found that for Klf1wt/ko animals, expression of εy-globin was significantly increased in >10-week-old animals (Figure 7C-D), while this genotype did not have a positive effect on ζ-globin expression at any stage of development analyzed (Figure 7A-B). Finally, we note that the expression pattern of the human ε-globin gene is very similar to that of the mouse εy-globin gene (supplemental Figure 3).
Expression of embryonic ζ- and εy-globins in compound mutant mice. (A,C) QRT-PCR analysis of mouse ζ- and εy-globin, absolute expression levels calculated relative to expression of mouse α-globin. (B,D) Expression levels relative to those observed in control mice of the same age group; expression of α-globin was used for normalization. N = 3 to 5 for each genotype and time point. Note logarithmic scales. *P < .05 between mutant and control groups; P < .05 between mutant groups is indicated by a color-matched asterisk.
Expression of embryonic ζ- and εy-globins in compound mutant mice. (A,C) QRT-PCR analysis of mouse ζ- and εy-globin, absolute expression levels calculated relative to expression of mouse α-globin. (B,D) Expression levels relative to those observed in control mice of the same age group; expression of α-globin was used for normalization. N = 3 to 5 for each genotype and time point. Note logarithmic scales. *P < .05 between mutant and control groups; P < .05 between mutant groups is indicated by a color-matched asterisk.
Collectively, the data on expression of the endogenous mouse embryonic globin genes are congruent with the observations on expression of the γ-globin genes residing in the human β-globin locus PAC8.1 transgene. BCL11A has a dominant role in silencing the embryonic/fetal globin genes during mouse ontogeny, which is further augmented by the preferential activation of β-globin expression by KLF1. The embryonic/fetal mouse globin genes remain expressed in adult Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko animals, but expression declines to quantitatively low, physiologically irrelevant levels. This shows that additional silencing mechanisms are operational in adult mouse erythropoiesis.
Discussion
In this report, we focused on the role of two transcription factors, KLF1 and BCL11A, in erythropoiesis and developmental regulation of globin expression. KLF1, an erythroid-specific protein, directly activates β-globin expression through binding to CACCC box sequences in the locus control region and the β-globin promoter.12 Although high-level expression of β-globin requires the presence of KLF1, the other globins, including γ-globin genes contained on human β-globin locus transgenes, are still highly expressed in the absence of KLF1.18,19 In addition to being a critical activator of β-globin expression, genome-wide gene expression analyses have revealed that KLF1 is a master regulator of genes activated during terminal erythroid differentiation.34-36 These target genes include heme synthesis enzymes, cell cycle regulators, and erythroid membrane and cytoskeleton proteins. It is therefore not surprising that Klf1 null embryos succumb to severe anemia. Embryonic lethality is not rescued by expression of a γ-globin transgene;37 this corrects globin chain imbalance but not hemolysis caused by deregulated expression of essential erythroid membrane and cytoskeleton proteins.
In humans, KLF1 mutations result in a spectrum of erythroid phenotypes.38 Haploinsufficiency for KLF1 is the underlying cause of the In(Lu) blood group,20 is associated with red cell zinc protoporphyria,21 increased HbA2,22 and HPFH.13 A dominant KLF1 missense mutation p.E325K also affects DNA binding and was reported to cause CDA.23 The two CDA patients with the p.E325K mutation displayed HbF levels of 31.6% and 44%. Remarkably, embryonic ζ- and ε-globin were also increased to very high levels.23 The ethylnitrosurea-induced mouse mutant Nan (neonatal anemia) carries a missense mutation, p.E339D, in the homologous position of mouse KLF1.39,40 This mutation causes a dominant hemolytic anemia, with markedly increased expression of embryonic globins in adult Nan animals.40 Collectively, these data support a model in which KLF1 activates β-globin expression and suppresses the embryonic/fetal β-like globin genes. Recently, it has been shown that expression of BCL11A is regulated by KLF1, suggesting an intricate mechanism for the developmental regulation of the β-likeglobin genes coordinately mediated by KLF1 and BCL11A.13,14 Ablation of BCL11A in mice demonstrated that it is the major repressor of embryonic/fetal β-like globin genes during ontogeny.8 Interestingly, the timing of expression of full-length BCL11A differs between mice and humans,8 providing an explanation for the observation that in mice carrying a human β-globin locus transgene, γ-globin silencing is already completed at the fetal liver stage.41
In this article, we have investigated the interplay of KLF1 and BCL11A in erythropoiesis and developmental regulation of globin gene expression. We find that BCL11A is the dominant repressor of embryonic/fetal globin genes in the mouse embryo from midgestation to term. While the endogenous embryonic globin genes are normally silenced at the onset of definitive erythropoiesis in the fetal liver, their expression is markedly increased in the absence of BCL11A. Quantitatively, their expression is still considerable at E18.5, just prior to birth. These data are in agreement with those reported on mouse embryos with a systemic BCL11A null mutation8 and further establish that this phenotype is intrinsic to the erythroid lineage.28 In combination with KLF1 haploinsufficiency, expression of the embryonic globin genes was also increased. However, expression of εy-globin declined to quantitatively low levels, suggesting that preferential activation of β-globin expression by KLF1 still occurs in adult Klf1wt/ko::Bcl11acko/cko adults and indicating that additional adult-stage silencing mechanisms exist. To achieve efficient silencing of the embryonic globin genes in fetal liver erythropoiesis, an intact KLF1-BCL11A axis is required: KLF1 activates BCL11A expression,13,14 and BCL11A represses the embryonic globin genes,8 thereby unleashing the full potential of KLF1 to activate β-globin expression. It is interesting to note that BCL11A also represses the embryonic ζ-globin gene, but KLF1 is not essential for high levels of α-globin expression. This provides another example of the contrasting mechanisms regulating the α-like and β-like globin loci.42
In mice carrying a human β-globin locus transgene, the switch from γ- to β-globin expression occurs at the early fetal liver stage.41 This is dependent on the presence of BCL11A;8 in our experiments with erythroid-specific ablation of BCL11A, we obtained similar results. In agreement with previous reports using a different human β-globin locus transgene (line 72),19 we find that haploinsufficiency for KLF1 delays γ- to β-globin switching leading to an approximately twofold increase in the γ/(γ+β) ratio at E14.5 and E18.5. Part of this increase can be explained by diminished BCL11A expression in embryos with KLF1 insufficiency.14 Remarkably, relative to Bcl11acko/cko embryos, there is a further increase in the γ/(γ+β) ratio in compound Klf1wt/ko::Bcl11acko/cko embryos (eg, from 0.37 to 0.48 at E18.5). This shows that even in the absence of BCL11A, KLF1 still preferentially activates the β-globin gene. Collectively, these data support the proposed role of the KLF1-BCL11A axis in γ-globin regulation.13,14,38
Genome-wide association studies have shown an association between BCL11A single nucleotide polymorphisms and erythroid parameters,43 suggesting a role for BCL11A in adult erythropoiesis beyond globin regulation. We therefore determined the effects on steady-state erythropoiesis and globin expression in adult animals. We found only minor deviations in the hematologic parameters of Klf1wt/ko, Bc11acko/cko, and Klf1wt/ko::Bcl11acko/cko animals. Klf1wt/ko animals display mild reticulocytosis but do not have increased Epo levels, indicating that the production of erythroid cells is adequate but that the maturation of reticulocytes takes more time, or alternatively that the reticulocytes are prematurely released in the circulation. Mild reticulocytosis is also one of the hallmarks of individuals with a Maltese pedigree with KLF1 haploinsufficiency.13 Bcl11acko/cko animals are displaying lower hematocrit, Hb, red blood cell counts, and slightly increased Epo levels. In the Klf1wt/ko::Bcl11acko/cko animals, these traits are exacerbated and, in addition, increased MCV, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration reached statistical significance. The most straightforward explanation for these observations is that the Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko genotypes provoke a mild anemia, which is compensated by increased Epo levels. KLF1 has a well-established role in the expression of erythroid membrane and cytoskeleton proteins,12 although a putative role for BCL11A in erythroid maturation remains to be further investigated. Importantly, Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko animals were obtained at the expected Mendelian ratios and, thus far, none of them have developed leukemia (n = 74). This is in contrast to animals reconstituted with BCL11A null hematopoietic cells, which succumb to T-cell leukemia at a high rate.29 We conclude that erythroid-specific ablation of BCL11A, alone or in combination with KLF1 haploinsufficiency, only mildly affects steady-state erythropoiesis in adult mice. This supports proposals for the modulation of the KLF1-BCL11A axis in β-thalassemia and SCA patients for reactivation of γ-globin expression.13,14,28
At first glance, the observation that expression of γ-globin declines in adult Bcl11acko/cko and Klf1wt/ko::Bcl11acko/cko animals is counterintuitive to this notion. However, there is evidence for species-specific differences in developmental regulation of globin expression. In mice, full-length BCL11A is already expressed in the fetal liver, while human fetal liver cells express only short isoforms of BCL11A.8 The full-length isoform is first observed in human adult erythroid progenitors. This difference in developmental timing of BCL11A expression provides a molecular explanation for the observation that γ- to β-globin switching occurs at the fetal liver stage in mouse embryos carrying a human β-globin locus transgene.8,41 Furthermore, treatment with drugs that raise HbF cells in human subjects fail to do so in human β-globin locus transgenic mice,44,45 suggesting that silencing of the γ-globin genes is much tighter in mice than it is in humans. We therefore strongly feel that this intrinsic property of the mouse model does not provide a convincing argument against proposals to target the KLF1-BCL11A axis as a therapeutic approach to increase HbF levels in patients with β-thalassemia and SCA.8,11,13,14 This notion is confirmed by the recent elegant demonstration that erythroid-specific ablation of BCL11A corrects disease in a mouse model for SCA.28
We suggest that the tight repression of the γ-globin genes in mice provides a window of opportunity for identification of additional factors involved in the silencing mechanism at the adult stage. Enforcement of repression of the embryonic/fetal program in adult erythropoiesis may be executed by, for instance, the transcription factors MYB46 and SOX6,47 the chromatin-bound FOP/CHTOP protein27 and NuRD complex,48 the orphan nuclear receptors TR2/TR4,49 and the protein arginine methyl transferase PRMT5,50 and is likely to include additional epigenetic mechanisms such as PcG complex recruitment and DNA methylation. Future work will be aimed at further elucidating the multilayered, repressive network of the embryonic/fetal program in the adult erythroid environment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to acknowledge Sylvia Dekker, Ralph Stadhouders, Rien van Haperen, and Divine Kulu for technical assistance, and the animal facility of Erasmus MC for animal husbandry.
This work was supported by the Netherlands Genomics Initiative (NGI). Research of the Busslinger group was supported by Boehringer Ingelheim and an Advanced Grant of the European Research Council, the Landsteiner Foundation for Blood Transfusion Research (LSBR; 1040), and the Netherlands Scientific Organization (NWO; DN 82-301 and 912-07-019).
Authorship
Contribution: S.P. was the principle investigator and was primarily responsible for the paper. F.E., N.G., I.B., E.v.d.A., I.C., and K.v.L. performed experiments. T.v.G. performed the statistical analysis. F.E., I.B., U.K., M.v.L., F.G., T.B.v.D., M.B., and S.P. designed experiments. The paper was written by F.E., E.v.d.A., M.B., and S.P.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for F.E. is Sanquin Research Department of Hematopoiesis, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands. The current affiliation for I.B. is Akron Molecules GmbH, Helmut-Qualtinger-Gasse 2, A-1030 Vienna, Austria.
Correspondence: Sjaak Philipsen, Department of Cell Biology Ee720, Erasmus MC, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands; email: j.philipsen@erasmusmc.nl.