Key Points
A murine model was developed for capsid-specific CD8 cell responses in AAV gene therapy for hemophilia.
Y-F mutant capsid minimizes the effect of anticapsid CD8+ T cells on hepatocyte-derived factor IX expression in mice and in human cells.
Abstract
Recent clinical trials have shown that evasion of CD8+ T-cell responses against viral capsid is critical for successful liver-directed gene therapy with adeno-associated viral (AAV) vectors for hemophilia. Preclinical models to test whether use of alternate serotypes or capsid variants could avoid this deleterious response have been lacking. Here, the ability of CD8+ T cells (“cap-CD8,” specific for a capsid epitope presented by human B*0702 or murine H2-Ld molecules) to target AAV-infected hepatocytes was investigated. In a murine model based on adoptive transfer of ex vivo expanded cap-CD8, AAV2-transduced livers showed CD8+ T-cell infiltrates, transaminitis, significant reduction in factor IX transgene expression, and loss of transduced hepatocytes. AAV8 gene transfer resulted in prolonged susceptibility to cap-CD8, consistent with recent clinical findings. In contrast, using an AAV2(Y-F) mutant capsid, which is known to be less degraded by proteasomes, preserved transgene expression and largely avoided hepatotoxicity. In vitro assays confirmed reduced major histocompatibility complex class I presentation of this capsid and killing of human or murine hepatocytes compared with AAV2. In conclusion, AAV capsids can be engineered to substantially reduce the risk of destruction by cytotoxic T lymphocytes, whereas use of alternative serotypes per se does not circumvent this obstacle.
Introduction
More than a decade of translational research has been dedicated to developing a gene transfer regimen for sustained therapeutic expression of coagulation factor IX (F.IX) to correct the X-linked bleeding disorder hemophilia B.1 Two recent phase 1/2 trials used in vivo adeno-associated viral (AAV) gene transfer to the liver. Preclinical studies in hemophilia B mice and dogs had demonstrated therapeutic multiyear expression following a single round of hepatic gene transfer.1 Studies in nonhuman primates further supported the approach.1,2 Additionally, the protocol resulted in induction of immune tolerance to F.IX in experimental animals, involving active suppression by regulatory T cells.3-7 Nonetheless, AAV vectors are not invisible to the immune system. Their DNA genome and capsid structure is being sensed by Toll-like receptors 9 and 2, respectively, and humans often have preexisting immunity.8-13 Activation of capsid-specific CD8+ T cells has now been observed in 4 clinical trials using AAV1, 2, or 8 vectors.11,14-18 These cytotoxic T lymphocytes (CTLs) have the capacity to eliminate AAV-transduced cells resulting in loss of therapeutic expression upon hepatic gene transfer.
The initial trial on AAV2-F.IX gene transfer to the liver uncovered 2 obstacles posed by capsid immunity: lack of hepatic transduction because of preexisting neutralizing antibodies to the virus and activation of memory CD8+ T cells against capsid.11,15 Humans are naturally infected with AAVs, and there is high conservation and cross-reactivity of T-cell epitopes among serotypes.11,16,19,20 Nonetheless, sustained therapeutic expression has now been achieved in 6 human subjects treated with varying doses of an AAV8 vector expressing F.IX from a hepatocyte-specific promoter.18 However, 4 patients developed T-cell responses against capsid, and 2 patients showed a rise in transaminases accompanied in 1 case by partial loss of F.IX transgene expression. Prompt administration of a course of steroids ablated the CD8+ T-cell response and resulted in rapid resolution of transaminitis, thus preventing complete loss of transduced hepatocytes.
Animal studies had not predicted such a response to input capsid.21 In vitro studies have been helpful in studying T-cell targeting of transduced human hepatocytes.22,23 However, development of a preclinical in vivo model of AAV vector immunogenicity has been frustrating and largely unsuccessful. Immunization against capsid by various methods designed to induce capsid-specific CD8+ T cells failed to eliminate transduced cells in mice,20,24-28 despite major histocompatibility complex class I (MHC I) presentation of capsid for several weeks following AAV gene transfer.29 A more recent study showed some effect when a strong heterologous CD8+ T-cell epitope was incorporated into the capsid.30 Surprisingly, nonhuman primates, who are also natural hosts for AAVs, have also not been suitable models. This may be explained by several phenotypic differences between capsid-specific CTLs in humans and nonhuman primates.9,31
Our new study sought to establish a suitable murine model for preclinical testing of the effect of capsid-specific CD8+ T cells on therapeutic hepatocyte-derived F.IX expression and to identify immunologically stealthier capsid candidates. Following cellular entry, AAV capsid is phosphorylated, a signal for ubiquitination and subsequent proteosomal degradation.32-34 Presentation of resulting peptides in the context of MHC I would be predicted to flag transduced hepatocytes for destruction by capsid-specific CD8+ T cells.23 However, shunting of the capsid toward proteasomal degradation can be substantially reduced by elimination of potential phosphorylation sites through mutation of surface-exposed tyrosine residues to phenylalanine.34,35 Here, we hypothesized that such Y-F mutant capsids would be less efficiently MHC I presented by hepatocytes. Using a novel in vivo mouse model, supplemented with in vitro studies of human hepatocytes, we demonstrate that (1) AAV2-transduced hepatocytes are targeted for CTL-mediated destruction, and (2) AAV8-transduced hepatocytes are targeted for a more prolonged period of time, whereas (3) Y-F mutant capsid antigen is less effectively presented, resulting in substantially reduced killing. Therefore, AAV capsids can be engineered to escape capsid-specific CD8+ T cells, whereas use of alternative serotypes may alter timing of capsid antigen presentation rather than solve the problem.
Methods
This study was approved under University of Florida Institutional Animal Care and Use Committee protocol #201003971.
AAV vectors
Generation of murine capsid-specific CD8+ T cells (“cap-CD8”)
Male BALB/c mice (6-8 weeks old; Jackson Laboratory, Bar Harbor, ME) were intramuscularly injected with AAV2-LacZ and boosted 7 days later with 5 µg of the dominant CD8+ T-cell epitope VPQYGYLTL (AnaSpec, Fremont, CA) emulsified in complete Freund’s adjuvant (CFA, Sigma, St Louis, MO) by subcutaneous injection. Splenocytes were isolated 10 days later, and cap-CD8 cells were expanded in vitro as follows: 2 × 107 splenocytes were cultured with 6 mL stimulation media over a 5- to 6-day period at 37°C in 15 mL Falcon tubes (BD Biosciences, Bedford, MA) with the caps loosened to allow gas exchange; stimulation media was exchanged after 3 days. Stimulation media was RPMI-1640 (Invitrogen, Carlsbad, CA) media with 10% fetal bovine serum and 1% penicillin and streptomycin with 10 µg/mL of VPQYGYLTL, 25 ng/mL of interleukin 15, and 10 ng/mL of interleukin 21. Control-CD8+ cells (“con-CD8”) were generated by immunization and in vitro expansion with H2-Ld–restricted CD8+ influenza A epitope (IYATVAGSL). CD8+ cells were magnetically purified from freshly expanded cells using Miltenyi reagents (Cambridge, MA) and used for in vitro and in vivo studies.
In vitro CTL and antigen presentation assays for hepatocytes
The assay by Höppner et al37 was adapted to measure killing of murine H2.35 cells. Capsid antigen presentation and CTL assays (killing by effector CD8+ T cells expanded in vitro from human peripheral blood mononuclear cells) for human hepatocyte cell line HHL5 were performed as described.22,23 Further details are provided in the supplemental data (“Materials”; see the Blood Web site).
Mouse model for in vivo targeting of AAV-transduced hepatocytes
C.129S7(B6)-Rag1tm1Mom/J mice (male, 6-8 weeks old) were purchased from Jackson Laboratories. AAV viral vectors were delivered by tail vein (intravenous) injection. Ex vivo expanded cap-CD8 or con-CD8 were adoptively transferred at a dose of 2 × 106 cells per mouse by intravenous injection 24 hours later (or up to 14 days later). A lipopolysaccharide (LPS) regimen of 3 intraperitoneal injections (10 ng per injection) was used to provide additional activation signals. The first injection was 30 minutes prior to adoptive transfer, followed by injections at 24 and 48 hours. Liver lobes were collected 7 or 28 days after CD8+ T-cell transfer and cryopreserved in Optimal Cutting Temperature tissue-freezing medium. Proteasome inhibition studies were performed by 2 injections of 25 μg of bortezomib (intravenously at the time of vector administration and intraperitoneally 3 days later).
F.IX expression and pathology
Immunohistochemistry of liver cryosections was performed with antibody stains for hF.IX expression (goat anti-hF.IX; Affinity Biologicals, Ancaster, ON, Canada) (donkey α-goat IgG-Alex Fluor-568; Invitrogen) and for CD8+ T cells (using rat α-CD8a, clone 53.6.7 and donkey α-rat IgG-Alex Fluor-488; Invitrogen) as published.5,38 Images were captured with Nikon Eclipse 80i fluorescence microscope (Nikon, Tokyo, Japan) and Retiga 2000R digital camera (QImaging, Surrey, BC, Canada) and analyzed with Nikon Elements software. Percent hF.IX expressing hepatocytes in liver cross sections was determined with Volocity software (Perkin Elmer, Waltham, MA). Systemic hF.IX levels were measured by enzyme-linked immunosorbent assay as published.38 Alanine transaminase (ALT) and aspartate transaminase (AST) levels in plasma were measured by Small Animal Veterinary diagnostics laboratory at the University of Florida in a blinded fashion.
Results
A novel mouse model demonstrates that in vivo elimination of AAV-hF.IX–transduced hepatocytes by capsid-specific CD8+ T cells is blunted by use of AAV2(Y-F) capsid
The dominant, Ld-restricted CD8+ epitope for AAV2 in BALB/c mice (H-2d haplotype), VPQYGYLTL, is conserved among several serotypes (including AAV8) and is also an epitope in humans, where it is presented by B*0702.11,19,20 Therefore, we decided to develop a BALB/c-based model to study the effect of capsid-specific CTLs on therapeutic F.IX expression in vivo. Previously, we found that a combination of 3 Y-F mutations at AAV2 capsid amino acid positions 444, 500, and 730 [referred to as AAV2(Y-F) capsid here] increased gene transfer to hepatocytes.36 We hypothesized that hepatocytes transduced with this capsid variant may be less prone to being killed by capsid-specific CTLs. Although the mutations in AAV2(Y-F) do not affect the VPQYGYLTL epitope, in silico analyses suggested that 2 additional novel epitopes (RPKRLNFKL, EPRPIGTRF) might have been created. However, immunization of BALB/c mice with AAV2 or AAV2(Y-F) vector particles resulted in activation of interferon γ–producing, VPQYGYLTL-specific T cells at identical frequency, whereas no responses to the other 2 potential epitopes were observed (supplemental Figure 1). Therefore, Y-F mutations did not alter the magnitude of CD8+ T-cell activation or immunodominance of the VPQYGYLTL epitope.
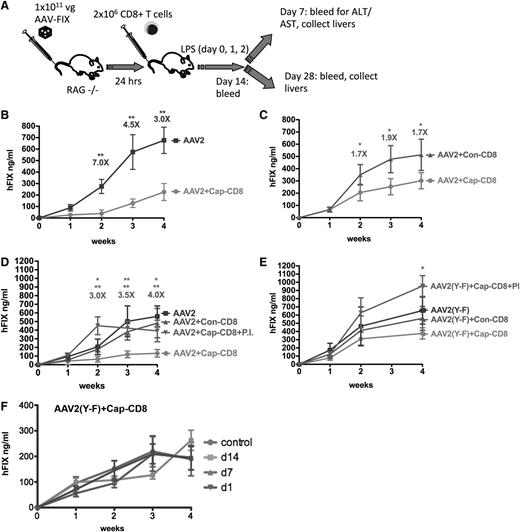
Thus far, studies in mice failed to demonstrate in vivo elimination of AAV-transduced hepatocytes by capsid-specific CTLs.21 Interestingly, in models of colitis, aggressor cells are studied by adoptive transfer to recombinase-activating gene–deficient mice.39 Therefore, we chose Rag1−/− BALB/c mice for hepatic gene transfer with AAV2 or AAV2(Y-F) vectors (1 × 1011 vg per mouse) followed by adoptive transfer of 2 × 106 BALB/c-derived cap-CD8 cells 24 hours later (Figure 1A). Cap-CD8 represents CD8+ T cells enriched for VPQYGYLTL-specific cells that had been generated by immunization of BALB/c mice and subsequent in vitro expansion as described in “Methods.”
Effects of AAV capsid-specific CD8+ T cells on systemic liver-derived hF.IX expression in vivo. (A) Diagram of in vivo model and experimental outline: In vitro expanded capsid-specific, Ld-restricted CD8+ T cells (cap-CD8) isolated from BALB/c mice were adoptively transferred by tail vein injection into Rag-1−/− BALB/c mice 24 hours after administration of AAV-ApoE/hAAT-hF.IX vector (1 × 1011 vg per mouse; n = 4 per experimental group). As negative control cells, CD8+ T cells specific for the influenza dominant epitope (con-CD8) were generated in BALB/c mice and in vitro expanded in parallel. Mice received cap-CD8, con-CD8, or no cells as indicated in each panel. Following T- cell transfer, mice were treated with LPS on days 0, 1, and 2 to provide an additional activation signal. Systemic hF.IX levels were measured as a function of time after administration of the following vectors: AAV2 (B), AAV2 (without LPS administration) (C), AAV2 in absence or presence of proteasome inhibitor bortezomib (PI) (D), or AAV2(Y-F) in absence or presence of PI (E). Fold differences of hF.IX levels for mice treated with con-CD8 and cap-CD8 are indicated (except for panel B, which is no CD8 compared with cap-CD8). Data are average ± standard deviation (SD) for each time point with n = 5 per experimental group. *P < .05; **P < .01 using Student t test for each time point. (F) Systemic hF.IX levels as a function of time after AAV2(Y-F) vector administration to Rag-1−/− BALB/c mice that received cap-CD8 cells at the indicated time points or no cells (control; n = 4 per group).
Effects of AAV capsid-specific CD8+ T cells on systemic liver-derived hF.IX expression in vivo. (A) Diagram of in vivo model and experimental outline: In vitro expanded capsid-specific, Ld-restricted CD8+ T cells (cap-CD8) isolated from BALB/c mice were adoptively transferred by tail vein injection into Rag-1−/− BALB/c mice 24 hours after administration of AAV-ApoE/hAAT-hF.IX vector (1 × 1011 vg per mouse; n = 4 per experimental group). As negative control cells, CD8+ T cells specific for the influenza dominant epitope (con-CD8) were generated in BALB/c mice and in vitro expanded in parallel. Mice received cap-CD8, con-CD8, or no cells as indicated in each panel. Following T- cell transfer, mice were treated with LPS on days 0, 1, and 2 to provide an additional activation signal. Systemic hF.IX levels were measured as a function of time after administration of the following vectors: AAV2 (B), AAV2 (without LPS administration) (C), AAV2 in absence or presence of proteasome inhibitor bortezomib (PI) (D), or AAV2(Y-F) in absence or presence of PI (E). Fold differences of hF.IX levels for mice treated with con-CD8 and cap-CD8 are indicated (except for panel B, which is no CD8 compared with cap-CD8). Data are average ± standard deviation (SD) for each time point with n = 5 per experimental group. *P < .05; **P < .01 using Student t test for each time point. (F) Systemic hF.IX levels as a function of time after AAV2(Y-F) vector administration to Rag-1−/− BALB/c mice that received cap-CD8 cells at the indicated time points or no cells (control; n = 4 per group).
Administration of cap-CD8 substantially reduced systemic hF.IX expression from AAV2 vector (Figure 1B). Similar results were obtained when cap-CD8–treated mice were compared with mice that received con-CD8 cells (which were generated against an irrelevant influenza A virus epitope; Figure 1D). In either comparison, an approximately fourfold reduction in hF.IX levels was seen. However, when cap-CD8 cells were given to AAV2(Y-F)-transduced mice at different time points, only a minor decrease of ≤1.5-fold was observed, failing to reach statistical significance (Figure 1E-F). In order to provide optimal inflammatory signals that lead to upregulation of MHC I expression,40 a 3-day course of LPS administration was included, starting the day before cells were injected. This was not absolutely required, as Figure 1C shows that a less substantial but still significant loss of AAV2-derived hF.IX expression was obtained even in the absence of LPS. No loss of hF.IX expression was detected when cap-CD8 cells were given to mice 1 week after AAV2 gene transfer or later, indicating a difference between the murine model and humans (supplemental Figure 2). Cap-CD8 did not affect hF.IX expression from an adenoviral vector in mice, further confirming their specificity for AAV capsid (supplemental Figure 3).
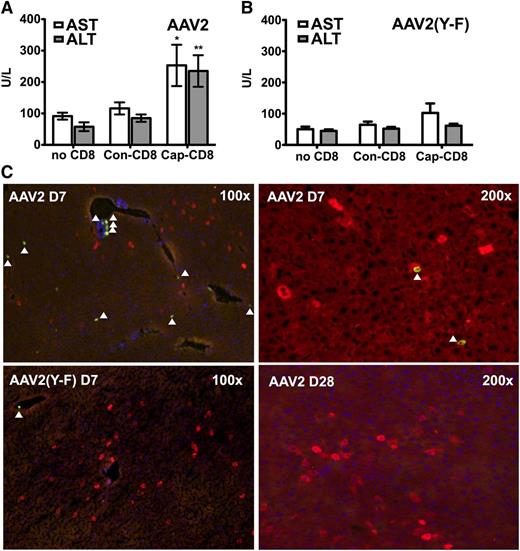
To address the question of liver toxicity, ALT and AST levels were measured 7 days after T-cell administration. Cap-CD8 induced a significant increase in systemic levels of both liver enzymes for AAV2 (Figure 2A), which was not seen for AAV2(Y-F)-transduced mice (Figure 2B). Immunohistochemical analysis of liver sections from the same time point revealed CD8+ T-cell infiltration in AAV2-transduced mice that had received cap-CD8 (Figure 2C, upper panels), which was absent in AAV2(Y-F)-transduced livers (lower left panel and data not shown). Infiltrating CD8+ T cells represented adoptively transferred cells, as Rag1−/− lack endogenous T cells. No more CD8+ infiltrates were detected 1 month after gene transfer (Figure 2C and data not shown). When liver sections were stained for hF.IX 1 month after gene/T-cell transfer, a 50% reduction in percent of hF.IX-expressing hepatocytes was seen in AAV2-transduced recipients of cap-CD8 (Figure 3A,C). Importantly, no loss of hF.IX+ hepatocytes was evident in mice that had received AAV2(Y-F) vector at various time points, and CD8+ T-cell infiltrates were also not seen at this time point (Figure 3B,D and data not shown).
Liver toxicity caused by AAV capsid-specific CD8+ T cells. Circulating liver enzyme (AST and ALT) levels in Rag-1 −/− BALB/c mice were measured in serum collected 7 days after adoptive transfer of CD8+ T cells (as outlined in Figure 4A: AAV capsid-specific, cap-CD8; control cells, con-CD8; or no cells; n = 4 per experimental group). (A) AAV2. (B) AAV2(Y-F). Data are average ± SD. *P < .05; **P < .01 using Student t test (comparison between mice treated with cap-CD8 and con-CD8). (C) Liver cryosections were generated for the same day-7 time point. Antibody stains show CD8+ T cells (green) and hF.IX+ hepatocytes (red). Representative examples at the indicated original magnification are shown for mice having received cap-CD8 cells and the following vector capsid: AAV2 (upper panels); AAV2(Y-F) (lower left panel); day 28 after AAV2 vector administration (lower right panel). Arrows depict CD8+ cells. Original magnifications are as indicated for each panel.
Liver toxicity caused by AAV capsid-specific CD8+ T cells. Circulating liver enzyme (AST and ALT) levels in Rag-1 −/− BALB/c mice were measured in serum collected 7 days after adoptive transfer of CD8+ T cells (as outlined in Figure 4A: AAV capsid-specific, cap-CD8; control cells, con-CD8; or no cells; n = 4 per experimental group). (A) AAV2. (B) AAV2(Y-F). Data are average ± SD. *P < .05; **P < .01 using Student t test (comparison between mice treated with cap-CD8 and con-CD8). (C) Liver cryosections were generated for the same day-7 time point. Antibody stains show CD8+ T cells (green) and hF.IX+ hepatocytes (red). Representative examples at the indicated original magnification are shown for mice having received cap-CD8 cells and the following vector capsid: AAV2 (upper panels); AAV2(Y-F) (lower left panel); day 28 after AAV2 vector administration (lower right panel). Arrows depict CD8+ cells. Original magnifications are as indicated for each panel.
Quantification of hF.IX+ hepatocytes 1 month after adoptive transfer of cap-CD8 or con-CD8 cells. Mice had been transduced with AAV2-hF.IX (A) or AAV2(Y-F)-hF.IX (B) vector. Percent decrease of hF.IX levels from cap-CD8 cells is indicated. Data from individual mice (n = 3-4 per group, with 10 random low-power fields for each liver) as well as averages are graphed. P values for significant differences are indicated. Representative hF.IX stains (red) are shown for AAV2 (C) and AAV2(Y-F) (D).
Quantification of hF.IX+ hepatocytes 1 month after adoptive transfer of cap-CD8 or con-CD8 cells. Mice had been transduced with AAV2-hF.IX (A) or AAV2(Y-F)-hF.IX (B) vector. Percent decrease of hF.IX levels from cap-CD8 cells is indicated. Data from individual mice (n = 3-4 per group, with 10 random low-power fields for each liver) as well as averages are graphed. P values for significant differences are indicated. Representative hF.IX stains (red) are shown for AAV2 (C) and AAV2(Y-F) (D).
Further evidence that murine hepatocytes transduced with AAV2(Y-F) vector are less effectively killed by capsid-specific CTLs
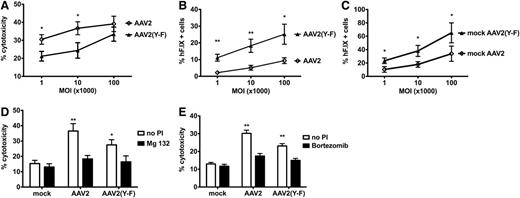
In order to confirm that AAV2(Y-F)-transduced hepatocytes are less effectively killed by cap-CD8, an in vitro model was established. Murine BALB/c-derived H2.35 hepatocytes were transduced with AAV2-hF.IX or AAV2(Y-F)-hF.IX vector at different multiplicities of infection (MOIs). The next day, expanded cap-CD8 cells were added for in vitro CTL assay (supplemental data [“Materials”] and supplemental Figure 4). For AAV2 capsid, a similar level of lysis of 30% to 40% at the highest effector:target ratio was observed over a wide range of MOIs (103-105; Figure 4). At MOI = 105, lysis was marginally reduced for AAV2(Y-F) (Figure 4A). However, significant differences were observed at lower MOIs (103-104; Figure 4A). For all MOIs, the percent hF.IX+ hepatocytes that had survived the killing assay was consistently and substantially higher for AAV2(Y-F), reflecting both increased transduction efficiency and decreased killing (Figure 4B-C; supplemental Figure 5).
In vitro killing of AAV-pulsed murine hepatocytes by capsid-specific CD8+ T cells. (A) Percent death of target cells (adult BALB/c hepatocyte cell line H2.35), pulsed with either AAV2 or AAV2(Y-F), as a function of MOI (at effector to target ratio of 80:1). (B) Percent surviving hF.IX+ H2.35 hepatocytes (as determined by flow cytometry after completion of in vitro killing assay) pulsed with either AAV2 or AAV2(Y-F), as a function of MOI (at effector to target ratio of 80:1). (C) Percent hF.IX+ H2.35 hepatocytes transduced with either AAV2 or AAV2(Y-F) (in the absence of effector cells) as a function of MOI. Data are average ± SD for quadruplicate measurements. Panels are representative examples of at least 2 experiments. Statistically significant differences between AAV2 and AAV2(Y-F) for a specific target:effector ratio: *P < .05; **P < .01, unpaired, 2-tailed t test. (D-E) Effects of proteasomal inhibition on liver target cell death. Shown are percent dead H2.35 target cells that had been pulsed with either AAV2 or AAV2(Y-F) at MOI of 104 vg per cell and treated with proteasomal inhibitor (PI). Liver cells were treated with 100 nM bortezomib (D) or 300 nM MG-132 (E). Controls include mock-transduced target cells and cells that were not treated with PI. Assays were again performed in quadruplicate.
In vitro killing of AAV-pulsed murine hepatocytes by capsid-specific CD8+ T cells. (A) Percent death of target cells (adult BALB/c hepatocyte cell line H2.35), pulsed with either AAV2 or AAV2(Y-F), as a function of MOI (at effector to target ratio of 80:1). (B) Percent surviving hF.IX+ H2.35 hepatocytes (as determined by flow cytometry after completion of in vitro killing assay) pulsed with either AAV2 or AAV2(Y-F), as a function of MOI (at effector to target ratio of 80:1). (C) Percent hF.IX+ H2.35 hepatocytes transduced with either AAV2 or AAV2(Y-F) (in the absence of effector cells) as a function of MOI. Data are average ± SD for quadruplicate measurements. Panels are representative examples of at least 2 experiments. Statistically significant differences between AAV2 and AAV2(Y-F) for a specific target:effector ratio: *P < .05; **P < .01, unpaired, 2-tailed t test. (D-E) Effects of proteasomal inhibition on liver target cell death. Shown are percent dead H2.35 target cells that had been pulsed with either AAV2 or AAV2(Y-F) at MOI of 104 vg per cell and treated with proteasomal inhibitor (PI). Liver cells were treated with 100 nM bortezomib (D) or 300 nM MG-132 (E). Controls include mock-transduced target cells and cells that were not treated with PI. Assays were again performed in quadruplicate.
Proteasome inhibition has been shown to reduce MHC I presentation of capsid antigen, prevent killing by CTLs, and also increase transduction/transgene expression from AAV vectors.22,32,41 Pretreatment of target H2.35 hepatocytes with proteasome inhibitors, bortezomib or Mg-132, effectively prevented killing of AAV2- or AAV2(Y-F)-transduced cells by cap-CD8 in vitro (Figure 4D-E). Similarly, bortezomib prevented the loss of hF.IX expression from AAV2 vector upon cap-CD8 administration in vivo (Figure 1D). In AAV2(Y-F)-treated mice, bortezomib treatment increased hF.IX levels even beyond those seen in the absence of cap-CD8 (Figure 1E).
Human hepatocytes transduced with AAV2(Y-F) present less capsid antigen and are more resistant to killing by capsid-specific CTLs
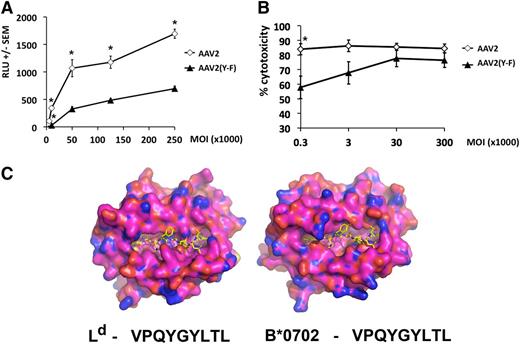
The choice of the VPQYGYLTL epitope allowed us to demonstrate the relevance of our findings for MHC I presentation by human hepatocytes.11,19,22 Comparison of the crystal structure of murine Ld and modeled human HLA-B*0702 molecules suggests that although the overall antigen-binding clefts are different, both have structural features complementary to the peptide epitope with residues 2 (proline) and 9 (lysine) being the critical anchoring residues (Figure 5C and data not shown; see supplemental data [“Materials”] for methodology). Levels of MHC I capsid antigen presentation were measured in human hepatocytes transduced with vector overnight at different MOIs. Subsequently, cells were washed and incubated with a reporter T-cell line, which recognizes VPQYGYLTL in the context of the HLA-B*0702 and, upon TCR engagement, expresses luciferase in amounts proportional to the levels of antigen presentation.22 Over a wide range of vector doses, AAV2(Y-F) vector yielded levels of capsid antigen presentation significantly lower than those measured on hepatocytes transduced with AAV2 vector at identical MOIs (Figure 5A). Quantitative real-time polymerase chain reaction to detect vector genome copy numbers confirmed that the difference in antigen presentation was not due to differences in cellular entry (data not shown).
Capsid antigen presentation and killing assay in vitro in AAV-transduced human hepatocytes. HHL5 human hepatocytes were transduced in vitro at increasing MOI with AAV2 or AAV2(Y-F) vectors. (A) Levels of antigen presentation measured with Jurma-VPQ reporter cells 24 hours after vector transduction. Jurma-VPQ cells were added in culture overnight at a ratio of 10:1 reporter:target. RLU, relative light units. (B) CTL assay. HHL5 target cells were transduced overnight and cocultured for 4 hours with effector cells derived by peripheral blood mononuclear cells at an effector:target ratio of 10:1. Percent cytotoxicity was measured relative to a maximum lactate dehydrogenase release (tritonX-treated targets) after background subtraction. All results are reported as average ± standard error of the mean. *P < .05, unpaired, 2-tailed t test. (C) The peptide VPQYGYLTL is shown bound to H2-Ld based on the crystal structure of SPLDSLWWI bound to H2-Ld in Protein Data Bank code 3TJH. VPQYGYLTL is shown as yellow sticks for carbon, blue for nitrogen, and red for oxygen. The peptide VPQYGYLTL is shown bound to HLA-B*-07:02 as modeled from the crystal structure of HLA-B8 from PDB code 3SPV (95.7% identical to HLA-B*07:02:01). The molecular surfaces of H2-Ld and HLA-B*07:02:01 are shown as orange for carbon, blue for nitrogen, and red for oxygen.
Capsid antigen presentation and killing assay in vitro in AAV-transduced human hepatocytes. HHL5 human hepatocytes were transduced in vitro at increasing MOI with AAV2 or AAV2(Y-F) vectors. (A) Levels of antigen presentation measured with Jurma-VPQ reporter cells 24 hours after vector transduction. Jurma-VPQ cells were added in culture overnight at a ratio of 10:1 reporter:target. RLU, relative light units. (B) CTL assay. HHL5 target cells were transduced overnight and cocultured for 4 hours with effector cells derived by peripheral blood mononuclear cells at an effector:target ratio of 10:1. Percent cytotoxicity was measured relative to a maximum lactate dehydrogenase release (tritonX-treated targets) after background subtraction. All results are reported as average ± standard error of the mean. *P < .05, unpaired, 2-tailed t test. (C) The peptide VPQYGYLTL is shown bound to H2-Ld based on the crystal structure of SPLDSLWWI bound to H2-Ld in Protein Data Bank code 3TJH. VPQYGYLTL is shown as yellow sticks for carbon, blue for nitrogen, and red for oxygen. The peptide VPQYGYLTL is shown bound to HLA-B*-07:02 as modeled from the crystal structure of HLA-B8 from PDB code 3SPV (95.7% identical to HLA-B*07:02:01). The molecular surfaces of H2-Ld and HLA-B*07:02:01 are shown as orange for carbon, blue for nitrogen, and red for oxygen.
To test whether the amounts of AAV capsid antigen being presented were sufficient to flag transduced cells for recognition and killing by CD8+ T cells, a CTL assay was performed as published.23 Human hepatocytes were transduced overnight at varying MOIs followed by coculture with effector CTLs.15,18 Percent killing at lower MOIs (ie, those close to the in vivo setting) was significantly decreased in targets transduced with AAV2(Y-F) compared with AAV2 vector (Figure 5B). Hence, at MOIs similar to those used clinically, reduced levels of capsid antigen presentation on MHC I with AAV(Y-F) vectors correlated with reduced CTL-mediated clearance.
AAV2(Y-F) particles traffic to the hepatocyte nucleus more efficiently than AAV2
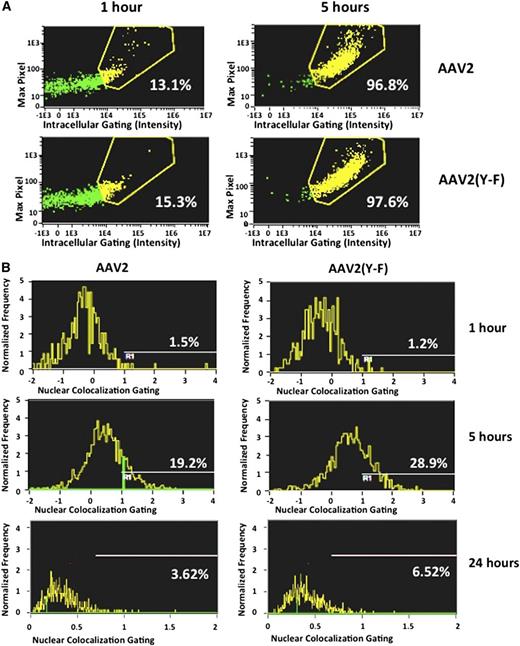
We next compared the uptake of AAV2 and AAV2(Y-F) vectors by human hepatocytes using flow-imaging analysis as described in the supplemental data (“Materials”). In a time course of vector internalization, cellular entry in the human hepatocyte cell line HHL5 and in human dendritic cells was comparable for AAV2 and AAV2(Y-F) (Figure 6A; supplemental Figure 6A-D). In hepatocytes, only ∼15% of the virus was internalized at 1 hour, whereas after 5 hours >95% of both AAV2 and AAV2(Y-F) virions were localized intracellularly. Nuclear colocalization of viral particles showed a higher amount of AAV2(Y-F) virions localized within the nucleus compared with AAV2 at 5 and 24 hours after starting the time course (Figure 6B). These results are in agreement with previously published work showing that tyrosine mutant AAV2 traffics more efficiently to the nucleus.35,42
Cell entry and nuclear colocalization of AAV vectors. The human hepatocyte cell line HHL5 was incubated with AAV vectors at an MOI of 105 in a tissue culture incubator and assayed at 1, 5, and 24 hours for virus nuclear colocalization on an ImageStream imaging flow cytometer. (A) Virus intracellular localization. Five hours after the time course started, virtually all virions were internalized into hepatocytes. (B) Colocalization of fluorescently labeled viral particles and nuclei, shown as percent of total viral particles. Results from 1 representative experiment. All experiments were repeated and analyzed at least twice.
Cell entry and nuclear colocalization of AAV vectors. The human hepatocyte cell line HHL5 was incubated with AAV vectors at an MOI of 105 in a tissue culture incubator and assayed at 1, 5, and 24 hours for virus nuclear colocalization on an ImageStream imaging flow cytometer. (A) Virus intracellular localization. Five hours after the time course started, virtually all virions were internalized into hepatocytes. (B) Colocalization of fluorescently labeled viral particles and nuclei, shown as percent of total viral particles. Results from 1 representative experiment. All experiments were repeated and analyzed at least twice.
AAV8-derived hF.IX expression is vulnerable to capsid-specific CD8+ T cells for a prolonged period of time
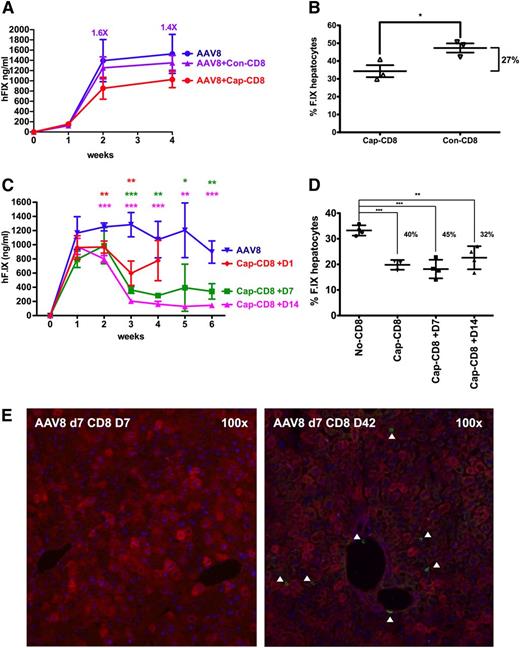
Earlier preclinical studies suggested that MHC I presentation of capsid antigen could be avoided by use of capsids lacking heparin-binding sites such as AAV8.28 However, T-cell responses and loss of hF.IX expression were observed in a recent clinical trial following hepatic AAV8 gene transfer.18 AAV8 has strong tropism for the liver and is therefore widely considered for clinical applications, including current and future trials in hemophilia.18,43,44 Poor in vitro transduction with AAV8 kept us from performing assays in hepatocyte cultures. However, because the dominant capsid epitopes for AAV2 and AAV8 are nearly identical and highly cross-reactive in BALB/c mice,19 our in vivo model allowed us to test for the effect of a serotype switch. Upon administration of cap-CD8 at day 1 after AAV8 gene transfer, a 33% decrease in systemic expression and a significant reduction in hF.IX+ hepatocytes (by ∼27%) was observed (Figure 7A-B). A similar result was obtained in a second experiment (Figure 7C-D). In contrast to AAV2, delayed administration of the cap-CD8 (on day 7 or day 14) had a substantial and even greater effect on hF.IX levels, resulting in a three- to fivefold loss of expression and a reduction in hF.IX+ hepatocytes by up to 45% (Figure 7C-D). Also in contrast to AAV2, CD8+ T-cell infiltrates were not seen at day 7 after T-cell transfer but were prominent at later time points, regardless of the time point of T-cell transfer (Figure 7E; supplemental Figure 7). One of the T-cell transfers resulted in a minor but statistically significant increase in liver enzymes at day 7 (supplemental Figure 8), whereas the others did not (data not shown), suggesting that the time course of hepatotoxicity was altered as well.
Effects of capsid-specific CD8+ T cells on hF.IX expression from an AAV8 vector. In vitro expanded capsid-specific, Ld-restricted CD8+ T cells (cap-CD8) isolated from BALB/c mice were adoptively transferred by tail vein injection into Rag-1 −/− BALB/c mice after administration of AAV8-ApoE/hAAT-hF.IX vector (1 × 1011 vg per mouse; n = 4 per experimental group). Cells were given 1 day (A-B) or 1 day, 7 days, or 14 days (C-D) after vector administration. (A,C) Systemic hF.IX expression. (B) Percent hF.IX+ hepatocytes 4 weeks after gene transfer. Statistically significant differences to the control group: *P < .05; **P < .01; ***P < .001. (D) Percent hF.IX+ hepatocytes 6 weeks after gene transfer. (E) Immunostains for hF.IX and CD8+ T cells 1 and 6 weeks after cap-CD8 T-cell administration to mice that had received AAV8 vector 7 days prior. Analyses were performed as for Figure 1.
Effects of capsid-specific CD8+ T cells on hF.IX expression from an AAV8 vector. In vitro expanded capsid-specific, Ld-restricted CD8+ T cells (cap-CD8) isolated from BALB/c mice were adoptively transferred by tail vein injection into Rag-1 −/− BALB/c mice after administration of AAV8-ApoE/hAAT-hF.IX vector (1 × 1011 vg per mouse; n = 4 per experimental group). Cells were given 1 day (A-B) or 1 day, 7 days, or 14 days (C-D) after vector administration. (A,C) Systemic hF.IX expression. (B) Percent hF.IX+ hepatocytes 4 weeks after gene transfer. Statistically significant differences to the control group: *P < .05; **P < .01; ***P < .001. (D) Percent hF.IX+ hepatocytes 6 weeks after gene transfer. (E) Immunostains for hF.IX and CD8+ T cells 1 and 6 weeks after cap-CD8 T-cell administration to mice that had received AAV8 vector 7 days prior. Analyses were performed as for Figure 1.
Discussion
A novel murine model for targeting of AAV-transduced liver by capsid-specific CTLs
Capsid-specific CD8+ T cells have been identified as a major hurdle and potential source of immunotoxicity in human recipients of AAV gene transfer. However, their ability to target AAV-transduced human hepatocytes in vivo has been indirectly inferred from correlations between their emergence in peripheral blood and onset of transaminitis,11,18 whereas animal models failed to show this effect. Data presented here in a highly defined setting clearly demonstrate that capsid-specific CD8+ T cells can target AAV-transduced liver cells, resulting in reduction of transgene expression by up to 80% and of transduced hepatocytes by up to 50%. Expanded CD8+ T cells infiltrated the liver and caused transaminitis in AAV-hF.IX–transduced mice. The greater loss of systemic hF.IX expression than of hF.IX+ hepatocytes suggests that the in vivo effects of cap-CD8 may not entirely be explained by hepatocyte killing. Interestingly, Breous et al45 reported that inflammation could silence AAV-derived gene expression in the liver via a tumor necrosis factor α–dependent mechanism. Several reasons may explain why capsid-specific CTLs were effective in our mouse model while previous experimental approaches were not. In vitro expanded T cells are expected to be more antigen specific and highly and more uniformly active. Aggressor T cells are best studied in Rag−/− mice, where confounding effects of endogenous T cells or regulatory T cells are eliminated. Increased inflammatory signals further enhanced the effect of the T cells. In future studies, we can test other specific T-cell types, use cells from knockout mice, or transfer cells into Rag−/− mice lacking additional gene functions, thereby addressing questions about specific immune functions in a defined manner.39 One potential caveat of the model is that immune-deficient mice may show somewhat altered transduction efficiencies. For example, AAV2 is inferior to AAV2(Y-F) vector in immune-competent mice (supplemental Figure 9) but, for unknown reasons, shows more similar hF.IX expression levels in the Rag−/− mice.
CTL-mediated killing is less efficient against hepatocytes transduced with Y-F capsid
Using in vitro assays, hepatocytes transduced with AAV2 vectors were effectively killed by cap-CD8 over a wide range of vector doses, suggesting efficient MHC I presentation of input capsid, which is consistent with our prior studies.23 Under conditions of limited phosphorylatation of capsid following cellular entry, vector performance improves because of reduced ubiquitination and proteasomal degradation.34-36,46 Here, we find that capsids engineered to minimize tyrosine phosphorylation are also less presented to CD8+ T cells. At MOIs similar to those expected to be therapeutic in vivo, we clearly observed reduction in MHC I presentation and in killing of hepatocytes. Moreover, the murine model confirmed the protective effect of the modified capsid on hF.IX-transduced hepatocytes in vivo. Thus, 3 models consistently support use of Y-F mutant capsids. Our studies also indicate that uptake, processing, and T-cell activation is unchanged for AAV2(Y-F) capsid; however, tyrosine mutations enhance virus trafficking to the nucleus of hepatocytes.35 Therefore, the difference in antigen processing and presentation by the target cells (ie, hepatocytes) likely explains the increased resistance to CTLs. Capsids that are not processed by proteasomes may be degraded via other proteases such as cathepsins.47
Similarities and differences between humans and the murine model
Similar to data in humans, our murine model exhibits targeting of AAV-transduced liver by capsid-specific CTLs, resulting in hepatotoxicity and loss of therapeutic expression. However, our data also indicate differences between humans and mice. The fact that we were not able to obtain loss of gene expression when CD8+ T cells were given 1 week or later after AAV2 gene transfer suggests that AAV2 capsid antigen presentation by murine hepatocytes is only sufficient for targeting by CTLs in the first days after vector administration. Previous studies from our team demonstrated that epitopes displayed by AAV2 capsid can trigger proliferation of CD8+ T cells with corresponding receptors for several weeks following hepatic transfer of the vector.29 It is possible that cell lysis as tested here requires higher levels of surface-expressed MHC I–peptide complexes compared with T-cell proliferation as was tested in our previous studies. Nonetheless, there is another interesting similarity between the mouse model and clinical studies. With the caveat that the number of vector-treated patients is small, responses to AAV2 occurred ∼1 month after vector administration, whereas the response to AAV8 was markedly delayed to 8 to 10 weeks.15,18 Our novel mouse data support delayed and prolonged MHC I presentation of AAV8 capsid antigen in the liver (compared with AAV2), which allowed us to generate a time course of hF.IX expression more similar to what was observed in humans (ie, an initial rise followed by a decline). Despite more rapid uncoating,48 AAV8 capsid-derived peptides appear to be presented for an extended period of time. These results further support use of this particular murine model to study differences in capsid antigen presentation for alternate serotypes.
Implications for human gene therapy
The impact of CD8+ T-cell responses on AAV gene transfer to human liver is evident from the complete loss of hF.IX expression in a subject treated with AAV2.15 Gene transfer in this patient had raised hF.IX levels from <1% to ∼11% prior to transaminitis. Another subject recently treated with an AAV8 vector expressing hF.IX initially expressed levels of 6% to 8% of hF.IX, which declined to 2%.18 Following the onset of transaminitis, the steroid drug prednisolone was given in an attempt to blunt the T-cell response. Both patients had been treated with a vector dose of 2 × 1012 vg/kg (similar to the 4 × 1012 vg/kg used in our mouse studies). Clinical studies suggest that the risk of T-cell responses and transaminitis is vector-dose dependent.15,17,18 Therefore, development of capsid variants that have increased efficacy and reduced antigen presentation by hepatocytes is highly desirable. Our models would predict that AAV2(Y-F) vector minimize but not entirely eliminate the risk of CTL-mediated loss of transduced hepatocytes. Effectiveness of the proteasome inhibitor indicates a residual level of proteasomal processing of AAV2(Y-F) capsid. Interestingly, the combination of AAV2(Y-F) vector and bortezomib yielded the most optimal results for transgene expression (Figures 1E and 4E; supplemental Figure 9A). Contribution of other nonimmunoproteasomes in the cell (located in the cytoplasm or the nucleus) or other effects of proteasome inhibitors (such as generation of reactive oxygen species as discussed elsewhere)22 may have also contributed to these findings.
Our study sheds light on findings from recent clinical results,18 indicating that use of AAV serotype 8 alters the time frame but does not eliminate the risk of losing expression due to capsid-specific CTLs, which needs to be considered for trial design. Finally, the model will be useful to design pharmacologic maneuvers that may reduce T-cell activation or reduce or alter processing and/or presentation of capsid-derived antigen.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants (P01 HD078810 [C.T., H.C.J.E., K.A.H., R.W.H., and S.Z.], R01 HL097088 [A.S. and R.W.H.], and F32 HL096281 [D.M.M.]), and by the Howard Hughes Medical Institute (K.A.H.).
Authorship
Contribution: A.T.M., E.B.-T., D.M.M., J.D.F., C.H., and S.Z. performed experiments; A.T.M., E.B.-T., D.M.M., C.T., D.A.O., K.A.H., F.M., and R.W.H. designed experiments; A.T.M., E.B.-T., D.M.M., H.C.J.E., C.T., D.A.O., K.A.H., F.M., and R.W.H. interpreted data; A.S. provided critical reagents; K.A.H., F.M., and R.W.H. supervised and coordinated the study; and A.T.M., D.M.M., C.T., D.A.O., H.C.J.E., K.A.H., F.M., and R.W.H. wrote the manuscript.
Conflict-of-interest disclosure: R.W.H. has received royalty payments from Genzyme Corp for license of AAV-FIX technology. K.A.H. is an inventor on issued and pending patents on AAV gene transfer technologies.
Correspondence: Roland Herzog, University of Florida, Cancer and Genetics Research Center, 2033 Mowry Rd, Room 203, Gainesville, FL 32610; e-mail: rherzog@ufl.edu; Federico Mingozzi, Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, Colket Translational Research Building, Suite 5064, Philadelphia, PA 19104; e-mail: fmingozzi@genethon.fr; and Katherine A. High, Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, Colket Translational Research Building, Suite 5060, Philadelphia, PA 19104; e-mail: high@e-mail.chop.edu.
References
Author notes
A.T.M., E.B.-T., D.M.M., K.A.H., F.M., and R.W.H. contributed equally to the study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal