In this issue of Blood, Jourdan and colleagues from the French AML Intergroup demonstrate the prognostic value of minimal residual disease (MRD) in adult patients with core binding factor (CBF) acute myeloid leukemia (AML).1
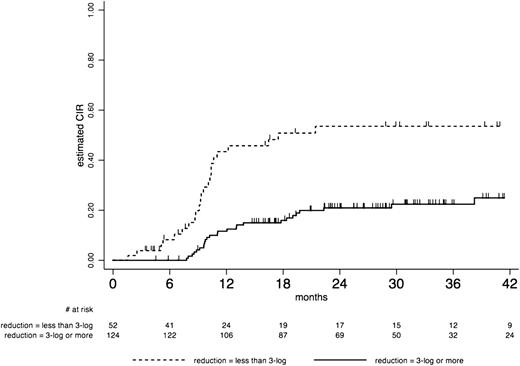
Outcome by “Minimal Residual Disease response” after the first consolidation course in CBF AML cumulative incidence of relapse (CIR): At 36 months CIR was estimated at 22% (95% CI, 16-32) in patients who achieved a 3 log MRD reduction vs 54% (95% CI, 39-69) in those who did not. See figure 5A in the article by Jourdan et al that begins on page 2213.
Outcome by “Minimal Residual Disease response” after the first consolidation course in CBF AML cumulative incidence of relapse (CIR): At 36 months CIR was estimated at 22% (95% CI, 16-32) in patients who achieved a 3 log MRD reduction vs 54% (95% CI, 39-69) in those who did not. See figure 5A in the article by Jourdan et al that begins on page 2213.
Currently, the most important prognostic factors for AML are based on cytogenetics and molecular abnormalities, which are assessed at diagnosis.2 Although these factors have been shown to be of utmost importance in risk stratification, the treatment outcome of patients within the thus-defined risk groups is still highly variable. New prognostic factors that, apart from diagnosis parameters, may include treatment and response related factors are needed.
MRD, defined as the persistence of leukemic cells after chemotherapy at numbers below the sensitivity detection level of routine morphology, represents the sum of the effect of all relevant cellular resistance mechanisms, pharmacokinetic resistance, dosage and compliance, and other unknown factors affecting the effectiveness of treatment. Relapses still are a major cause of dismal outcome in AML treatment and are generally thought to be the result of outgrowth of these persisting leukemic cells.
Many studies have shown that MRD cell frequency after different cycles of therapy offers a highly independent prognostic factor, both in adult and childhood AML. In adult AML, these data are mostly derived from retrospective correlative studies.
In this prospective study, patients with CBF-AML carrying the t(8;21) or inv(16)/t(16;16) chromosomal abnormality, characterized by the presence of RUNX1-RUNX1T1 or CBFB-MYH11 fusion transcripts, respectively, were monitored for MRD. CBF-AML has a favorable prognosis with a cure rate of 65% with chemotherapy alone.2 Frequent receptor tyrosine kinase mutations are present in CBF-AML, where especially mutations in KIT and FLT3 have been associated with a worse outcome. In the present study, KIT, FLT3, and N/K-RAS gene mutations were examined at diagnosis.1 Patients were randomly allocated either to an intensive induction or a standard treatment arm. Subsequently, when a complete remission (CR) was achieved, patients received 3 postremission cycles consisting of high-dose ARA-C. After each consolidation course, the levels of MRD were monitored for RUNX1-RUNX1T1 or CBFB-MYH11 transcripts by real-time quantitative polymerase chain reaction. It was planned by protocol to perform an allogeneic transplant in patients who did not achieve at least a 3-log MRD reduction. However only 12 of 52 patients, who based on MRD level classified for an allogeneic transplant, actually received one. Reasons for that are not mentioned by the investigators, but most likely are due to reluctance of individual physicians to transplant these good-risk patients who, at least for inv(16) AML, usually have a rather good prognosis if transplanted in second remission. The clinical outcome of the study confirmed the good prognosis of CBF-AML, but intensified induction was not associated with a better survival. Striking were the findings associated with the MRD monitoring: although higher WBC, RTK gene mutations, and a <3-log MRD reduction after the first consolidation cycle individually were associated with a higher risk of relapse, MRD response remained the only significant prognostic factor in multivariate analysis. In patients who achieved 3-log MRD reduction vs patients who did not, the 3-year cumulative incidence of relapse was 22% and 54%, respectively, and 3-year relapse-free survival was 73% and 44%, respectively. The same applied for the absolute 0.1% MRD level.
This is in line with recent observations by the UK MRC trial group, who also concluded that MRD monitoring by quantitative reverse-transcription polymerase chain reaction in CBF-AML at different time points identified patients at high risk for relapse.3
Clearly, a new definition of CR is emerging in CBF-AML. How this should be implemented and whether preemptive therapeutic intervention would be of benefit is not established given the fact that the majority of patients can be rescued after relapse.
In a recent ELN recommendation, a patient-specific application of allogeneic hematopoietic stem cell transplantation (HSCT) in patients with AML in first CR was proposed integrating the risk for relapse and nonrelapse mortality. The recommendation aims for a disease-free survival benefit of at least 10% for the individual patient as compared with consolidation by a nonallogeneic HSCT approach.4 It could be argued that a patient with CBF-AML and a <3-log reduction of MRD level associated with a relapse risk of around 50% should be offered an allogenic HSCT if the estimated transplant-related mortality is 10% to 15%.
Can we extrapolate to AML groups other than the CBF-AML? The German Austrian AML study group showed that NPM1(mut) transcript levels were significantly associated with prognosis after each treatment cycle.5 Mutations in FLT3, WT1, and CEBPα offer other molecular markers potentially useful for MRD detection. However, robust data in prospective studies are currently lacking.
Another valuable method to monitor MRD is by flow cytometry that relies on the expression of “leukemia-associated immunophenotypes” defined as the presence of a combination of antigens and/or other flow-cytometric abnormalities that are absent in normal cells. It is widely applicable (in >90% of AML), quick, and relatively cheap, but usually less sensitive than molecular MRD.
Studies showing the prognostic value of flow-cytometric MRD were mostly performed in a single-institute setting, resulting in well-known potential pitfalls such as bias in patient groups and subjective judgment.
The next logical and obvious step would be to perform the studies in a prospective and preferably multicenter way. In childhood AML, such a recent study showed that after initial induction chemotherapy, MRD was detected in one-third of patients without morphologic evidence of disease, which in turn was highly correlated with relapse and an independent predictor of outcome.6 In adult AML, prospective studies are about to be published. However, based on the existent data, many AML trial groups are in the process of implementation MRD monitoring (flow cytometry and molecular) in new clinical trials.
Fine-tuning of techniques and merging of flow and molecular genetic assays may ultimately bring us closer to the final goal of real individualized risk assessment and therapy in patients with AML.
MRD is at the edge to offer a new definition for CR and is possibly useful as a surrogate end point for outcome of studies investigating new drugs in AML.
Conflict-of-interest disclosure: The authors declare no competing financial interests.