To the editor:
Ly6G is a marker of neutrophils, but the function of this GPI-linked receptor has remained mysterious. Wang et al provided data implicating Ly6G in regulating leukocyte migration.1 These investigators found that low doses of antibodies (10 μg total administration) directed against Gr-1 or Ly6G reduced experimental arthritis, an effect not due to neutrophil depletion. Instead, anti–Gr-1 or anti-Ly6G antibodies were proposed to inhibit neutrophil recruitment, leading to a reduction in arthritis by acting on the β2-integrins CD11a and CD11b and on ICAM-1 binding levels.
Our laboratory uses intravital imaging to assess the entire leukocyte recruitment cascade directly in real time in vivo. Although granulocyte depletion is achieved at high anti–Gr-1 antibody doses (150-250 μg), we visualize leukocyte recruitment using lower IV doses (1-40 μg) of fluorochrome-labeled anti–Gr-1 (clone RB6-8C5) or anti-Ly6G (clone 1A8). Exploiting these antibodies as imaging tools, we have characterized rolling, adhesion, intravascular crawling, transmigration, emigration, phagocytosis, and tissue NETosis in multiple tissues including skin, liver, brain, and muscle.2-6 Despite not observing defects in leukocyte recruitment with these antibodies, we performed new experiments to compare neutrophil recruitment directly in transgenic LysM-eGFP mice, in which peripheral blood neutrophils are visualized without antibodies to Ly6G, with LysM-eGFP mice treated with a fluorochrome-labeled anti–Gr-1 antibody (Figure 1 and supplemental Video 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
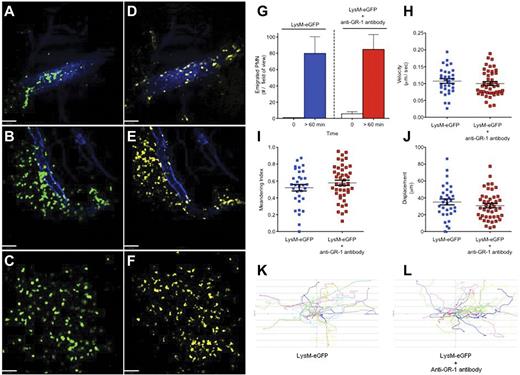
Direct real-time imaging of leukocyte recruitment in vivo using spinning disk confocal intravital microscopy. (A-F) Staphylococcus aureus–infected (intradermal 1 × 108 CFU of methicillin-resistant S aureus, USA300-2406) LysM-eGFP mouse skin microvasculature was imaged in vivo. Images shown in panels A through C demonstrate neutrophils defined by LysM-eGFP expression (green), and the corresponding side-by-side images shown in panels D through F demonstrate neutrophils defined by the anti–Gr-1 antibody (5 μg IV) conjugated to Alexa Fluor 750 (yellow) at identical time points. Endothelium was visualized using an anti–PECAM-1 antibody (10 μg IV) conjugated to Alexa Fluor 647 (blue). (A,D) Images of neutrophils rolling and adhering within a vessel captured immediately after administration of live S aureus into the mouse skin. (B,E) Images of neutrophils transmigrating out of a vessel captured 60 minutes after S aureus administration. (C,F) Images of neutrophils emigrating and chemotaxing within the parenchyma 60 minutes after S aureus administration. Images were captured using an Olympus BX51 upright microscope equipped with a 10×/0.3 numeric aperture air objective. The microscope was equipped with a confocal light path (WaveFx; Quorum) based on a modified CSU-10 head (Yokogawa Electric). Laser excitation at 488, 649, and 730 nm (Cobalt) was used in rapid succession with the appropriate long-pass filters (Semrock). A 512 × 512 pixel back-thinned EMCCD camera (C9100-13; Hamamatsu) was used for fluorescence detection. Volocity Acquisition Version 6.0 software (Improvision) was used to drive the confocal microscope. Images captured using the spinning disk were processed and analyzed in Volocity with linear adjustments to the black-and-white points for improved image quality. Bars represent 60 μm. Images are representative of 3 independent experiments. (G-L) Neutrophil emigration (G), parenchymal tissue crawling velocity (H), meandering index (I), displacement over 10 minutes (J), and tissue neutrophil crawling tracks (K-L) after live S aureus administration in LysM-eGFP mice without antineutrophil antibodies and LysM-eGFP mice that received IV anti–Gr-1 (5 μg) immediately before imaging. For cell quantification, 2 independent LysM-eGFP experiments were compared with 3 independent LysM-eGFP + anti–Gr-1 antibody experiments. All procedures performed were approved by the University of Calgary Animal Care Committee and were in accordance with the Canadian Guidelines for Animal Research.
Direct real-time imaging of leukocyte recruitment in vivo using spinning disk confocal intravital microscopy. (A-F) Staphylococcus aureus–infected (intradermal 1 × 108 CFU of methicillin-resistant S aureus, USA300-2406) LysM-eGFP mouse skin microvasculature was imaged in vivo. Images shown in panels A through C demonstrate neutrophils defined by LysM-eGFP expression (green), and the corresponding side-by-side images shown in panels D through F demonstrate neutrophils defined by the anti–Gr-1 antibody (5 μg IV) conjugated to Alexa Fluor 750 (yellow) at identical time points. Endothelium was visualized using an anti–PECAM-1 antibody (10 μg IV) conjugated to Alexa Fluor 647 (blue). (A,D) Images of neutrophils rolling and adhering within a vessel captured immediately after administration of live S aureus into the mouse skin. (B,E) Images of neutrophils transmigrating out of a vessel captured 60 minutes after S aureus administration. (C,F) Images of neutrophils emigrating and chemotaxing within the parenchyma 60 minutes after S aureus administration. Images were captured using an Olympus BX51 upright microscope equipped with a 10×/0.3 numeric aperture air objective. The microscope was equipped with a confocal light path (WaveFx; Quorum) based on a modified CSU-10 head (Yokogawa Electric). Laser excitation at 488, 649, and 730 nm (Cobalt) was used in rapid succession with the appropriate long-pass filters (Semrock). A 512 × 512 pixel back-thinned EMCCD camera (C9100-13; Hamamatsu) was used for fluorescence detection. Volocity Acquisition Version 6.0 software (Improvision) was used to drive the confocal microscope. Images captured using the spinning disk were processed and analyzed in Volocity with linear adjustments to the black-and-white points for improved image quality. Bars represent 60 μm. Images are representative of 3 independent experiments. (G-L) Neutrophil emigration (G), parenchymal tissue crawling velocity (H), meandering index (I), displacement over 10 minutes (J), and tissue neutrophil crawling tracks (K-L) after live S aureus administration in LysM-eGFP mice without antineutrophil antibodies and LysM-eGFP mice that received IV anti–Gr-1 (5 μg) immediately before imaging. For cell quantification, 2 independent LysM-eGFP experiments were compared with 3 independent LysM-eGFP + anti–Gr-1 antibody experiments. All procedures performed were approved by the University of Calgary Animal Care Committee and were in accordance with the Canadian Guidelines for Animal Research.
Using a mouse model of Staphylococcus aureus cellulitis,6 we imaged neutrophil recruitment directly using spinning disk confocal intravital microscopy. LysM-eGFP mice received an intradermal injection of live S aureus and fluorochrome-conjugated anti–Gr-1 antibody (5 μg IV) or Ly6G clone 1A8 (data not shown), followed immediately by intravital imaging. Neutrophils were visualized rolling and adhering within the dermal microvasculature in both the LysM-eGFP (green) laser channel and the anti–Gr-1 (yellow) laser channel immediately after infection (Figure 1A,D and supplemental Video 1). Within 60 minutes of infection, LysM-eGFP/anti–Gr-1 double-positive peripheral blood neutrophils transmigrated into the parenchyma (Figure 1B,E) and continued to chemotax through the skin (Figure 1C,F). Therefore, we conclude that the entire recruitment cascade, from rolling to chemotaxis, occurs in anti–Gr-1 antibody-treated animals.
To ensure that the anti–Gr-1 antibody did not have subtle quantitative effects on neutrophil recruitment, we compared LysM-eGFP mice with and without anti–Gr-1 antibody (Figure 1G-L). The number of emigrated neutrophils was similar between the 2 groups (Figure 1G). Once emigrated, neutrophils from each group crawled with identical velocities (Figure 1H), meandering index (a measurement of the ability of a cell to move in a straight line; Figure 1I), and displacement (Figure 1J). Leukocyte crawling tracks are shown for tissue neutrophils in LysM-eGFP mice (Figure 1K) and LysM-eGFP mice treated with anti–Gr-1 (Figure 1L). These data demonstrated that no quantifiable difference in leukocyte recruitment and behavior occurred after anti–Gr-1 administration. A fluorochrome-conjugated anti-Ly6G antibody (1A8 clone) did not disrupt any of the leukocyte recruitment measurements (data not shown).
Our ability to visualize all aspects of the leukocyte recruitment cascade directly in vivo demonstrates that low-dose anti–Gr-1 antibodies do not interfere with neutrophil migration. Anti–Gr-1 administration in vitro inhibited stimulation-induced up-regulation of β2-integrins above baseline; however, cell-surface levels continued to be high, as were ICAM-1–binding levels. It is likely that these levels continue to mediate normal recruitment under in vivo physiologic models. The abrogation of arthritis reported by Wang et al is an interesting observation that highlights the need to better characterize Ly6G. However, given our data, we do not believe that the mechanism proposed by the authors adequately explains their observations. We demonstrate that fluorochrome-conjugated antineutrophil antibodies against Gr-1 can be used to investigate leukocyte recruitment without interfering with cell migration.
Authorship
The online version of this article contains a data supplement.
Acknowledgments: The Canadian Institute of Health Research provided the operating grants to support this work. P.K. is an Alberta Innovates Health Solutions Scientist, Canada Research Chair, and the Snyder Chair in Critical Care Medicine. B.G.Y is a Clinical Scholar in the Department of Critical Care Medicine (University of Calgary), an Alberta Innovates Health Solutions Clinical Fellow, and a Canadian Institute of Health Research Fellow.
Contribution: B.G.Y designed and performed the experiments, analyzed the results, produced the figures, and wrote the manuscript; and P.K designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Bryan G. Yipp, McCaig Tower, 3134 Hospital Drive NW, Calgary, AB, Canada T2N 2T9; e-mail: bgyipp@ucalgary.ca; or Dr Paul Kubes, HRIC 4AA16, 3330 Hospital Drive NW, Calgary, AB, Canada T2N 4N1; e-mail: pkubes@ucalgary.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal