In this issue of Blood, Zeng and colleagues demonstrate in SIV-infected macaques and HIV-infected humans that CD4+ T cells actively maintain the fibroblastic reticular cell network in lymphoid tissues and that this network in turn is needed to maintain normal T-cell homeostasis and function.1
How HIV causes immune dysfunction and disease has never been fully elucidated. This is true in antiretroviral untreated disease, in which HIV (or its cousin SIV) causes progressive loss of CD4+ T-cells and a broad range of immunologic abnormalities. This is also true in long-term antiretroviral treated disease, where despite complete or near complete suppression of viral replication for years, immune dysfunction, chronic inflammation, and excess morbidity often persist.
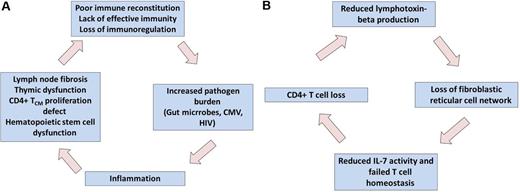
Through a series of remarkably elegant and consistent experiments over the past decade, the Haase, Schacker, and Estes laboratories have developed a comprehensive model that may explain some of the most proximal mechanisms by which HIV causes disease (see figure). The focus of their work has been those areas of lymphoid tissues where antigen presentation occurs and T-cell homeostasis is regulated.
Two connected self-perpetuating cycles of inflammation, lymphoid tissue fibrosis, and immunodeficiency might be central to pathogenesis of antiretroviral untreated and treated HIV infection. Systemically, HIV-mediated increases in inflammation results in T-cell homeostasis, immunodeficiency, increased pathogen burden, and more inflammation (A). Within the local lymphoid tissues, HIV-associated CD4+ T-cell loss results in reduced production of lymphotoxin-beta, which is essential in maintaining the architecture necessary to produce new T cells (B). Professional illustration by Paulette Dennis.
Two connected self-perpetuating cycles of inflammation, lymphoid tissue fibrosis, and immunodeficiency might be central to pathogenesis of antiretroviral untreated and treated HIV infection. Systemically, HIV-mediated increases in inflammation results in T-cell homeostasis, immunodeficiency, increased pathogen burden, and more inflammation (A). Within the local lymphoid tissues, HIV-associated CD4+ T-cell loss results in reduced production of lymphotoxin-beta, which is essential in maintaining the architecture necessary to produce new T cells (B). Professional illustration by Paulette Dennis.
During acute HIV/SIV infection, the virus directly infects CCR5-expressing CD4+ T cells, leading to their rapid depletion. This appears to occur where these cells are most densely populated, such as the mucosal surfaces in the gut and in secondary lymphoid tissues (eg, spleen, lymph nodes, and mucosa lymphoid tissues). Virus-mediated loss of cells leads directly and indirectly to breakdown of the local host defenses in gut, which in turn leads to chronic systemic exposure to microbial products.2 The loss of CD4+ T cells also leads to a systemic immune deficiency, which in turn leads to an excessive amount of pathogens (including HIV itself, as well as other persistent infections such as CMV).3 Suppression of HIV replication with antiretroviral therapy reverses this process, but the effect is often incomplete. The chronic inflammatory process and persistent immunodeficiency that persist during antiretroviral therapy predict a number of complications, including vascular disease, atherosclerosis, nerve damage, liver disease, kidney disease, and bone disease.4 There is now a broad evidence base that supports this intuitive model.5
A major unanswered question is why does the immune system fail to simply replace those CD4+ T cells that are lost as a consequence of infection. After the acute phase of the disease, only a small fraction of cells are infected and killed on a daily basis. Such cells should readily be replaced through normal homeostatic mechanisms, but this clearly does not occur. Once an effective antiretroviral regimen is administered, these same homeostatic mechanisms should result in eventual restoration of a normal immune system. Although this may eventually happen in some optimally treated patients, a significant proportion of the antiretroviral-treated population exhibits durable and perhaps life-long defects, including low numbers of CD4+ T-cell counts, elevated inflammation, and clinical immunodeficiency. Determining why therapy fails to restore health is among the most compelling and urgent questions in HIV therapeutics.
Zeng and colleagues have previously argued that a self-perpetuating vicious circle is initiated during early infection that prevents restoration of immune function, even after complete suppression of HIV replication is achieved.6 The site of the problem is the parafollicular T-cell zone of lymph nodes and other secondary lymphoid structures. A well-organized and highly regulated fibroblastic reticular cell (FRC) network within this zone provides the mechanical infrastructure that enables antigen-presenting cells to activate CD4+ T cells and regulates the expression and activity of those cytokines like interleukin-7 that maintain T-cell homeostasis. In what may be a central problem in HIV pathogenesis, the HIV-mediated inflammatory response activates a potent and sustained immunosuppressive T regulatory cell response. These immunoregulatory cells produce TGF-β, which in turn causes collagen deposition, tissue fibrosis, and the loss of the FRC network.7 This progressive and possibly irreversible loss of lymphoid microarchitecture means that T-cell homeostasis cannot be properly regulated.6 A self-perpetuating cycle of T-cell dysfunction, increased pathogen loads, increased inflammation, lymphoid fibrosis, and more T-cell dysfunction is eventually established. Although antiretroviral therapy reduces much of the pro-inflammatory signaling that started the process, the damage is done, and inflammatory signals persist that act to prevent restoration of immune function.9
As outlined by Zeng and colleagues in this journal, a second related vicious circle contributes to this process. The FRC network is maintained in part by lymphotoxins. To determine with more precision the source of these proteins, Zeng et al used antibodies to specifically deplete CD4+ or CD8+ T cells, and found that the loss of the former was causally associated with reduced lymphotoxin production and the loss of the FRC network.1 In addition, the natural hosts of SIV infection, which typically maintain normal T-cell homeostasis despite high-level viremia, have an intact FRC network. Finally, in a nice work of pure translational investigation, Zeng et al observed that treatment of cancer with chemotherapy or radiation causes CD4+ T-cell depletion and damage to the FRC, an observation that can be explained by many mechanisms but which is generally consistent with the nonhuman primate data. The end result of all of this is a second self-perpetuating cycle in which CD4+ T-cell loss results in less immunotoxin. This in turn results in loss of the FRC-maintained reticular microarchitecture and ultimately further loss ofCD4+ T cells.
Persistently low CD4+ T-cell counts and elevated levels of inflammatory biomarkers are both highly predictive of morbidity and mortality in HIV infected adults.4,10,11 Fortunately, the model of disease pathogenesis provided by Zeng and colleagues can direct clinical investigators toward a number of potential interventions that can reverse 1 or both of the aforementioned self-perpetuating cycles.1 These experimental interventions include drugs that remove pro-inflammatory pathogens and microbial products (eg, valganciclovir for CMV, rifaximin for gut microbes, sevelamer for lipopolysaccharide), drugs that directly prevent fibrosis (eg, angiotensin II receptor antagonists and ACE inhibitors), and drugs that have more broad effects in reducing inflammation (eg, statins, nonsteroidal antinflammatory drugs, methotrexate, and mesalamine). Because disease is often easier to prevent than treat and the untreated phase of the infection is associated with persistent, high-level inflammation and presumably progressive lymphoid fibrosis, then a reasonable conclusion from the body of work summarized here is for patients to start antiretroviral therapy as soon as possible. This rationale is clearly shared by some treatment guidelines, particularly those in the US, and will likely continue to shape how we approach the management of this infection.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal