While idiotype vaccines have shown promise for B-cell malignancies, production is cumbersome; thus, targeting a common antigen on malignant B cells using an off-the-shelf approach would provide significant logistical advantages.
Bendandi et al demonstrated early on that complete molecular remission in lymphoma patients who had received a patient-specific idiotype vaccine is possible.1 In a follow-up randomized phase 3 study, Schuster et al showed that vaccination with a patient-specific anti-idiotype vaccine led to improved disease-free survival in patients with follicular lymphoma.2 Patients enrolled in study who achieved a complete response to chemotherapy were randomly assigned to vaccine with idiotype conjugated to keyhole limpet hemocyanin (KLH) with local GM-CSF versus KLH control. The median disease-free survival for vaccine-treated patients was 44.2 months versus 30.6 months for patients in the control arm (hazard ratio 0.62; P = .047). However, this patient-specific vaccine required a significant amount of time to produce (6-12 months was allowed). Only 69% of patients randomized remained in complete response to chemotherapy by the time vaccine was available, making the remaining 31% ultimately ineligible to receive vaccine or placebo. Nevertheless, the promising disease-free survival seen among vaccinated patients provides hope that therapeutic vaccines will prove to be a well-tolerated treatment associated with clinical benefit in the setting of minimal disease.
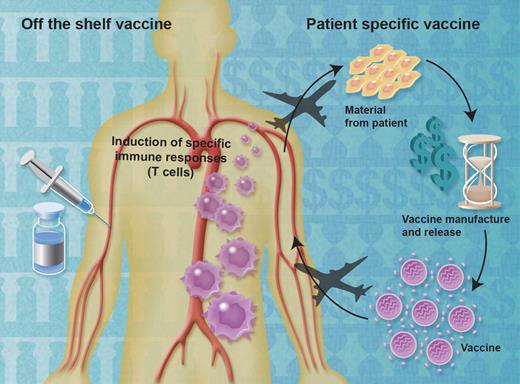
The complexity of making a patient-specific vaccine can lead to significant financial and temporal costs (see figure). The only currently approved therapeutic vaccine for cancer is sipuleucel-T, which showed a statistically significant and clinically meaningful 22% reduction in risk of death in patients with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer.3 The median 4.1-month improvement in survival was seen without significant side effects (only 1.5% of patients had to stop treatment because of toxicity). However, patients treated with sipuleucel-T must undergo apheresis at 3 time points and the vaccine is subsequently manufactured from the apheresis products at a central processing facility capable of rapid turnaround. This complexity adds to the price of the vaccine: $93 000 US for a complete course of treatment.4 Several off-the-shelf therapeutic vaccines have shown preliminary evidence of efficacy,5–7 providing hope that improvements in patient outcomes with this modality may lead to therapeutic options that are less resource-intense.
Patient-specific therapeutic vaccines are generated from immune cells or tumor material obtained from cancer patients. This process is resource-intensive, but has yielded therapeutic vaccines with clinical benefit. In contrast, large numbers of doses of off-the-shelf vaccines can be manufactured at one time, then stored until needed. In theory, off-the-shelf vaccines should be cheaper to manufacture while simplifying time and supply lines. Professional illustration by Alice Y. Chen.
Patient-specific therapeutic vaccines are generated from immune cells or tumor material obtained from cancer patients. This process is resource-intensive, but has yielded therapeutic vaccines with clinical benefit. In contrast, large numbers of doses of off-the-shelf vaccines can be manufactured at one time, then stored until needed. In theory, off-the-shelf vaccines should be cheaper to manufacture while simplifying time and supply lines. Professional illustration by Alice Y. Chen.
Identification of a common antigen on B-cell malignancies that is not present on normal B cells thus offers the potential for an off-the-shelf vaccine for lymphomas that avoids the resource-intense manufacture and release of patient-specific vaccines. In a convincing set of experiments described in this issue of Blood, Weng et al demonstrate that T-cell leukemia/lymphoma 1 (TCL1) oncoprotein is overexpressed on a wide range of human B-cell lymphomas, but only selectively expressed on normal B cells.8 They demonstrate not only that TCL1 peptide-specific T cells could be generated from normal donors, but that TCL1-specific T cells were present in the blood of patients with lymphoma, and that these T cells could be expanded and, in an HLA-A2–restricted manner, could lyse autologous lymphoma cells but not normal B cells. This approach should help catalyze intensive translational efforts to rationally design off-the-shelf therapeutic vaccines that, alone or in combination with other therapies, can efficiently propel clinically significant antilymphoma immunity in minimal disease settings without significant side effects.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health