Abstract
The discovery of JAK2617F mutation paved the way for the development of small molecule inhibitors of JAK1/2 resulting in first approved JAK1/2 inhibitor, ruxolitinib, for the treatment of patients with myelofibrosis (MF). Although JAK1/2 inhibitor therapy is effective in decreasing the burden of symptoms associated with splenomegaly and MF-related constitutional symptoms, it is neither curative nor effective in reducing the risk of leukemic transformation. Presently, allogeneic hematopoietic cell transplantation (HCT) is the only curative therapy for MF. A significant risk of regimen-related toxicities, graft failure, and GVHD are major barriers to the success of HCT in MF. Because of significant HCT-associated morbidity and mortality, divergent opinions regarding its appropriate role in this clinical situation have emerged. In this review, the risk-benefit ratios of modern drug therapy compared with HCT in MF patients are analyzed. A risk-adapted approach individualized to each patient's biologic characteristics and comorbidities is described, which is currently warranted in determining optimal treatment strategies for patients with MF. Inclusion of JAK1/2 inhibitor therapy in future transplant conditioning regimens may provide an opportunity to overcome some of these barriers, resulting in greater success with HCT for MF patients.
Introduction
Primary myelofibrosis (PMF) is a myeloproliferative neoplasm that originates at the level of the hematopoietic stem cell and is characterized by cytopenias, extramedullary hematopoiesis, megakaryocytic hyperplasia, reactive marrow fibrosis, and systemic symptoms resulting from elevated levels of inflammatory and proangiogenic cytokines.1 The median age at diagnosis is 67 years,2 and only 13% of patients are 50 years of age or younger at the time of referral.3 PMF is notorious for its heterogeneity, and its clinical course varies from an indolent course persisting for almost a decade in some to others with rapidly progressive disease with a survival of 12 to 24 months.4-6 A form of myelofibrosis (MF) indistinguishable from PMF can occur as part of the natural history of both polycythemia vera (PV) and essential thrombocythemia (ET), and are referred to as post-PV or ET-related MF. These 3 disorders will be collectively referred to as MF in this report.
Allogeneic hematopoietic cell transplantation (HCT) is the only curative treatment for MF at present. The therapeutic efficacy of HCT in patients with MF is mediated partly through the antineoplastic effect of pretransplant conditioning regimen and through an alloimmune GVL effect. The significant morbidity and mortality associated with HCT in MF have led to divergent opinions regarding its appropriate role.7,8 Several important issues, including patient selection, timing of HCT, optimal conditioning regimens, role of prior splenectomy, and its appropriate use in older persons, remain unresolved, resulting in considerable diversity of application of HCT in MF patients.
Evolution of HCT for MF over time
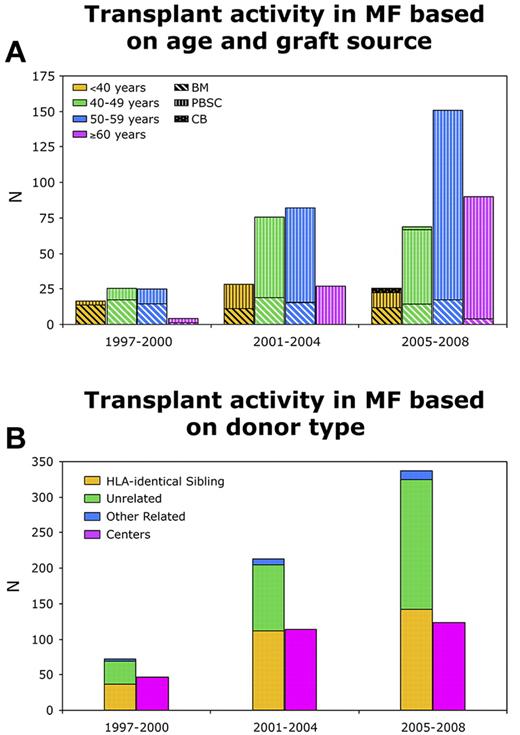
MF is a rare indication for HCT, and even major transplant centers perform limited numbers of transplantations for patients with MF. HCT was historically underused in MF as graft failure was thought intuitively to be highly likely in the setting of marrow fibrosis. Early studies demonstrated the feasibility of engraftment as well long-term disease control in patients with MF using myeloablative conditioning (MAC) regimens.9-11 The use of reduced intensity conditioning (RIC) has become widespread during the last decade. There has been a slow progressive increase in the use of HCT for MF as reflected in the trends generated by the Center for International Blood and Marrow Transplant Research (CIBMTR; Table 1; Figure 1A-B). These data provide a snapshot of current transplant practices in MF and demonstrate increasing use of HCT in older patients, greater use of peripheral blood stem cell grafts, and the growing popularity of RIC regimens.
Time trends in HCT for myelofibrosis: data from CIBMTR
| Characteristics of patients . | 1997-2000, N (%) . | 2001-2004, N (%) . | 2005-2008, N (%) . |
|---|---|---|---|
| No. of patients | 72 | 213 | 336 |
| No. of centers | 47 | 114 | 123 |
| Age, y | |||
| ≤ 9 | 1 (1) | 0 | 3 (1) |
| 10-19 | 1 (1) | 5 (2) | 8 (2) |
| 20-29 | 3 (4) | 3 (1) | 4 (1) |
| 30-39 | 12 (17) | 20 (9) | 11 (3) |
| 40-49 | 26 (36) | 76 (36) | 69 (21) |
| 50-59 | 25 (35) | 82 (38) | 151 (45) |
| 60-69 | 4 (6) | 25 (12) | 87 (26) |
| ≥ 70 | 0 | 2 (1) | 3 (1) |
| Donor group | |||
| HLA-identical sibling | 36 (50) | 111 (52) | 142 (42) |
| Other related | 3 (4) | 9 (4) | 12 (4) |
| Unrelated | 33 (46) | 93 (44) | 182 (54) |
| Graft type | |||
| BM | 48 (67) | 45 (21) | 48 (14) |
| PBSCs | 24 (33) | 168 (79) | 284 (85) |
| CB | 0 | 0 | 4 (1) |
| Conditioning regimen | |||
| Proportion of RIC | 15% | 37% | 45% |
| Characteristics of patients . | 1997-2000, N (%) . | 2001-2004, N (%) . | 2005-2008, N (%) . |
|---|---|---|---|
| No. of patients | 72 | 213 | 336 |
| No. of centers | 47 | 114 | 123 |
| Age, y | |||
| ≤ 9 | 1 (1) | 0 | 3 (1) |
| 10-19 | 1 (1) | 5 (2) | 8 (2) |
| 20-29 | 3 (4) | 3 (1) | 4 (1) |
| 30-39 | 12 (17) | 20 (9) | 11 (3) |
| 40-49 | 26 (36) | 76 (36) | 69 (21) |
| 50-59 | 25 (35) | 82 (38) | 151 (45) |
| 60-69 | 4 (6) | 25 (12) | 87 (26) |
| ≥ 70 | 0 | 2 (1) | 3 (1) |
| Donor group | |||
| HLA-identical sibling | 36 (50) | 111 (52) | 142 (42) |
| Other related | 3 (4) | 9 (4) | 12 (4) |
| Unrelated | 33 (46) | 93 (44) | 182 (54) |
| Graft type | |||
| BM | 48 (67) | 45 (21) | 48 (14) |
| PBSCs | 24 (33) | 168 (79) | 284 (85) |
| CB | 0 | 0 | 4 (1) |
| Conditioning regimen | |||
| Proportion of RIC | 15% | 37% | 45% |
PBSCs indicates peripheral blood stem cells; CB, cord blood; and RIC, reduced intensity conditioning.
CIBMTR reporting trends showing transplant activity in myelofibrosis. (A) Based on age and graft source. (B) Based on donor type.
CIBMTR reporting trends showing transplant activity in myelofibrosis. (A) Based on age and graft source. (B) Based on donor type.
HCT or JAK1/2 inhibitor therapy: a changing risk-benefit ratio
Advances in supportive care, conditioning regimens, GVHD prophylaxis, and high-resolution typing for the selection of unrelated donors, have improved the safety and outcomes of HCT.12 Application of RIC has expanded the scope of HCT to many older patients or those with multiple comorbidities that would have previously precluded them from the option of HCT.13
Significant progress has been made in the last few years in understanding the natural history of MF. The discovery of the JAK2V617F mutation in 2005 provided a significant impetus to the laboratory and translational research in MF, culminating in the Food and Drug Administration approving the first JAK 1/2 inhibitor (ruxolitinib; INCB018424) for the treatment of MF in November 2011. In a phase 3 randomized trial, 42% of patients treated with ruxolitinib experienced more than or equal to 35% reduction in spleen volume compared with 0.7% of patients receiving placebo (P < .001) regardless of JAK2 mutational status.14 In addition, 46% of ruxolitinib-treated patients experienced a more than or equal to 50% improvement in constitutional symptoms compared with 5% in the placebo group. After a median follow-up of 51 weeks, the ruxolitinib group experienced a significant reduction in mortality (hazard ratio = 0.50; 95% CI, 0.25-0.98; P = .04). Patients who received ruxolitinib had mean reduction in JAK2V617F allele burden of 10.9% at week 24 and 21.5% at week 48; patients who received placebo had a mean increase of 3.5% at week 24 and 6.3% at week 48. Another phase 3 trial comparing ruxolitinib with best available therapy reproduced the findings of improvement in splenomegaly and MF-related symptoms; however, a survival benefit was not demonstrated.15 Two case-control studies compared the survival of patients treated with ruxolitinib with historical controls and found contradictory results.16,17
Ruxolitinib was uniformly ineffective in reversing abnormalities in peripheral blood or histopathologic abnormalities in the marrow, eliminating marker cytogenetic abnormalities or reducing the JAK2V617F allele burden to a degree associated with tyrosine kinase inhibitor therapy of BCR/ABL1 for chronic myeloid leukemia.14,15 Other limitations have been the rapid return of splenomegaly and MF-related symptoms after discontinuation of the drug. Clinical trials with several alternative JAK inhibitors and other novel agents are underway in patients with MF to identify alternative strategies that might have more substantial effects on long-term survival.18
Because the currently available JAK inhibitor therapy is not curative, HCT remains an important therapeutic option for patients with advanced forms of MF. In this review, we evaluate the positioning of HCT in the management of MF in the light of changing risk/benefit ratios associated with HCT and emerging novel therapeutic options.
Optimal timing of therapeutic intervention in MF
Controversy over the optimal timing of HCT for MF remains. Many patients with MF have a prolonged life expectancy and enjoy a reasonable quality of life. Therefore, exposing such patients to the immediate risk of morbidity and mortality associated with HCT is not currently thought to be appropriate. However, most patients with MF will eventually develop cytopenias, symptomatic splenomegaly, and troublesome constitutional symptoms. Some patients transform to acute myeloid leukemia (AML), which further reduces the success of HCT. Therefore, understanding the natural history of MF, and risk factors associated with leukemic transformation (LT), are vital in deciding the optimal time to consider HCT to gain the greatest benefit to the patient from such a high-risk procedure.
Risk stratification strategies for MF
Prognostic factors for survival.
A variety of prognostic scoring systems based on clinical characteristics have been created with the aim of identifying higher-risk patients who would benefit from HCT or experimental therapeutics. These stratification schemas were developed with the hope of minimizing treatment-related risk for patients with anticipated prolonged survival until their disease acquired characteristics associated with sufficiently shortened survival to merit the risk associated with such potentially risky therapeutic options.
Among conventional scoring systems, the Lille scoring system has been the most widely used.19 Based on hemoglobin levels and the presence of leukopenia or leukocytosis, low-, intermediate-, and high-risk groups were identified with median survival of 93, 26, and 13 months, respectively. A new prognostic model known as International Prognostic Scoring System (IPSS) has been developed.4 Five independent risk factors, including age more than 65 years, hemoglobin less than 10 g/dL, WBC count more than 25 × 109/L, peripheral blood (PB) blasts more than 1%, and presence of constitutional symptoms at the time of diagnosis were predictive of survival of patients with PMF at the time of diagnosis. The presence of 0, 1, 2, and more than or equal to 3 factors are categorized as low-, intermediate-1–, intermediate-2–, and high-risk disease with a median survival of 135, 95, 48, and 27 months, respectively (Table 2).
Modern risk stratification systems for survival and leukemic transformation in primary myelofibrosis
| Risk stratification system . | Applicability . | Prognostic factors . | Risk score . | Median survival, mo . | Comments . |
|---|---|---|---|---|---|
| IPSS4 | Diagnosis | Age > 65 y | 1 | Low risk (0), 135 | |
| Anemia (Hb < 10 g/dL) | 1 | Intermediate 1 risk (1), 95 | |||
| WBC count > 25 × 109/L | 1 | Intermediate 2 risk (2), 48 | |||
| Blood blasts > 1% | 1 | High ( ≥ 3), 27 | |||
| Constitutional symptoms | 1 | ||||
| DIPSS6 | Any time in disease course | Age > 65 y | 1 | Low risk (0), not reached | DIPSS also predicts evolution to AML, Hazard ratios of 7.8 and 24.9 for Intermediate-2 and high-risk category compared with low risk23 |
| Anemia (Hb < 10 g/dL) | 2 | Intermediate 1 risk (1-2), 170 | |||
| WBC count > 25 × 109/L | 1 | Intermediate 2 risk (3-4), 48 | |||
| Blood blasts ≥ 1% | 1 | High (5-6), 18 | |||
| Constitutional symptoms | 1 | ||||
| DIPSS plus5 | Any time in disease course | DIPSS low risk | 0 | Low risk (0), 185 | First calculate DIPSS score6 and then add the score of transfusion dependency, cytogenetics, and thrombocytopenia to calculate final DIPSS plus score |
| DIPSS intermediate-1 | 1 | Intermediate 1 risk (1), 78 | |||
| DIPSS intermediate-2 | 2 | Intermediate 2 risk (2-3), 35 | |||
| DIPSS high-risk | 3 | High (≥ 4), 16 | Based on single institution data, needs validation | ||
| PLUS | |||||
| Transfusion dependency | 1 | ||||
| Unfavorable cytogenetics* | 1 | ||||
| Platelets < 100 × 109/L | 1 | ||||
| IWG risk model for LT25 | Any time in disease course | Risk factors: high-risk cytogenetics† | 2 | Risk of LT: low (score 0), 3% at 3 y | |
| Risk factors: blood blasts ≥ 2% | 1 | Risk of LT: intermediate (score 1), 10% at 3 y | |||
| Risk factors: platelets ≤ 50 × 109/L | 1 | Risk of LT: high (score 2), 35% at 3 y |
| Risk stratification system . | Applicability . | Prognostic factors . | Risk score . | Median survival, mo . | Comments . |
|---|---|---|---|---|---|
| IPSS4 | Diagnosis | Age > 65 y | 1 | Low risk (0), 135 | |
| Anemia (Hb < 10 g/dL) | 1 | Intermediate 1 risk (1), 95 | |||
| WBC count > 25 × 109/L | 1 | Intermediate 2 risk (2), 48 | |||
| Blood blasts > 1% | 1 | High ( ≥ 3), 27 | |||
| Constitutional symptoms | 1 | ||||
| DIPSS6 | Any time in disease course | Age > 65 y | 1 | Low risk (0), not reached | DIPSS also predicts evolution to AML, Hazard ratios of 7.8 and 24.9 for Intermediate-2 and high-risk category compared with low risk23 |
| Anemia (Hb < 10 g/dL) | 2 | Intermediate 1 risk (1-2), 170 | |||
| WBC count > 25 × 109/L | 1 | Intermediate 2 risk (3-4), 48 | |||
| Blood blasts ≥ 1% | 1 | High (5-6), 18 | |||
| Constitutional symptoms | 1 | ||||
| DIPSS plus5 | Any time in disease course | DIPSS low risk | 0 | Low risk (0), 185 | First calculate DIPSS score6 and then add the score of transfusion dependency, cytogenetics, and thrombocytopenia to calculate final DIPSS plus score |
| DIPSS intermediate-1 | 1 | Intermediate 1 risk (1), 78 | |||
| DIPSS intermediate-2 | 2 | Intermediate 2 risk (2-3), 35 | |||
| DIPSS high-risk | 3 | High (≥ 4), 16 | Based on single institution data, needs validation | ||
| PLUS | |||||
| Transfusion dependency | 1 | ||||
| Unfavorable cytogenetics* | 1 | ||||
| Platelets < 100 × 109/L | 1 | ||||
| IWG risk model for LT25 | Any time in disease course | Risk factors: high-risk cytogenetics† | 2 | Risk of LT: low (score 0), 3% at 3 y | |
| Risk factors: blood blasts ≥ 2% | 1 | Risk of LT: intermediate (score 1), 10% at 3 y | |||
| Risk factors: platelets ≤ 50 × 109/L | 1 | Risk of LT: high (score 2), 35% at 3 y |
Hb indicates hemoglobin; WBC, white blood cell; and IWG, International Working Group.
Unfavorable cytogenetics includes +8, −7/7q−, i(17q), −5/5q−, 12p−, Inv(3) or 11q23.
High-risk cytogenetics was defined as monosomal karyotype, Inv 3 or i(17q).
The risk factors of IPSS were also analyzed in a time-dependent fashion termed dynamic IPSS (DIPSS).6 Acquisition of anemia had a higher adverse impact on survival (roughly double) compared with other factors; therefore, anemia was assigned a score of 2 (Table 2). Thus, DIPSS differs from IPSS, which gave the same weight to each risk factor.
Marked interpatient variability within IPSS and DIPSS risk groups is observed, suggesting a potential role of other risk factors for precise risk stratification. Cytogenetics, transfusion dependency, and thrombocytopenia were incorporated into the DIPSS plus scoring system (Table 2).5 DIPSS plus has not been to date validated in another independent dataset.
For risk stratification, it is recommended to use IPSS at the time of diagnosis and the DIPSS anytime during the disease course in patients with PMF.6
Risk of LT.
LT after MF results in a resistant form of AML that is almost universally associated with poor outcomes.20-22 One of the goals of HCT for PMF patients is to transplant them before LT to avoid this complication. Patients with intermediate-2 and high-risk DIPSS groups had a 7.8-fold and 24.9-fold risk of LT compared with patients with low-risk disease.23 Patients with either thrombocytopenia (platelet count < 100 × 109/L) or an unfavorable karyotype (as discovered in “Cytogenetics”) had 18% and 31% risk of LT at 5 years and 10 years, respectively.5 The corresponding risk of LT was 6% and 12% in patients with platelet count more than or equal to 100 × 109/L and not having unfavorable karyotype. It also appears that risk of LT may be higher in patients with transfusion dependency.24 The risk of LT has been further evaluated by introducing a weighted scoring system.25 This proposed system described 3 risk factors for LT: high-risk karyotype [defined as monosomal karyotype, Inv 3 or i(17q); score 2]; PB blasts more than or equal to 2% (score 1); and thrombocytopenia (platelet count ≤ 50 × 109/L; score 1).25 Based on scores of these 3 factors, there were 3 distinct groups with scores of 0, 1, and more than or equal to 2, and corresponding risk of LT at 3 years was 3%, 10%, and 35%, respectively.
Available data highlight the importance of cytogenetics, PB blasts, and severity of thrombocytopenia in predicting LT in PMF.
Risk stratification in post-PV MF and post-ET MF.
It is important to note that IPSS, DIPSS, and DIPSS plus have been studied in patients with PMF. These scoring systems have not been validated in patients with post-PV MF and ET-related MF. Available studies addressing this topic are limited by small sample size.26,27 At present, same scoring systems are being used for risk stratification in patients with PPV-MF and PET-MF. Efforts are in progress to develop prognostic models for these patients. Until more data become available, risk stratification models used for PMF may be used for enrollment of these patients in clinical trials and therapeutic decision making.
Current risk models for survival and LT have been generated from retrospective studies in an era when therapeutic options were limited and HCT rarely used. Prospective validation and further improvement on these models by including other important variables, such as comorbidities in the setting of improving treatment options, will be desirable in future.
Cytogenetics and molecular aberrations in MF
Cytogenetics: a growing appreciation of its utility for prognostication of MF patients.
Several reports have highlighted the impact of cytogenetic abnormalities on the outcomes of patients with MF.28-31 Commonly observed cytogenetic abnormalities in MF and their prognostic impact are summarized in Table 3. Cytogenetic abnormalities are seen in approximately 35% to 43% of patients with MF.31-33 Karyotypic abnormalities involving one chromosome (sole), 2 chromosomes (double), or more than or equal to 3 chromosomes (complex) are observed in approximately 70%, 15%, and 15% patients, respectively.32 Based on a large series of 433 patients with PMF, a 2-tiered cytogenetic risk stratification system has been proposed identifying favorable and unfavorable karyotypes.32 Favorable karyotypes include: normal, sole 20q−, sole 13q−, sole chromosome 1 translocation/duplication, sole +9, other sole abnormalities (excluding those with unfavorable risk), and 2 abnormalities excluding unfavorable ones. Unfavorable karyotypes include: complex (≥ 3 abnormalities), sole +8, sole −7/7q−, sole 5/5q−, i(17q), inv(3), 12p− or 11q23, and 2 abnormalities including an unfavorable type. Patients with favorable and unfavorable karyotypes have median survivals of 5.2 years and 2 years, respectively and corresponding risk of LT at 5 years is 7% and 46%, respectively.
Impact of cytogenetics and molecular prognostic markers in patients with myelofibrosis
| Cytogenetics/molecular markers . | Frequency, % . | Impact on LT . | Impact on survival . | Comments . |
|---|---|---|---|---|
| Diploid karyotype32,33 | 57-71 | 7% at 5 y | 46 mo | |
| Sole abnormalities, standard risk | 15-22 | Comparable with diploid karyotype | Comparable survival with diploid karyotype | |
| Sole del 20q32,33 | 4-7 | Association noted between sole 20q− and leucopenia and thrombocytopenia32 | ||
| Sole del 13q32,33 | 2-4 | |||
| Sole +932,33 | 2-3 | |||
| Chromosome 1 translocation/duplication32 | 2-3 | |||
| Other miscellaneous sole abnormalities other than listed under unfavorable risk32 | 5 | |||
| Sole abnormalities, unfavorable risk | 4-7 | Significant high risk of LT compared with diploid karyotype (46% vs 7% at 5 y) | Poor survival comparable to diploid karyotype (15 mo vs 46 mo) | |
| Sole +830,32 | 2-3 | |||
| Sole −7/7q−/−5/5q−/Inv 332 | 1-2 | |||
| Chromosome 17 abnormalities33 | 1-2 | |||
| Double abnormalities* | ||||
| Standard risk32 | 2-3 | Comparable with diploid karyotype | Similar to diploid karyotype | |
| Significant high risk of LT compared with diploid karyotype (46% vs 7% at 5 y) | Similar to complex karyotype | |||
| Double abnormalities† | ||||
| Unfavorable risk32 | 2-3 | |||
| Complex karyotype (≥ 3 abnormalities)32,33 | 6-8 | Significant high risk of LT compared with diploid karyotype (46% vs 7% at 5 y) | Poor survival comparable to diploid karyotype (15 mo vs 46 mo) | ∼ 50% patients with CK have monosomal karyotype with very poor survival30 |
| JAK2V617F41-44 | 55-60 | No difference compared with WT | No difference compared with WT | Low JAK2V617F allele burden associated with inferior survival42,43 ; and associated with myelodepletive type of MF |
| MPL45 | 8-10 | No difference compared with JAK2V617F mutation positive or unmutated JAK2/MPL | No difference compared with JAK2V617F mutation positive or unmutated JAK2/MPL | MPL and JAK2 unmutated patients were significantly younger than JAK2-mutated patients45 |
| LNK39 | 2-3 | Not known | Not known | |
| TET286 | 7-17 | No | No | More frequent in older patients compared with younger patients86 |
| DNMT3A87 | 10-15 | No | No | |
| IDH88 | 4 | Shortened LFS in PMF | Short survival in PMF | |
| EZH289 | 6-9 | Shorter LFS in patients with PMF with EZH2 mutations | Associated with short survival in idiopathic MF, independent of IPSS | Associated with higher leukocyte count, blast cell counts, and larger splenomegaly |
| SUZ12/EED90,91 | 1-3 | Not clear | Not clear | May be associated with LT |
| Cytogenetics/molecular markers . | Frequency, % . | Impact on LT . | Impact on survival . | Comments . |
|---|---|---|---|---|
| Diploid karyotype32,33 | 57-71 | 7% at 5 y | 46 mo | |
| Sole abnormalities, standard risk | 15-22 | Comparable with diploid karyotype | Comparable survival with diploid karyotype | |
| Sole del 20q32,33 | 4-7 | Association noted between sole 20q− and leucopenia and thrombocytopenia32 | ||
| Sole del 13q32,33 | 2-4 | |||
| Sole +932,33 | 2-3 | |||
| Chromosome 1 translocation/duplication32 | 2-3 | |||
| Other miscellaneous sole abnormalities other than listed under unfavorable risk32 | 5 | |||
| Sole abnormalities, unfavorable risk | 4-7 | Significant high risk of LT compared with diploid karyotype (46% vs 7% at 5 y) | Poor survival comparable to diploid karyotype (15 mo vs 46 mo) | |
| Sole +830,32 | 2-3 | |||
| Sole −7/7q−/−5/5q−/Inv 332 | 1-2 | |||
| Chromosome 17 abnormalities33 | 1-2 | |||
| Double abnormalities* | ||||
| Standard risk32 | 2-3 | Comparable with diploid karyotype | Similar to diploid karyotype | |
| Significant high risk of LT compared with diploid karyotype (46% vs 7% at 5 y) | Similar to complex karyotype | |||
| Double abnormalities† | ||||
| Unfavorable risk32 | 2-3 | |||
| Complex karyotype (≥ 3 abnormalities)32,33 | 6-8 | Significant high risk of LT compared with diploid karyotype (46% vs 7% at 5 y) | Poor survival comparable to diploid karyotype (15 mo vs 46 mo) | ∼ 50% patients with CK have monosomal karyotype with very poor survival30 |
| JAK2V617F41-44 | 55-60 | No difference compared with WT | No difference compared with WT | Low JAK2V617F allele burden associated with inferior survival42,43 ; and associated with myelodepletive type of MF |
| MPL45 | 8-10 | No difference compared with JAK2V617F mutation positive or unmutated JAK2/MPL | No difference compared with JAK2V617F mutation positive or unmutated JAK2/MPL | MPL and JAK2 unmutated patients were significantly younger than JAK2-mutated patients45 |
| LNK39 | 2-3 | Not known | Not known | |
| TET286 | 7-17 | No | No | More frequent in older patients compared with younger patients86 |
| DNMT3A87 | 10-15 | No | No | |
| IDH88 | 4 | Shortened LFS in PMF | Short survival in PMF | |
| EZH289 | 6-9 | Shorter LFS in patients with PMF with EZH2 mutations | Associated with short survival in idiopathic MF, independent of IPSS | Associated with higher leukocyte count, blast cell counts, and larger splenomegaly |
| SUZ12/EED90,91 | 1-3 | Not clear | Not clear | May be associated with LT |
Two abnormalities, excluding unfavorable.
Two abnormalities, including unfavorable.
Our understanding of cytogenetics in MF is at early stages compared with other myeloid malignancies, and the scheme discribed in previous paragraph is likely to be validated and refined in the future.
Molecular aberrations in MF: are they useful in prognostication?
The majority of patients with MF have hematopoietic cells that are characterized by overactivation of the JAK-STAT pathway or mutations affecting chromatin structure (Table 3). JAK2 V617F is the most common mutation observed in MF patients.34-37 Additional mutations include JAK2 exon 12, MPL, and LNK.38-40 The prognostic significance of JAK2V617F has been evaluated in several studies.41-44 One study described the adverse impact of JAK2V617F in patients with PMF,41 and another study described a higher rate of LT in patients with JAK2V617F.44 Other studies with larger sample sizes have not shown a significant difference between JAK2 mutated and unmutated patients.42,43 Importantly, 2 studies have demonstrated the shortened survival of JAK2V617F-positive patients with a low allele burden,42,43 indicating that a low JAK2V617F allele burden was associated with a myelodepletive variant of PMF. Overall, the prognostic significance of JAK2V617F mutation remains unclear. The presence of MPL mutations does not impact survival or LT in PMF.45
As highlighted in Table 3, the biologic consequences of most of the other known mutations remain unclear. The oncogenetic events that transform myeloproliferative neoplasm (MPN) to AML are poorly characterized. Several genes were implicated in LT, as evidenced by mutational analysis of 63 patients with AML secondary to a preexisting MPN.46 Frequent mutations were identified in TET2 (26.3%), ASXL1 (19.3%), IDH1 (9.5%), and JAK2V617F (36.8%) mutations in AML, and all possible mutational combinations of these genes were observed. Analysis of 14 patients with paired samples during chronic-phase MPN and subsequent AML revealed that TET2 mutations were frequently acquired at the time of LT (6 of 14; 43%). In contrast, ASXL1 mutations were almost always detected in both the MPN and AML clones from individual patients. Mutations in TET2, ASXL1, and IDH1 were common in MPN-related AML. Although TET2/ASXL1 mutations may precede acquisition of JAK2 mutations by the MPN clone, mutations in TET2, but not ASXL1, are commonly acquired at the time of LT. These findings indicate that the mutational order of events in MPN and sAML varies in different patients and that TET2 and ASXL1 mutations have distinct roles in MPN pathogenesis and LT. Because some cases of AML have no preexisting JAK2/TET2/ASXL1/IDH1 mutations, it is probable that there are other mutations that are necessary for LT. Recently, recurrent mutations in the serine/arginine-rich splicing factor 2 (SRSF2) gene were described in AML transformed from MPNs.47 At present, none of these mutations can be used in developing more robust risk stratification for either survival or risk of LT.
Identifying higher-risk patients will aid in more accurate decision making. Every patient with MF should have a detailed risk assessment at regular intervals on an ongoing basis using modern risk stratification systems (Table 2).
Nontransplant therapeutic options in MF
Conventional options: limited efficacy and scanty prospective evaluation
Clinical symptoms of patients with MF can be grouped into 3 main categories: cytopenias (mainly anemia), splenomegaly, and constitutional symptoms. Nontransplant treatment options mainly include supportive therapy and the use of various drugs for symptomatic improvement. Hydroxyurea (HU) is the most commonly used conventional treatment for patients with MF and is sometimes useful in managing some of the hyperproliferative manifestations of MF, such as splenomegaly, extreme leukocytosis, and thrombocytosis.48 Clinical improvement with HU according to International Working Group for Myeloproliferative Neoplasms Research and Treatment criteria was seen in 40% of patients, and median duration of response was 13.2 months. Anemia or new-onset pancytopenia was observed in 45% patients. The usefulness of HU has been recently questioned. HU was the most commonly used best available therapy in COMFORT-II trial, and none of the patient treated with HU met the primary endpoint of more than or equal to 35% reduction in spleen volume.15 Various other agents, such as erythropoiesis-stimulating agents, androgens, busulfan, anagrelide, interferon, and corticosteroids, and immunomodulatory derivatives, such as thalidomide or lenalidomide, have been used with mixed success in MF.8 Limited efficacy and scanty prospective clinical data have prevented defining the exact role of each of these agents in the management of MF. None has been shown to modify the natural history of the disease, and their use is mainly physician dependent.
Other treatments for advanced splenomegaly include splenectomy and low-dose radiation therapy. Historically, splenectomy has been performed in approximately 10% of patients with MF in the pre-JAK inhibitor era4 and is associated with significant risk of perioperative complications (27.7%) and mortality (6.7%).49 With the wider availability of JAK inhibitors, the option of splenectomy is likely to decrease and will be used in selected patients who are unable to tolerate JAK inhibitor therapy because of severe cytopenias. Splenic radiation results in temporary short responses and is associated with significant cytopenias.
Novel drugs in MF
JAK1/2 inhibitor therapy: benefits and limitations.
The clinical benefits of the JAK1/2 inhibitors are related to reducing the burden of troublesome symptoms of MF by reduction in splenomegaly and amelioration of constitutional symptoms.14,15,50-52 These agents mainly inhibit dysregulated JAK-STAT signaling present in JAK2V617F-positive and -negative patients and therefore are equally efficacious irrespective of JAK2 mutation status. Anti-JAK1–mediated reduction of proinflammatory and proangiogenic cytokines is an important effect of these drugs.52,53 However, there are limited effects on survival, resolution of marrow fibrosis, cytogenetic abnormalities, JAK2V617F allele burden, or LT, indicating a lack of effect on disease progression. Other limitations of current JAK1/2 inhibitors are the occasional return of MF-related symptoms on their discontinuation, unpredictable response duration, and lack of long-term safety and efficacy data. Nevertheless, JAK inhibitor therapy is an important advance for patients with MF and has significant clinical value in decreasing the symptom burden and improvement in quality of life.
Apart from JAK1/2 inhibitors, several other novel agents are at various stages of clinical development in MF. The most prominent among these are third-generation immunomodulatory derivative pamolidomide (phase 3), histone deacetylase inhibitors, such as panobinostat and givonostat (phase 2), mTOR inhibitors (phase 1 or 2), inhibitor of hedgehog pathway (Saridegib, phase 1), AB0024, a monoclonal antibody inhibiting LOXL2 (phase 1), and a TGF-β signaling inhibitor (phase 1).
Transplantation for MF
Major studies of HCT outcomes with more than 20 patients with MF in chronic phase, and published in peer-reviewed journals are summarized in Table 4. These studies, except one,54 are retrospective in nature. Another prospective study completed by Myeloproliferative Diseases-Research Consortium has not been published in a peer-reviewed journal yet.55
Summary of major reports on HCT outcomes in MF
| Reference . | Timeline of HCT . | N . | Median age, y (range) . | Conditioning regimen . | % of patients with RIC . | % with MRD . | NRM . | PFS . | OS . | Comment . |
|---|---|---|---|---|---|---|---|---|---|---|
| Guardiola11 | 1979-1997 | 55 | 42 (4-53) | TBI based (63%) | 0 | 90 | 27% at 1 y | 39% at 5 y | 47% at 5 y | Hb < 10 g/dL and osteomyelosclerosis associated with lower survival |
| Deeg10 | 1980-2002 | 56 | 43 (10-66) | Bu/Cy in 78% | 0 | 64 | 14% at 3 mo | NR | 58 at 3 y | Targeted Bu use improved survival; cGVHD 59% at 2 y |
| Daly9 | 1990-2002 | 25 | 48 (45-50) | TBI based (92%) | 0 | 52 | 48% at 1 y | NR | 41 at 2 y | Prohibitive NRM; no benefit of splenectomy |
| Rondelli66 | NR | 21 | 54 (27-68) | Multiple | 100 | 85 | 10% at 1 y | 81% at 2.7 y | 85% at 2.7 y | Extensive cGVHD in 44%; 2 patients needed DLI for 100% donor chimerism; resolution of fibrosis and splenomegaly in majority |
| Kerbauy58 | NR | 104 | 49 (18-70) | Multiple, Bu/Cy (62%) | 9 | 50 | 35% at 5 y | NR | 61% at 5 y | 3 syngeneic donors, 54 of the patients overlapped with a prior report10 ; targeted Bu improved OS; comorbidity score had impact on survival |
| Patriarca65 | 1986-2006 | 100 | 49 (21-68) | Multiple, Bu/Cy 50% of full intensity; Thiotepa + Cy in 46% of RIC | 52 | 78 | 43% at 3 y | 35% at 3 y | 42% at 3 y | AHCT before 1995; unrelated donor and longer interval from diagnosis predicted worse outcome but not conditioning intensity; relapse at 2 y 41%, progressive decline in NRM over 20 y studied |
| Kroger54 | 2002-2007 | 103 | 55 (32-68) | Flu-Bu (100%) | 100 | 32 | 16% at 1 y | 51% at 5 y | 67% at 5 y | First prospective study in MF, cGVHD in 43%; 12% NRM for fully matched donor AHCT; age > 55 y and HLA mismatch adversely affected OS; JAK2-positive recipients had better EFS and OS; splenectomy increased risk of relapse |
| Gupta64 | 1998-2005 | 46 | 47 y MAC; 54 y RIC | Multiple, Cy TBI (96%) for MAC; Flu Bu (70%) for RIC | 50 | 54 | 48% for MAC and 27% for RIC at 3 y | 43% for MAC and 58% for RIC at 3 y | 48% for MAC and 68% for RIC at 3 y | RIC recipients had more advanced disease and poor KPS; low risk of relapse after either conditioning; lower GVHD with novel conditioning possibly related to use of ATG |
| Ballen56 | 1989-2002 | 289 | 47 (18-73) | Multiple, Bu/Cy (43%) | 21 | 56 | 35% siblings 50% for URD at 5 y | 33% siblings 27% for URD at 5 y | 37% siblings 30% for URD at 5 y | Relapse at 5 y, 32% in sibling and 23% in URD; performance status, peripheral blasts sibling donor status impacted OS; RIC was similar in outcomes, except early NRM |
| Alchalby71 | 1999-2009 | 162 | 56 (32-73) | Flu-Bu in 96% | 100 | 27 | 22% at 1 y | 46% at 5 y | 62% at 5 y | 82 patients reported previously54 ; age and HLA mismatch impacted NRM; 23% relapse at 3 y; clearance of mutated JAK2 at median of 96 days, and this reduced relapse risk |
| Bacigalupo63 | 1994-2007 | 46 | 51 (24-67) | Thiotepa-Cy + melphalan | 100 | 65 | 24% at 5 y | NR | 45% at 5 y | A risk score based on transfusion history, spleen > 22 cm and alternative donor use predicted lower OS; no benefit for splenectomy |
| Stewart76 | 1989-2005 | 51 | 49 (19-64) | Multiple, RIC in 47% | 47 | 65 | 41% at 2 y | 44% and 24% at 3 y for MAC and RIC | 44% and 31% at 3 y for MAC and RIC | |
| Robin92 | 1997-2008 | 147 | 53 (20-68) | Multiple | 69 | 61 | 39% at 4 y | 32% at 4 y | 39% at 4 y | 19% patients had LT; poor outcome with mismatched donor |
| Samuelson13 | 1999-2007 | 30 | 65 (60-78) | Multiple | 63 | 50 | 13% at day 100 | 40% at 3 y | 45% at 3 y | Studied outcomes in patients ≥ 60 y, 7 patients had preceding LT |
| Abelsson67 | 1982-2009 | 92 | 46 for MAC, 55 for RIC | Multiple | 56 | 40 | 32% for MAC and 24% for RIC at 2 y | NR | 49% for MAC and 59% for RIC at 5 y | Overall NRM similar, but 5-year OS superior for RIC younger than age 60 years; less advanced MF associated with better OS |
| Nivison-Smith69 | 1993-2005 | 57 | 47 (16-71) | Multiple | 26 | 68 | 25% at 1 y | 58% at 5 y | Poor outcome in patients > 50 y | |
| Ditschkowski70 | 1994-2010 | 76 | 50.5 (22-67) | Multiple | NR | 35 | 36% at 5 y | 50% at 5 y | 53% at 5 y | Significant high risk of relapse in patients without cGvHD; |
| DIPSS was predictive of survival | ||||||||||
| Scott57 | 1990-2009 | 170 | 51.5 (12-78) | Multiple | NR | 50 | 34% at 5 y | 57% at 5 y | 57% at 5 y | Post-HCT success was dependent on pre-HCT DIPSS scores |
| Reference . | Timeline of HCT . | N . | Median age, y (range) . | Conditioning regimen . | % of patients with RIC . | % with MRD . | NRM . | PFS . | OS . | Comment . |
|---|---|---|---|---|---|---|---|---|---|---|
| Guardiola11 | 1979-1997 | 55 | 42 (4-53) | TBI based (63%) | 0 | 90 | 27% at 1 y | 39% at 5 y | 47% at 5 y | Hb < 10 g/dL and osteomyelosclerosis associated with lower survival |
| Deeg10 | 1980-2002 | 56 | 43 (10-66) | Bu/Cy in 78% | 0 | 64 | 14% at 3 mo | NR | 58 at 3 y | Targeted Bu use improved survival; cGVHD 59% at 2 y |
| Daly9 | 1990-2002 | 25 | 48 (45-50) | TBI based (92%) | 0 | 52 | 48% at 1 y | NR | 41 at 2 y | Prohibitive NRM; no benefit of splenectomy |
| Rondelli66 | NR | 21 | 54 (27-68) | Multiple | 100 | 85 | 10% at 1 y | 81% at 2.7 y | 85% at 2.7 y | Extensive cGVHD in 44%; 2 patients needed DLI for 100% donor chimerism; resolution of fibrosis and splenomegaly in majority |
| Kerbauy58 | NR | 104 | 49 (18-70) | Multiple, Bu/Cy (62%) | 9 | 50 | 35% at 5 y | NR | 61% at 5 y | 3 syngeneic donors, 54 of the patients overlapped with a prior report10 ; targeted Bu improved OS; comorbidity score had impact on survival |
| Patriarca65 | 1986-2006 | 100 | 49 (21-68) | Multiple, Bu/Cy 50% of full intensity; Thiotepa + Cy in 46% of RIC | 52 | 78 | 43% at 3 y | 35% at 3 y | 42% at 3 y | AHCT before 1995; unrelated donor and longer interval from diagnosis predicted worse outcome but not conditioning intensity; relapse at 2 y 41%, progressive decline in NRM over 20 y studied |
| Kroger54 | 2002-2007 | 103 | 55 (32-68) | Flu-Bu (100%) | 100 | 32 | 16% at 1 y | 51% at 5 y | 67% at 5 y | First prospective study in MF, cGVHD in 43%; 12% NRM for fully matched donor AHCT; age > 55 y and HLA mismatch adversely affected OS; JAK2-positive recipients had better EFS and OS; splenectomy increased risk of relapse |
| Gupta64 | 1998-2005 | 46 | 47 y MAC; 54 y RIC | Multiple, Cy TBI (96%) for MAC; Flu Bu (70%) for RIC | 50 | 54 | 48% for MAC and 27% for RIC at 3 y | 43% for MAC and 58% for RIC at 3 y | 48% for MAC and 68% for RIC at 3 y | RIC recipients had more advanced disease and poor KPS; low risk of relapse after either conditioning; lower GVHD with novel conditioning possibly related to use of ATG |
| Ballen56 | 1989-2002 | 289 | 47 (18-73) | Multiple, Bu/Cy (43%) | 21 | 56 | 35% siblings 50% for URD at 5 y | 33% siblings 27% for URD at 5 y | 37% siblings 30% for URD at 5 y | Relapse at 5 y, 32% in sibling and 23% in URD; performance status, peripheral blasts sibling donor status impacted OS; RIC was similar in outcomes, except early NRM |
| Alchalby71 | 1999-2009 | 162 | 56 (32-73) | Flu-Bu in 96% | 100 | 27 | 22% at 1 y | 46% at 5 y | 62% at 5 y | 82 patients reported previously54 ; age and HLA mismatch impacted NRM; 23% relapse at 3 y; clearance of mutated JAK2 at median of 96 days, and this reduced relapse risk |
| Bacigalupo63 | 1994-2007 | 46 | 51 (24-67) | Thiotepa-Cy + melphalan | 100 | 65 | 24% at 5 y | NR | 45% at 5 y | A risk score based on transfusion history, spleen > 22 cm and alternative donor use predicted lower OS; no benefit for splenectomy |
| Stewart76 | 1989-2005 | 51 | 49 (19-64) | Multiple, RIC in 47% | 47 | 65 | 41% at 2 y | 44% and 24% at 3 y for MAC and RIC | 44% and 31% at 3 y for MAC and RIC | |
| Robin92 | 1997-2008 | 147 | 53 (20-68) | Multiple | 69 | 61 | 39% at 4 y | 32% at 4 y | 39% at 4 y | 19% patients had LT; poor outcome with mismatched donor |
| Samuelson13 | 1999-2007 | 30 | 65 (60-78) | Multiple | 63 | 50 | 13% at day 100 | 40% at 3 y | 45% at 3 y | Studied outcomes in patients ≥ 60 y, 7 patients had preceding LT |
| Abelsson67 | 1982-2009 | 92 | 46 for MAC, 55 for RIC | Multiple | 56 | 40 | 32% for MAC and 24% for RIC at 2 y | NR | 49% for MAC and 59% for RIC at 5 y | Overall NRM similar, but 5-year OS superior for RIC younger than age 60 years; less advanced MF associated with better OS |
| Nivison-Smith69 | 1993-2005 | 57 | 47 (16-71) | Multiple | 26 | 68 | 25% at 1 y | 58% at 5 y | Poor outcome in patients > 50 y | |
| Ditschkowski70 | 1994-2010 | 76 | 50.5 (22-67) | Multiple | NR | 35 | 36% at 5 y | 50% at 5 y | 53% at 5 y | Significant high risk of relapse in patients without cGvHD; |
| DIPSS was predictive of survival | ||||||||||
| Scott57 | 1990-2009 | 170 | 51.5 (12-78) | Multiple | NR | 50 | 34% at 5 y | 57% at 5 y | 57% at 5 y | Post-HCT success was dependent on pre-HCT DIPSS scores |
MRD indicates matched related donor; PFS, progression-free survival; OS, overall survival; TBI, total body irradiation; Hb, hemoglobin; Bu, busulfan; Cy, cyclophosphamide; cGvHD, chronic GVHD; NR, not reported; DLI, donor lymphocyte infusion; AHCT, allogeneic HCT; EFS, event-free survival; KPS, Karnofsky performance status; and ATG, antithymocyte globulin.
The early era of HCT in MF: feasible and curative, but associated with a high mortality
In the early era of HCT for MF, outcomes of relatively small number of patients were reported.9-11 These studies established the feasibility and curative potential of HCT in MF and demonstrated that severe marrow fibrosis was not a barrier to engraftment.9-11 High-dose cyclophosphamide with busulfan or total body irradiation were the most commonly used conditioning regimens.56 Several important observations were made from these studies. Splenomegaly and marrow fibrosis resolved slowly in the majority after successful engraftment. Regimen-related toxicities and nonrelapse mortality (NRM) were high, and expected long-term survival was in the range of 30% to 40%, restricting the use of this option to younger patients. Patients older than 50 years, those receiving alternative donor grafts, and those with higher-risk MF were more likely to experience treatment failure. The outcomes from these reports performed more than 15 to 20 years ago are not generally applicable today, although they are sometimes used as justification for the reluctance to refer MF patients for transplantation.
The modern era of HCT: novel conditioning strategies and establishing the feasibility of reduced intensity conditioning
During the last decade, several important advances have been made in the traditional MAC regimens. Advances, such as the use of intravenous busulfan, targeted dose of busulfan, and reversed order conditioning with cyclophosphamide followed by busulfan, reduced early regimen-related toxicities associated with HCT.10,12,57,58
The introduction of a variety of newer and lower-intensity conditioning regimens shifted the emphasis of pretransplantation conditioning therapy from myeloablation to establishing an immune-suppressive effect sufficient to establish donor hematopoiesis. These regimens, developed with the aim of reducing transplantation-related morbidity and mortality, were rapidly adopted in MF. Several lines of data suggest an immunologically mediated GVL effect as the reported success of donor-lymphocyte infusions in MF, and the success of RIC in establishing donor engraftment and resolution of marrow fibrosis over time.54,59-61
Even among the lower-intensity regimens, intensity can vary from minimal (usually 20%-25% of full intensity) or truly nonmyeloablative to reduced intensity (∼ 40%-50% of full intensity).62 We will use the term RIC collectively for minimal intensity and reduced intensity regimens here. Published literature and CIBMTR reporting trends indicate that fludarabine in combination with busulphan/melphalan or total body irradiation are the most commonly used RIC regimens in patients with MF.54,55,63-66 At present, there are no data to indicate the superiority of one regimen over the other.
Intensity of conditioning therapy for MF: is RIC better than MAC?
There are no prospective studies comparing MAC and RIC in MF. Several retrospective studies have compared MAC and RIC regimens.56,64,65,67 Similar outcomes were reported in all except one.67 This study reported a more favorable outcome of patients younger than 60 years undergoing RIC transplantation.67 Lack of statistical power, retrospective nature, and long time interval studied are major issues in these reports. Moreover, there are significant differences in patient populations as patients undergoing RIC are usually much older, have significant comorbidities, and have worse performance scores. NRM with RIC regimens are usually in the range of 15% to 20%; however, relapse is a major cause of failure observed in approximately 30% to 35% patients (Table 4).
A snapshot of various recently reported studies would indicate that progression-free survival is observed in approximately 45% to 50% at 3 years in patients undergoing HCT using modern conditioning regimens (Table 4).
Prognostic factors for outcome of HCT in MF
The outcome of HCT is usually determined by a complex summation of various patient-, disease-, and transplant-related factors. Data validating the utility of prognostic factors in predicting the outcome of MF patients undergoing HCT are limited and conflicting.
Patient-related factors
Age has been identified as an important prognostic factor for survival in several transplantation studies.11,54,58,67-69 In the early transplantation era, high NRM was a barrier for successful outcome in patients older than 50 years. With modern conditioning regimens and RIC, many centers consider the option of HCT in the sixth and seventh decade.13 Age more than 55 years/more than or equal to 57 years was identified as an independent prognostic factor (overall mortality 2.7 times) in studies from Germany using RIC consisting of fludarabine and busufan.54,68 Performance status at HCT56 and high comorbidity scores58 are other important patient-related factors affecting outcomes of HCT. The burden of comorbidities is usually higher in older patients.57 Therefore, it is prudent that older patients are selected for HCT only after careful evaluation of performance status and comorbidities.
MF-related factors
Do the independent risk factors described in IPSS/DIPSS/DIPSS plus scores have similar prognostic value for patients undergoing HCT for MF?
Anemia (hemoglobin < 100 g/dL)11,57 and a greater number of transfusions before HCT (> 20 U)63 have been identified as predictors for inferior survival after HCT. However, these findings have not been confirmed in other studies.68 WBC count, an important independent prognostic marker in all the risk stratification systems, did not impact transplant outcomes.57,68 The presence of blasts in PB was associated with inferior outcomes,56,57 mainly because of higher risk of relapse.57,71 However, prognostic value of PB blasts was not confirmed in a large study of patients treated with RIC.68 The presence of constitutional symptoms before HCT was associated with 2.8-fold higher risk of mortality in one study68 but did not influence the outcomes in another study.57 Adverse impact of cytogenetics in MF patients, described by some,10,11,70 has not been confirmed by others.58,65,68 Patients with abnormal cytogenetics are a small proportion in these studies for making meaningful conclusions. Thrombocytopenia (< 100 × 109/L) also appears to be an independent poor prognostic factor for survival in HCT recipients.58
Do other MF-related factors have any prognostic value in HCT patients?
The impact of JAK2V617F mutation status on the outcome of transplantation is not clear. Higher overall mortality and increased risk of graft dysfunction observed by some investigators in JAK2 wild-type patients68,72 were not confirmed by other groups.70-72 JAK2 mutational status may be used as a marker of minimal residual disease. Patients who were still positive for JAK2V617F mutation at 6 months after HCT had a significantly greater risk of progression (5% vs 30%).71
Higher grades of fibrosis have been associated with poor outcomes in the earlier studies,11,56 although the independent value of this variable has not been established.10,11,56,58,68 These studies are further limited by the lack of a uniform assessment of grading of fibrosis. Longer intervals between diagnosis and HCT have been associated with mortality in one study65 ; however, they were not confirmed in a further studies.57,68 Splenomegaly more than 22 cm has been associated with poor survival.63
Prognostic value of different risk stratification systems in HCT patients
The prognostic value of the Lille scoring system has been most extensively studied in HCT recipients.10,54,65,67,73 The patients with low-risk disease have better outcomes compared with intermediate- and high-risk patients. There appears to be a higher risk of relapse with high Lille scores.54
The Seattle group recently evaluated the usefulness of DIPSS score in HCT recipients.57 The HRs of post-HCT mortality and NRM were 4.11and 3.41 among DIPSS high-risk patients compared with low-risk patients. Median survival was not reached for DIPPS low and intermediate-1 risk cohorts, whereas it was 7 and 2.5 years, respectively, for the intermediate-2 and high-risk groups. This study highlights the dilemma of transplantation in MF in that those transplanted earlier in the course of are most likely to be cured, whereas these same patients are the ones who least need HCT, as the risk of death or LT from MF is low.5,24
A German group recently compared various risk models in a patients treated with a uniform RIC regimen.68 Advanced age (> 57 years), JAK2 V617F wild-type status, and constitutional symptoms were predictive of poor survival. For those with all 3 risk factors, the hazard of death was increased by 16-fold. The DIPSS system, although predictive, did not sufficiently distinguish between intermediate-1 and intermediate-2 risk groups in this study.
Transplant-related factors
Similar outcomes have been reported in patients undergoing matched sibling donor (MSD) and well matched (10 of 10) unrelated donor (URD) transplantation.54,58 The outcomes of mismatched donors are significantly inferior.54 Haploidentical or cord blood grafts are important alternative graft sources for hematologic malignancies. However, their utility has not been well established in patients with MF. A small study described the use of cord blood grafts in patients with severe marrow fibrosis; however, 11 of 14 patients in this study had fibrosis associated with AML or myelodysplastic syndrome,74 which are biologically distinct diseases than MF. There does not appear to be significant difference in outcomes of peripheral blood stem cell and marrow grafts in MF patients.56,57
The data herein highlight the difficulties in interpreting the conflicting results between various studies and making informed decisions. The reasons for such conflicting data are retrospective nature of studies, heterogeneity among patients, small sample sizes lacking statistical power, and thus inability to analyze these factors in multivariate analysis.
Splenectomy before HCT in MF: should we or should we not?
Conflicting data exist on the effect of pre-HCT splenectomy on relapse and survival. Earlier studies from several groups did not show an impact of prior splenectomy on survival9,56,58,75,76 ; however, recent studies evaluating a larger number of patients demonstrated beneficial effect of splenectomy on survival.57,77 Relapse risk was higher in splenectomized patients in a German study,54 whereas no impact was observed in the CIBMTR study.56 The discrepant results in various studies may be related to selection biases, as fitter patients who are able to undergo successful splenectomy may have a lower mortality after HCT. In addition, patients who have larger spleens may have more advanced disease, which may explain higher relapse rates.54 A consistent finding reported in several studies is the faster hematopoietic recovery in splenectomized patients.11,58,76,78 The procedure of splenectomy is associated with significant risk of perioperative complications (27.7%) and mortality (6.7%).49 Another disadvantage of splenectomy is immunologic issues in the post-HCT setting (eg, poor response to vaccines). Reduction of splenomegaly by JAK1/2 inhibitor therapy before HCT may be a reasonable alternative to splenectomy but without the risks of surgery related morbidity and mortality.
Given the lack of favorable data and significant perioperative complications associated with this procedure, routine splenectomy is not recommended before HCT.
Barriers to success of HCT in MF
Regimen-related toxicities: higher risk of early hepatotoxicity
Patients with MF are at a significantly higher risk of developing early hepatotoxicity.79 A case-control study from Toronto evaluated early hepatotoxicity in 53 patients undergoing HCT for MF.79 Compared with matched myelodysplastic syndrome patients, patients with MF had a significantly higher risk of moderate/severe hyperbilirubinemia (44% vs 21%, P = .02) and veno-occlusive disease (36% vs 19%, P = .05). Moderate/severe hyperbilirubinemia had an adverse impact on survival. Investigators hypothesized that a higher rate of hepatotoxicity in MF patients may be related to underlying asymptomatic portal hypertension. Screening for asymptomatic portal hypertension, using upper gastrointestinal endoscopy and abdominal Doppler ultrasound, should be considered in the pretransplantation workup of patients with MF.
Graft failure
The incidence of GF in MF patients is reported between 5% and 25%.55,56,64 In a large CIBMTR study, GF was significantly higher in patients undergoing HCT using donors other than MSD (MSD, 9%; other related, 27%; and unrelated, 20%).56 Similar trends were observed in a prospective study from Myeloproliferative Diseases-Research Consortium, which showed a significantly higher rate of primary GF in MF patients undergoing URD transplantation compared with MSD (25% vs 3%).55 TNF-α is a negative regulator of expansion and renewal of normal hematopoietic stem cells80,81 and may have a differential effect on MPN and normal hematopoietic cells.82 Patients with advanced MF have increased plasma levels of TNF-α.83 It is possible that the higher rate of GF may be related to cytokines associated with more advanced disease rather than donor factors alone. Biology and risk factors for GF are poorly understood in MF and need to be studied in well-designed studies.
GVHD
GVHD is one of the most debilitating complications of HCT. In the published literature, some studies have reported higher than expected rates of acute GVHD in MF patients,10,11,55,58,64 whereas in others the rates of GVHD do not appear much different from other hematologic malignancies.54,56 The impact of conditioning on acute GVHD is not clear in MF. In a prospective study from Myeloproliferative Diseases-Research Consortium, the incidence of severe acute GVHD (grade 3 or 4) was 12% and 21% in patients undergoing RIC transplantation using MSD and URD, respectively.55 An alarmingly high incidence of severe GVHD in URD transplants in this study is of concern despite the use of thymoglobulin for GVHD prophylaxis. Several convergent lines of evidence have suggested that inflammatory cytokines act as mediators of acute GVHD.84 Inflammatory cytokines are implicated in pathophysiology of MF and cause debilitating symptoms as well as mediate higher mortality.83 Cytokines may also influence the proliferative advantage of neoplastic clone.82 Could patients with MF be at additional risk of GVHD because of high levels of inflammatory cytokines?
Poor PS
Symptomatic splenomegaly, debilitating constitutional symptoms, and anemia commonly impair the performance status (PS) of patients with MF. Poor PS at HCT is an independent predictor of a higher NRM and poor survival in MF patients.56
How will the availability of JAK1/2 inhibitors impact the application of HCT in MF?
Although JAK1/2 inhibitor therapy is of significant clinical value in patients with MF, their use neither is curative nor decreases the risk of LT. The impact of the wider availability of JAK1/2 inhibitor therapy on the referral pattern for HCT is not clear. It is possible that some patients who are responding well to JAK1/2 inhibitor therapy may be delayed from consideration for transplantation and may have more advanced-stage disease at the time of actual referral. We think that patients whose therapeutic goal is cure should still be referred for a transplantation consultation, even when they are responding to JAK1/2 inhibitor therapy. This approach is not contradictory because there is a sound theoretical rationale for combining JAK1/2 inhibitor therapy with the transplant conditioning regimen.
Exploring the benefits of combining JAK1/2 inhibitor therapy with HCT
JAK1/2 inhibitor therapy presents an opportunity to address some of the barriers for the success of HCT in MF patients mentioned as discovered in the previous section. JAK1/2 inhibitor therapy is effective in decreasing the burden of troublesome symptoms in patients with MF by reduction of splenomegaly, amelioration of constitutional symptoms, and improvement in PS and well-being.14,15,50-52 Reduction in splenomegaly may help in faster hematologic recovery in the posttransplantation period. Drugs, such as ruxolitinib, are effective in rapid down-regulation of inflammatory cytokine levels because of anti-JAK1–mediated effect, resulting in improvement of constitutional symptoms.52 Down-regulation of cytokines may potentially have beneficial impact on GF and acute GVHD.
Improvement in pretransplantation PS and the possible beneficial impact on GF and GVHD make these agents attractive agents for clinical trials in transplant-related strategies for MF. Potential harmful effects of JAK1/2 inhibitor therapy in the transplantation setting may include negative impact on hematologic recovery or explosive splenomegaly or cytokine excesses on withdrawal. Therefore, it is important that the strategy of using JAK1/2 inhibitor therapy in combination with transplantation be explored in a well-designed clinical trial setting wherever possible.
Optimal timing for JAK inhibition in the context of a transplantation strategy appears to be in the pretransplantation setting. In contrast to BCR-ABL inhibitors, which are commonly used to eradicate minimal residual disease in the posttransplantation setting for Philadelphia-positive leukemias, current JAK1/2 inhibitors are not effective in reversing histologic abnormalities or reducing the JAK2V617F allele burden in MF and, therefore, may have limited value in the posttransplantation setting.
Selection of therapeutic options for MF patients in the JAK inhibitor era
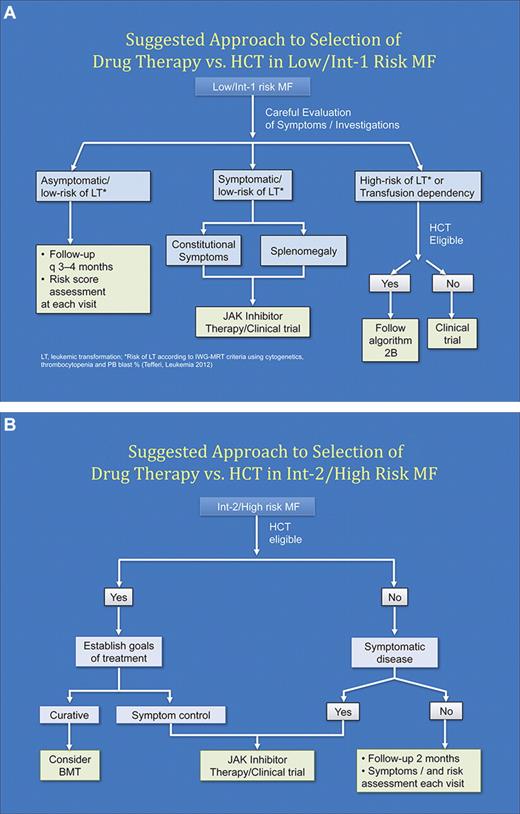
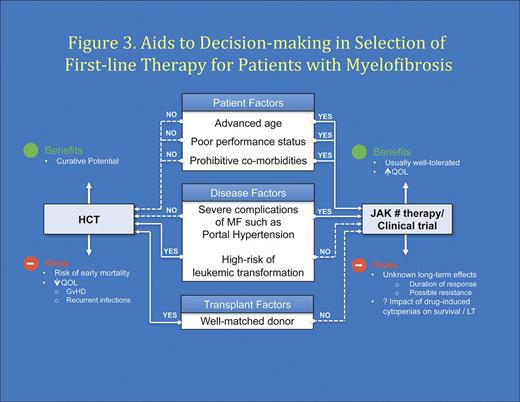
MF is a rare chronic hematologic malignancy. Optimal management of MF will involve close collaboration between community hematologist/oncologist and a center with expertise in MPN. The author's perspectives on optimal modern management of MF are summarized in Table 5. In patients, where the goal of therapy is curative, the option of HCT should be considered and a donor search initiated. As proposed by the European LeukemiaNet group, HCT should be considered in patients whose anticipated survival is less than 5 years (usually Intermediate-2/high-risk disease).85 In addition, transfusion dependency or risk of LT more than or equal to 35% at 3 years would be reasonable indications for considerations for HCT in lower-risk patients. Candidates not eligible for HCT should be offered JAK1/2 inhibitor therapy or clinical trials when symptomatic (Figure 2A-B). Within the framework of this approach, the decision about candidacy for HCT should be evaluated after careful consideration of the risk posed by disease itself versus risk from transplantation taking into consideration patient-, disease-, and transplant-related factors (Figure 3). Continued study of novel therapeutic strategies, including HCT, is required to optimize patient outcomes in MF.
Authors' perspective on optimal modern management of MF
|
|
Suggested algorithm for approach to selection of first-line therapy (drug therapy vs HCT). (A) Low/Intermediate-1 risk MF. (B) Intermediate-2/High risk MF.
Suggested algorithm for approach to selection of first-line therapy (drug therapy vs HCT). (A) Low/Intermediate-1 risk MF. (B) Intermediate-2/High risk MF.
Aids to decision making in selection of initial therapy (drug therapy vs HCT) in patients with MF.
Aids to decision making in selection of initial therapy (drug therapy vs HCT) in patients with MF.
Acknowledgments
The authors thank CIBMTR for the data on trends in transplantation for MF as well as Dr Hans Messner (Princess Margaret Hospital) for review of this manuscript and helpful suggestions.
Authorship
Contribution: V.G. prepared the initial draft of the manuscript; P.H. and R.H. provided further knowledge, insights, discussions, and helped in critical review; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: V.G. received clinical trial research funding from Incyte, Novartis, Celgene, YM Biosciences, and Sanofi-Aventis; served on advisory board of Incyte, Novartis, YM Biosciences, and Sanofi-Aventis; and received consulting fees from Novartis, YM Biosciences, and Sanofi-Aventis. R.H. received research support from Novartis, Celgene, Incyte, Genzyme, AstraZeneca, Bristol Meyers Squibb, and Roche/Genentech. P.H. declares no competing financial interests.
Correspondence: Vikas Gupta, Blood and Marrow Transplant Program, Princess Margaret Hospital, Suite 5-217, 610 University Ave, Toronto, ON, Canada, M5G 2M9; e-mail: vikas.gupta@uhn.ca.