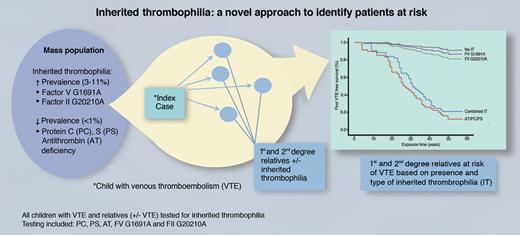
In this issue of Blood, Holzhauer et al have determined a novel method of identifying patients with protein C, protein S, and antithrombin deficiency who are at increased risk of developing venous thromboembolism (VTE; see figure). Children with VTE and their relatives were screened for inherited thrombophilia including proteins C and S and antithrombin deficiency; and Factor (F)V G1691A and FII G20210A. Their study demonstrates that relatives with proteins C and S and antithrombin deficiency are at a significantly higher risk of developing VTE compared with those without inherited thrombophilia.1
Within the population at large, there are at least 2 groups of inherited thrombophilias based on prevalence; those with a higher prevalence, 3% to 11% (FV G1691A and FII G20210A) and those with a lower prevalence, < 1% (protein C, protein S, and antithrombin deficiency). Diagnosis of a child with venous thromboembolism can be used to identify first-degree relatives with inherited thrombophilia who are at increased risk of venous thromboembolism; most notably protein C, protein S, and antithrombin deficiency, and to a much lesser extent FV G1691A and FII G20210A. PC indicates protein C deficiency; PS, protein S deficiency; AT, antithrombin deficiency; VTE, venous thromboembolism; IT, inherited thrombophilia; and combined IT, combined inherited thrombophilia (rare). See also Figure 2 in the article by Holzhauer et al that begins on page 1510.1 Professional illustration by Marie Dauenheimer.
Within the population at large, there are at least 2 groups of inherited thrombophilias based on prevalence; those with a higher prevalence, 3% to 11% (FV G1691A and FII G20210A) and those with a lower prevalence, < 1% (protein C, protein S, and antithrombin deficiency). Diagnosis of a child with venous thromboembolism can be used to identify first-degree relatives with inherited thrombophilia who are at increased risk of venous thromboembolism; most notably protein C, protein S, and antithrombin deficiency, and to a much lesser extent FV G1691A and FII G20210A. PC indicates protein C deficiency; PS, protein S deficiency; AT, antithrombin deficiency; VTE, venous thromboembolism; IT, inherited thrombophilia; and combined IT, combined inherited thrombophilia (rare). See also Figure 2 in the article by Holzhauer et al that begins on page 1510.1 Professional illustration by Marie Dauenheimer.
Previous studies have demonstrated that VTE risk in families is dependent on the type of thrombophilia.2 The importance of identifying patients at risk for VTE cannot be overstated because of associated mortality, morbidity, and increased costs to society. However, mass screening for these inherited thrombophilias in the healthy population is not warranted as the prevalence of a deficiency in proteins C and S and antithrombin is less than 1%.3 In addition, thrombophilia testing is expensive and has ethical ramifications. There is a paucity of studies demonstrating the utility of testing or the safety and efficacy of prophylaxis.
There are numerous factors that increase the likelihood of the development of VTE including blood stasis, abnormal vascular endothelium resulting from surgery, trauma, immobility, sepsis, and others; as well as abnormalities in hemostasis. Hemostasis, the normal ongoing process that repairs damaged vascular endothelium, relies on normal protein components.4 Abnormalities in proteins involved in clot formation, such as FV G1691A and prothrombin G20210A polymorphisms, as well as dysfunctional changes (low level or decreased activity) to hemostatic modulating proteins, proteins C and S and antithrombin deficiency, can result in VTE.3
Preventive strategies for VTE in established high-risk populations are supported by studies demonstrating safety and effectiveness; for example, hip and knee arthroplasty,5 trauma, surgery,6 and hospitalized nonsurgical patients.7 However, currently there are no studies supporting VTE prophylaxis in the setting of low-risk scenarios, even in a patient with a documented inherited thrombophilia but who has never been diagnosed with VTE. In fact, Holzhauer and colleagues found transient risk factors (not defined in the study) to be present in most children with VTE who were identified to have inherited thrombophilia.1 This information may provide data supporting studies using targeted preventive strategies in certain cohorts of patients with inherited thrombophilia who have additional risk factors.
Increasing age is a known risk factor for VTE.8 Supporting this fact, Holzhauer et al demonstrated that the incidence of VTE in relatives 15 years of age and older was increased relative to those younger than 15 for all study participants, those with and without inherited thrombophilia.1 Children have a lower incidence of VTE than adults but higher-risk groups exist, with the presence of a central line being the most common.9 Using a child with VTE as an index case to identify relatives that may have inherited thrombophilia presents challenges that involve the following areas: developmental hemostasis, current laboratory testing methods for inherited thrombophilia, and ethical considerations. Children have physiologic differences in hemostasis compared with adults, including lower levels of proteins C and S and antithrombin, all approaching adult levels in late childhood.10 Defining age-appropriate normal ranges for these proteins has difficulties. Current laboratory testing methods for levels of proteins C and S and antithrombin use a clot-based or colorimetric assay.10 Studies have demonstrated that age-appropriate pediatric ranges in healthy children for levels of proteins C and S and antithrombin may vary according to reagents, type of laboratory test system, and, potentially, ethnicity.10 Consequently, definitively diagnosing inherited thrombophilia, proteins C and S and antithrombin deficiency, in childhood may not be possible. In addition, ethical considerations exist because a child is unable to give informed consent to be tested, which if positive, may result in life-long emotional, financial, and life-style changes.
The medical community continues to search for efficient methods to ascertain populations at risk for VTE and when identified, to provide safe and effective prevention. Holzhauer and colleagues have developed a unique method of identifying a known high-risk population without mass screening. However, due to the rarity of proteins C and S and antithrombin deficiency,3 the homogeneity of the study population1 and the challenges of using pediatric index cases, further studies are needed. Importantly, this study also demonstrated that patients with FV G1691A and FII G20210A abnormalities did not have a significantly increased VTE risk compared with patients without these abnormalities,1 yet in the general population these are the two most common inherited thrombophilic abnormalities tested for today. As a result of this new information, screening for these inherited abnormalities should be re-examined because the utility of testing may be in question. Identifying which individuals to test for thrombophilia still needs to be fully clarified; however, Holzhauer et al have increased our understanding of thrombophilia and the associated consequences and have generated new ideas for further studies.
Conflict-of-interest disclosure: A.B. declares no competing financial interests. P.M. was a nonpaid consultant for Berlin Heart and a paid consultant for Levitronix, Sanofi Aventis, Bayer, and Astra Zeneca. She is on the Data Monitoring Board of a Sanofi pharma study and was the chair of the Data Monitoring Committee for the Berlin Heart IDE FDA study. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal