Abstract
Mutations in the nucleophosmin gene (NPM1mut) are one of the most frequent molecular alterations in acute myeloid leukemia (AML), and immune responses may contribute to the favorable prognosis of AML patients with NPM1mut. In the present study, we were able to demonstrate both CD4+ and CD8+ T-cell responses against NPM1mut. Ten peptides derived from wild-type NPM1 and NPM1mut were subjected to ELISPOT analysis in 33 healthy volunteers and 27 AML patients. Tetramer assays against the most interesting epitopes were performed and Cr51-release assays were used to show the cytotoxicity of peptide-specific T cells. Moreover, HLA-DR–binding epitopes were used to test the role of CD4+ T cells in NPM1 immunogenicity. Two epitopes (epitopes #1 and #3) derived from NPM1mut induced CD8+ T-cell responses. A total of 33% of the NPM1mut AML patients showed immune responses against epitope #1 and 44% against epitope #3. Specific lysis of leukemic blasts was detected. To obtain robust immune responses against tumor cells, the activation of CD4+ T cells is crucial. Therefore, overlapping (OL) peptides were analyzed in ELISPOT assays and OL8 was able to activate both CD8+ and CD4+ T cells. The results of the present study show that NPM1mut induces specific T-cell responses of CD4+ and CD8+ T cells and therefore is a promising target for specific immunotherapies in AML.
Introduction
Mutations in the nucleophosmin 1 gene (NPM1mut) represent some of the most common gene mutations in acute myeloid leukemia (AML).1,2 Falini et al first described the abnormal cytoplasmic localization of the NPM1 protein caused by mutations in exon 12 of the gene.3 In AML patients with normal cytogenetics, the incidence of NPM1mut was reported in up to 60% of the patients.1-3 NPM1 constitutes an important prognostic marker, especially in the context of FMS-related tyrosine kinase internal tandem duplication (FLT3-ITD). In more than 90% of AML patients harboring NPM1mut, the 3 different NPM1mut types (A, B, and D) were found.1,2,4-6 AML patients harboring an NPM1mut without an FLT3-ITD mutation showed improved survival when treated with intensive chemotherapy.2 Most AML patients with NPM1mut seem not to benefit from an allogeneic stem cell transplantation as a first-line treatment.2,7 However, this issue remains to be elucidated in the context of minimal residual disease detection8 and the coexistence of other molecular markers. The functional role of NPM1mut for the improved clinical outcome remains to be elucidated. Immune responses may contribute to clinical outcome by lysis of residual leukemic cells through specific T cells after chemotherapy. Leukemia-associated antigens (LAAs) can be targeted by the immune system in a specific manner, leading to the hypothesis that the expression of LAAs might also influence the clinical outcome of AML patients. mRNA expression of at least 1 of the 3 LAA, receptor for hyaluronic acid-mediated motility (RHAMM), preferentially expressed antigen in melanoma (PRAME) or G250, was demonstrated to be associated with a favorable prognosis in AML patients.9 Similar results were found for the coexpression of other different LAAs in AML.10 Therefore, the expression of distinct LAAs on leukemic blasts may lead to the eradication of residual disease after intensive chemotherapy. Several LAAs can induce specific cytotoxic CD8+ T cells that are able to lyse autologous leukemic blasts.11-17 Several LAAs are ideal targets for immunotherapeutic approaches because they are involved in critical mechanisms of cell differentiation and tumor-cell proliferation.16 Therefore, clinical trials were started to target some of these LAAs in different hematologic malignancies. The LAA receptor for RHAMM, proteinase 3, and Wilms tumor antigen 1 (WT-1) were tested in clinical peptide vaccination trials. In these clinical phase 1 trials, immunologic and clinical responses could be detected in patients with different hematologic malignancies.18-21 Because of its exquisite specificity in leukemia, NPM1mut might constitute an ideal target structure for individualized immunotherapeutic approaches. In the present study, we investigated the existence and recognition of CD4+ and CD8+ T-cell epitopes derived from NPM1mut in patients with NPM1mut AML.
Methods
CD8+ T-cell epitope prediction
The entire amino acid sequences of the NPM1 wild-type protein (accession number NM_002520) as well as of the mutated cytoplasmic NPM1 types A, B, C, and D (AY740634, AY740635, AY740636, and AY7406379, respectively) were screened for HLA-A*0201–binding T-cell epitopes using the SYFPEITHI (www.syfpeithi.de), Rankpep (imed.med.ucm.es), and HLA-Bind (www-bimas.cit.nih.gov) software programs. The 10 nonamer peptides with the highest scores for human HLA-A*0201 binding were produced by a solid-phase synthesizer (GL Biochem). In addition, the influenza matrix protein derived peptide IMP (GILGFVFTL) was synthesized and served as a positive control.
Generation of NPM1-specific cytotoxic T cells
All samples from healthy volunteers (HVs) and AML patients were obtained by written informed consent in accordance with the Declaration of Helsinki (application number 334/09, approved by the local ethical committee). Ficoll-Paque (Biochrom AG) gradient density centrifuged PBMCs were cryopreserved in a standard RPMI 1640 medium (Biochrom AG) supplemented with 20% FCS serum (PAN Biotech) and 10% DMSO (Sigma-Aldrich) and stored in liquid nitrogen. The HLA-A and HLA-B alleles were routinely serotyped and the HLA-DR alleles were genotyped using high-resolution PCR on extracted genomic DNA by the Institute for Clinical Transfusion Medicine and Immunogenetics at the University of Ulm.
HLA-A2+ PBMCs were thawed and selected by anti-CD8 Abs coupled with MicroBeads using MACS columns (Miltenyi Biotec). The purity of each fraction was > 95%. Subsequently, mixed lymphocyte peptide cultures (MLPCs) were performed as described previously.14 Briefly, CD8− cells as APCs were irradiated with 30 Gy before pulsing with 2.5 μg/mL of β2-microglobulin (Sigma-Aldrich) and 10 μg/mL of individual peptide (peptide #1 to #9 and wild-type; Figure 1) for 2 hours. Peptide-pulsed CD8− APCs (2 × 106/500 μL) were then coincubated with autologous CD8+ T cells (5 × 105 cells/500 μL) at 37°C and 5% CO2. On day 1, 10 IU/mL of IL-2 and 20 ng/mL of IL-7 (Sigma-Aldrich) were added to the culture medium. Such peptide-stimulated CD8+ T cells were tested in ELISPOT assays on day 8 of culture. The remaining CD8+ T cells were restimulated by peptides on a weekly basis on days 7 and 14. Such repeatedly stimulated CD8+ T cells were subjected to Cr51-release assays on day 21. All PBMCs were cultured in RPMI 1640 medium supplemented with human 10% AB serum, 2mM l-glutamine, and 100 U/mL each of penicillin and streptomycin (Sigma-Aldrich).
Definition of NPM1 exon 12 mutations by mutation-specific RT-PCR
Genomic DNA was isolated from PBMCs of AML patients at the time of diagnosis using DNAzol (Gibco-BRL). NPM1 exon 12 gene sequences were amplified with primers NPM1-F (5′-TTAACTCTCTGGTGGTAGAATGAA-3′) and NPM1-R (5′-CAAGACTATTTGCCATTCCTAAC-3′). Purified PCR products were sequenced directly with primers NPM1-R2 (5′-GGCATTTTGGACAACACA-3′), as described previously.5
ELISPOT and cytotoxicity assays
ELISPOT and cytotoxicity assays were performed as described previously.9,15,18 On day 8, CD8+ T cells (effector cells) were coincubated with peptide-loaded T2 cells (target cells) at an effector/target (E/T) ratio of 1:4 for 18-24 hours. The secretion of IFN-γ and granzyme B was measured in parallel by ELISPOT assays following the manufacturer's instructions (BD Biosciences and Mabtech). Nonpeptide-pulsed T2 cells served as a negative control and IMP-pulsed T2 cells as a positive control.
The frequency of NPM1#1- and NPM1#3-specific CD8+ T lymphocytes was determined after 8 days of MLPC by staining with anti-CD8/CD4 and HLA-A2#1 or HLA-A2#3 tetramer PE, as described previously.19 HLA-A2#1 or HLA-A2#3 tetramer PE was synthesized at the Lausanne Branch of the Ludwig Institute for Cancer Research.
On day 14 or 21, a standard Cr51-release assay was performed to assess the peptide-specific cytotoxicity.15 NPM1 peptide–stimulated CD8+ T cells were coincubated with 51CrO4 (Cr51)–labeled target cells (peptide loaded T2 cells, T2 cells alone, and K562 cells as HLA-A2 negative controls and blasts from different AML patients) at various E/T ratios for 18-24 hours. The Cr51 release into the supernatant and thus specific lysis was determined by a γ-scintillation counter (Bio-Rad). The counts were standardized by the following formula: (measured release − spontaneous release)/(maximum release − spontaneous release).
Selection of MHC class II binding peptides
HLA-DRB1*0101-, HLA-DRB1*0301-, HLA-DRB1*0401-, HLA-DRB1*0701-, HLA-DRB1*1101-, and HLA-DRB1*1501–binding motifs derived from either intact or NPM1mut sequences were predicted for their specific binding capacity using the SYFPEITHI program for the prediction of specific binding. 15mer sequences and overlapping peptides were submitted to peptide synthesis.
CD4+, CD4−CD8+, and CD4−CD8− cell fractions were separated from PBMCs of HLA-A2+DR*0701+ HVs using a combination of CD4 and CD8 MicroBeads (Miltenyi Biotec). CD4−CD8− cells as APCs were pulsed with 10 μg/mL of HLA-DR overlapping (OL) peptide (OL1-OL8), and then APCs were coincubated with CD4+ T cells or CD8+ T cells as described in “Generation of NPM1-specific cytotoxic T cells.” Peptide-sensitized CD4+ T cells and CD8+ T cells were cocultured, and an IL-2 and IL-7 cocktail was added to the cell culture medium the following day. Peptide stimulation was repeated weekly and ELISPOT assays were performed on day 21. For the HLA-A2–blocking experiments, purified anti–human HLA-A2 mAb BB7.2 (BD Biosciences) or mouse IgG was added to the effector cell culture 1 hour before the coculture with target cells.
Results
Prediction of NPM1mut-derived peptides binding to HLA-A*0201 molecules
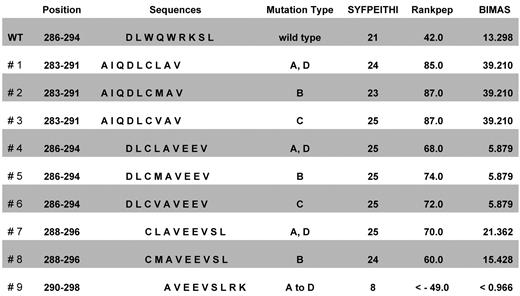
The intact and mutated NPM1 proteins were screened for HLA-A*0201–binding CD8+ T-cell epitopes using 3 different algorithm software programs. NPM1mut types A, B, C, and D amino acid sequences were ranked within the top 10 by all 3 software programs used (data not shown). By Rankpep and SYFPEITHI algorithms, 8 of 9 mutation-derived sequences and 5 of 9 mutation sequences in the HLA-Bind program obtained higher scores than wild-type sequences (Figure 1). Seven NPM1-derived epitopes presented above the unique binding threshold level in the Rankpep program.
Positions, sequences, and ranking of epitopes derived from the mutational region of NPM1. The entire amino acid sequences of the NPM1 wild-type protein and of the mutated cytoplasmic NPM1 types A, B, C, and D were screened for HLA-A*0201–binding T-cell epitopes using several software programs. The ten 9 mer peptides NPM1#1 to NPM1#9 and wild-type with the highest scores for human HLA-A*0201 binding were used for further experiments.
Positions, sequences, and ranking of epitopes derived from the mutational region of NPM1. The entire amino acid sequences of the NPM1 wild-type protein and of the mutated cytoplasmic NPM1 types A, B, C, and D were screened for HLA-A*0201–binding T-cell epitopes using several software programs. The ten 9 mer peptides NPM1#1 to NPM1#9 and wild-type with the highest scores for human HLA-A*0201 binding were used for further experiments.
Generation of NPM1mut-specific T cells characterized by secretion of IFN-γ and granzyme B
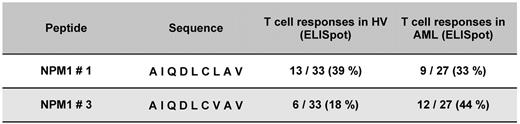
To assess the potential cytolytic activity of NPM1mut-specific T cells, the peptide-specific secretion of type cytokines was measured. IFN-γ is a surrogate marker for activation and granzyme B to demonstrate the lytic potential. CD8+ T cells isolated from HVs and NPM1mut AML patients were stimulated with NPM1-derived epitopes from the mutational region peptide #1 to #9 and wild-type. A significant increase of specific CD8+ T cells secreting IFN-γ and granzyme B for the NPM1 peptides #1 and #3 was found. For the epitope NPM1#1 13 of 33 (39%) HVs and 9 of 27 (33%) patients showed a specific immune response, whereas for NPM1#3, 6 of 33 (18%) HVs and 12 of 27 (44%) patients did (Figure 2). NPM1mut AML patients showed a significantly higher frequency of T-cell responses against peptide #3 compared with HVs (P = .046), whereas for peptide #1, the frequency of T-cell responses of AML NPM1mut patients and HVs was not significantly different.
Frequency of specific immune responses against different NPM1-derived epitopes from the mutational region in HVs and in AML patients with an NPM1 mutation. CD8+ T cells separated from 33 HVs and 27 AML patients with NPM1mut were stimulated with NPM1#1 to NPM1#9 and wild-type in a MLPC. Epitope recognition by these T cells was measured as IFN-γ and granzyme B secretion by ELISPOT. Significant frequencies of CD8+ T cells specific for epitopes #1 and #3 derived from the mutational region NPM1 could be detected in HVs and in AML patients with NPM1mut. Whereas the frequency of peptide #1–specific CD8+ T cells was equal in HVs and NPM1mut AML patients (nonsignificant), responses to peptide #3 were more frequent in AML patients than in HVs (P = .046).
Frequency of specific immune responses against different NPM1-derived epitopes from the mutational region in HVs and in AML patients with an NPM1 mutation. CD8+ T cells separated from 33 HVs and 27 AML patients with NPM1mut were stimulated with NPM1#1 to NPM1#9 and wild-type in a MLPC. Epitope recognition by these T cells was measured as IFN-γ and granzyme B secretion by ELISPOT. Significant frequencies of CD8+ T cells specific for epitopes #1 and #3 derived from the mutational region NPM1 could be detected in HVs and in AML patients with NPM1mut. Whereas the frequency of peptide #1–specific CD8+ T cells was equal in HVs and NPM1mut AML patients (nonsignificant), responses to peptide #3 were more frequent in AML patients than in HVs (P = .046).
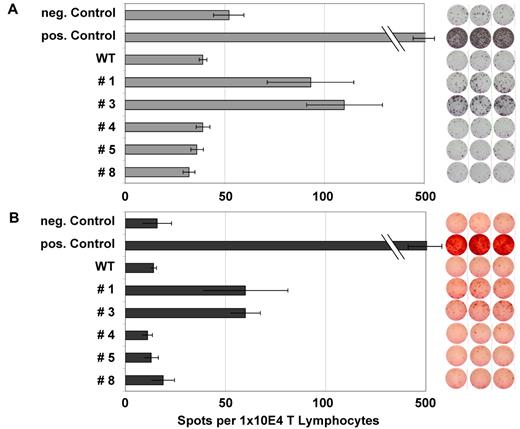
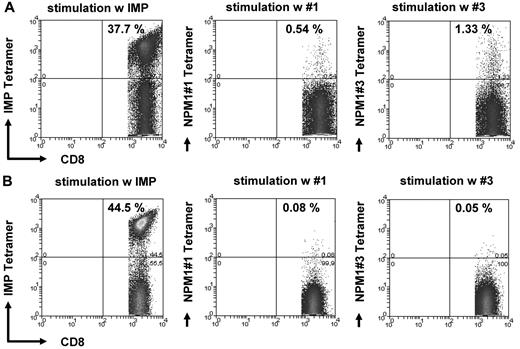
Figure 3A and B show an example of the frequency of specific T lymphocytes for peptides #1 and #3 in an NPM1mut AML patient. These findings were confirmed by FACS analysis of HLA-A2/NPM1#1 tetramer or NPM1#3+/CD8+ T lymphocytes (Figure 4). Figure 4B shows the negative control, a patient who was negative against NPM1 peptides #1 and #3 in ELISPOT analysis. IMP was used as a positive control.
ELISPOT analysis for IFN-γ and granzyme B of several NPM1-derived peptides in an NPM1mut AML patient. ELISPOT assays for the release of IFN γ (A) and granzyme B (B) were performed after stimulation with several NPM1-derived peptides. Stimulation with IMP served as a positive control and no peptide stimulation as a negative control. Peptide-specific T-cell activity was measured by IFN release, whereas granzyme B secretion indicated the lytic potential of the T cells. This patient showed a significant increase in frequency for the epitopes #1 and #3 derived from the mutational region of NPM1.
ELISPOT analysis for IFN-γ and granzyme B of several NPM1-derived peptides in an NPM1mut AML patient. ELISPOT assays for the release of IFN γ (A) and granzyme B (B) were performed after stimulation with several NPM1-derived peptides. Stimulation with IMP served as a positive control and no peptide stimulation as a negative control. Peptide-specific T-cell activity was measured by IFN release, whereas granzyme B secretion indicated the lytic potential of the T cells. This patient showed a significant increase in frequency for the epitopes #1 and #3 derived from the mutational region of NPM1.
Tetramer staining of CD8+ T cells specific for the NPM1mut-derived peptides #1 and #3. The frequency of CD8+ T lymphocytes specifically recognizing NPM1mut peptides #1 and #3 was assessed by counterstaining CD8 T cells with tetramers with the respective epitope peptide in an AML patient with a NPM1mut (A). (B) Negative controls using an individual who was negative against NPM1 peptides #1 and #3 in ELISPOT analysis. IMP tetramer staining served as a positive control for the experiments shown in both A and B.
Tetramer staining of CD8+ T cells specific for the NPM1mut-derived peptides #1 and #3. The frequency of CD8+ T lymphocytes specifically recognizing NPM1mut peptides #1 and #3 was assessed by counterstaining CD8 T cells with tetramers with the respective epitope peptide in an AML patient with a NPM1mut (A). (B) Negative controls using an individual who was negative against NPM1 peptides #1 and #3 in ELISPOT analysis. IMP tetramer staining served as a positive control for the experiments shown in both A and B.
In all experimental groups, an immune response after stimulation with peptides #1 and #3 resulted in a relatively stable cytokine secretion. Therefore, peptides #1 and #3 were considered to be the most significant epitopes. In the view of the clinical impact of a potential immune therapy for AML, the HLA-A2–restricted peptide #1 (NPM1#1) was selected as the target epitope for further experiments in this study.
NPM1 peptide#1–specific cytotoxicity
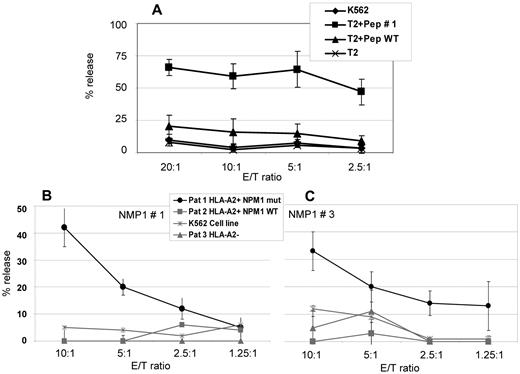
To examine peptide recognition and antigen-specific cell lysis of NPM1#1–stimulated CD8+ cells, CD8+ T cells were isolated from 4 HVs and stimulated weekly with peptide #1. Cr51-release assays were performed on day 21. Spontaneous release of target cells in all experiments were < 10% and maximum release induced by 10% Triton X-100 were > 800 cpm. NPM1#1 CD8+ T cells showed 57.1%-71.3% of peptide #1–specific cell killing at an E/T ratio of 20:1 compared with 6.8%-24.9% of nonspecific cell lysis against wild-type peptide–pulsed T2 cells (Figure 5A). Cell lysis of nonpeptide-pulsed T2 cells and K562 cells (HLA-A2−) was < 10.0% at each E/T ratio. To prove the specific lysis of NPM1mut blasts, Cr51-release assays with blasts from AML patients with NPM1mut versus NPM1 wild-type were performed. To prove the HLA-A2 restriction, A2+ and A2− patients were included in the study. K562 cells served as a control to determine to what extent natural killer cells were involved in the killing. Figure 5B shows results from NPM1#1 and Figure 5C from NPM1#3 peptide.
Cr51-release assays for the assessment of cytotoxic activity of CD8+ T lymphocytes of 4 HVs against the peptide NPM1#1. (A) NPM1#1–specific cytotoxic T lymphocytes (CTLs) were generated from 4 HVs and cytotoxic activity was assessed by Cr51-release assays using peptide-pulsed T2 cells as target cells. NPM1#1–specific CTLs showed a specific lysis of 57.1%-71.3% at an E/T ratio of 20:1. The counts were verified in 4 different HVs, as indicated by the error bars. T2 cells pulsed with a wild-type peptide or no peptide served as negative controls. The lytic potential of CTLs specifically recognizing epitope peptides derived from NPM1 mutations was further assessed in an autologous setting using PBMCs from AML patients with a blast percentage > 80% as targets. Only blasts with NPM1 mutations (“NPM1 mut”) #1 (B) and #3 (C) were lysed in a context of HLA-A2. Blasts from patients without NPM1 mutations (wild-type; “NPM1 WT”) or without HLA-A2 expression were not recognized. K562 cells lacking HLA-A2 were used as a negative control. These experiments clearly demonstrate that epitope peptides derived from NPM1 mutations #1 and #3 are naturally processed and recognized in the context of HLA-A2.
Cr51-release assays for the assessment of cytotoxic activity of CD8+ T lymphocytes of 4 HVs against the peptide NPM1#1. (A) NPM1#1–specific cytotoxic T lymphocytes (CTLs) were generated from 4 HVs and cytotoxic activity was assessed by Cr51-release assays using peptide-pulsed T2 cells as target cells. NPM1#1–specific CTLs showed a specific lysis of 57.1%-71.3% at an E/T ratio of 20:1. The counts were verified in 4 different HVs, as indicated by the error bars. T2 cells pulsed with a wild-type peptide or no peptide served as negative controls. The lytic potential of CTLs specifically recognizing epitope peptides derived from NPM1 mutations was further assessed in an autologous setting using PBMCs from AML patients with a blast percentage > 80% as targets. Only blasts with NPM1 mutations (“NPM1 mut”) #1 (B) and #3 (C) were lysed in a context of HLA-A2. Blasts from patients without NPM1 mutations (wild-type; “NPM1 WT”) or without HLA-A2 expression were not recognized. K562 cells lacking HLA-A2 were used as a negative control. These experiments clearly demonstrate that epitope peptides derived from NPM1 mutations #1 and #3 are naturally processed and recognized in the context of HLA-A2.
Identification of HLA-DR–binding epitopes and augmentation of NPM1-specific CD8+ T-cell response by MHC-class II peptide stimulation
To obtain a robust and effective immune response against tumor cells, the activation of CD4+ helper T cells is crucial. Therefore, NPM1#1 MHC class II OL epitopes were searched by a primary structure analysis program. Using the SYFPEITHI program, one identical epitope, CFRMTDQEAIQDLWQ, located at the C-terminus of intact NPM1, was predicted to be processed to HLA-DR*0101 and HLA-DR*1501, but not to HLA-DR*0301, HLA-DR*0401, or HLA-DR*1101 (Figure 6). HLA-A*0201–restricted NPM1#1, encompassing three 15 mer peptides derived from NPM1mut A, such as CFRMTDQEAIQDLCL, IQDLCLAVEEVSLRK, and MTDQEAIQDLCLAVE, was ranked within the top 10 and scored > 15. These epitopes were predicted to mimic HLA-DR molecules, and we focused on one common HLA-DR allele, HLA-DR*0701, to compare immunogenicity between 2 CD4+ T-cell epitopes. Like NPM1mut A, single amino acid–replaced epitopes were found to bind to HLA-DR molecules from the mutation C variant, a maternal protein of peptide #3. Based on a plenary search, 8 overlapping peptides, OL1-OL8, were synthesized (Figure 6) and exploited for CD4+ T-cell stimulation.
Peptide design of NPM-1–derived peptides for CD4+ and/or CD8+ T-cell induction assays. Several HLA-DRB1–binding motifs derived from either intact or mutated NPM1 sequences were predicted for their specific binding capacity using the SYFPEITHI program.
Peptide design of NPM-1–derived peptides for CD4+ and/or CD8+ T-cell induction assays. Several HLA-DRB1–binding motifs derived from either intact or mutated NPM1 sequences were predicted for their specific binding capacity using the SYFPEITHI program.
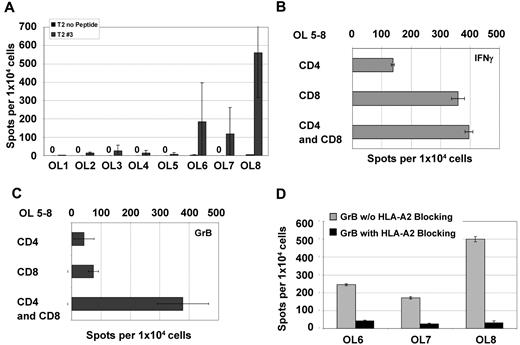
NPM1#1–specific CD8+ T cells were boosted in the presence of OL8 peptide stimulated with CD4+ T cells (Figure 7A). OL1-OL7 did not induce specific CD8+ T cells efficiently. NPM1#1–specific CD8+ T cells were copulsed with the peptide mixture of mutant #1 OL5-OL8, and the immune response against NPM1#1 was examined by ELISPOT on day 8 for CD4 and CD8 individually and for CD4/CD8 mixed in with the MLPCs (Figure 7B-C). NPM1#1 CD8+ T cells copulsed with OL5-OL8 and boosted by CD4+ T cells showed higher IFN-γ and granzyme B secretion.
OL analysis for CD4 and/or CD8 recognition. All OLs were analyzed by ELISPOT with PBMCs from 8 HVs individually for granzyme B (A). NPM1#1 CD8+ T cells were assessed for IFN-γ (B) and granzyme B (C) secretion activity in settings of coculture with HLA-DR*0701/binding OL mixture of 5-8. Secretion of CD8+ T cells was diminished by specifically blocking the HLA-A2 receptor as assessed by granzyme B ELISPOT (D).
OL analysis for CD4 and/or CD8 recognition. All OLs were analyzed by ELISPOT with PBMCs from 8 HVs individually for granzyme B (A). NPM1#1 CD8+ T cells were assessed for IFN-γ (B) and granzyme B (C) secretion activity in settings of coculture with HLA-DR*0701/binding OL mixture of 5-8. Secretion of CD8+ T cells was diminished by specifically blocking the HLA-A2 receptor as assessed by granzyme B ELISPOT (D).
To ensure that Th1 cytokine secretion, under the condition of a CD8+ and CD4+ T-cell mixed culture, resulted from NPM1#1 CD8+ T cells and not from HLA-DR epitope–stimulated CD4+ T-cell activation, an HLA-A2–blocking effect was confirmed using the ELISPOT assay (Figure 7D). NPM1#1 CD8+ T cells copulsed with OL6, OL7, and OL8 showed a reduction of IFN-γ secretion after HLA-A2–blocking Ab exposure of 73%, 35%, and 57%, respectively. Granzyme B secretion levels were reduced by 83%-94% through HLA-A2 blockade. This demonstrates that NPM1#1 CD8+ T cells are most probably producers of IFN-γ and granzyme B and that CD4+ T cells help CD8+ T cells in terms of NPM1-directed reactivity.
Discussion
NPM1-directed immune responses might be involved in the rejection of leukemic blasts containing NPM1mut. AML patients with mutations in the NPM1 gene without an FLT3-ITD mutation showed an improved overall survival,2,4,7,22,23 but the mechanisms still have to be elucidated. In elderly AML patients, NPM1mut seems to be a positive predictive marker for response to all-trans retinoic acid (ATRA) given as an adjunct to intensive chemotherapy.24 Wild-type NPM1 (and other proteins such as nucleolin) act as “hub” proteins that may interact with other proteins or facilitate their presentation to the immune system.25,26 In the present study, we investigated whether mutated NPM1 is immunogenic and may contribute to the survival benefit of NPM1mut AML patients.
The entire amino acid sequences of the NPM1 wild-type protein and of the NPM1mut types A, B, C, and D were screened for HLA-A*0201–binding CD8+ T-cell epitopes. Among 10 epitope peptides with the most favorable characteristics in algorithmic analysis (Figure 1), 2 peptides derived from the NPM1mut protein (Figure 2) induced high frequencies of specific CD8+ T-cell responses in HVs and AML patients. In NPM1mut AML patients, 33% showed immune responses of CD8+ T cells against peptide #1 and 44% against peptide #3 in ELISPOT assays (Figure 3). In HVs, we found that 39% versus 18% showed CD8+ T-cell reactivity against peptide #1 versus #3, respectively, in ELISPOT assays (Figure 2). Peptide #1 has some advantages compared with peptide #3: (1) mutation A, from which peptide #1 is derived, constitutes the most common mutation of NPM1; and (2) the probability of increasing peptide #1–specific CD8+ T cells as specific donor lymphocyte infusions from the stem cell donor to the AML patient who relapsed after allogeneic stem cell transplantation was higher for peptide #1 than for peptide #3. Both peptide #1 and peptide #3 were naturally processed and recognized by specific CD8+ T cells in tetramer assays (Figure 4) as well as in Cr51-release assays (Figure 5).
Immunotherapeutic approaches against LAAs showed encouraging results. The LAAs RHAMM, proteinase 3, and WT-1 were tested in clinical peptide vaccination trials.18-21,27 In these trials, immunologic but also clinical responses could be detected in patients with different hematologic malignancies. Several LAAs have been identified in AML and other malignant myeloid disorders such as BAGE, BCL2, G250, hTERT, proteinase 3 (PR3), RHAMM, survivin, and WT-1. These LAAs can be recognized by specific CD8+ T cells, and for some antigens, humoral immune responses have also been demonstrated.8,16 LAAs might enable the immune system to more easily eliminate minimal residual disease after chemotherapy, and therefore LAA expression might also influence clinical outcomes. The expression of at least 1 of the 3 LAAs—RHAMM, PRAME, or G250—proved to be favorable for the prognosis of AML patients.9 Similar results were demonstrated for the coexpression of other specific LAAs.10 Most of these LAAs are involved in pathways critical for leukemia cell proliferation.16
To obtain a robust and continuous T-cell reaction, the help of CD4+ T cells is indispensable.28 Therefore, in the present study, we investigated the increase of these CD8+ T-cell responses by the activation of CD4+ T cells stimulated with longer peptides (Figure 6). Potent HLA-DR epitopes were predicted to sustain LAA-specific CD8+ T cells, including epitopes detectable from CD8 and CD4 T cells. The overlapping peptide OL8 (MTDQEAIQDLCLAVE) stimulated CD4+ T cells and showed notable CD8+ T-cell activation in ELISPOT assays (Figure 7). Several favorable overlapping peptides, OL1-OL8, were synthesized and exploited for CD4+ T-cell stimulation. In the granzyme B ELISPOT assay, OL8-copulsed NPM1#1 CD8+ T cells indicated positive T-cell responses.
Our algorithm-based CD8+ and CD4+ T-cell epitopes derived from NPM1mut were demonstrated to be feasible to elicit a coordinative immune response against NPM1mut AML cells. NPM1mut is a favorable prognostic marker for patients with AML and is detectable in the BM and peripheral blood as a marker for minimal residual disease.29 Therefore, immunotherapeutic approaches are promising strategies for NPM1mut patients for maintenance treatment or therapy of positive minimal residual disease.
In a randomized, phase 3 clinical study investigating the effect of ATRA, the addition of ATRA to conventional therapy improved significantly the complete remission rate, event-free survival, and overall survival in untreated elderly AML patients with NPM1mut without FLT3-ITD mutation.30 AML patients with NPM1mut in particular seem to benefit from ATRA treatment,24 and therefore ATRA has been implemented into different clinical trials of NPM1mut AML patients (eg, the AMLSG 09-09 trial) and is routinely used in several cancer centers for NPM1mut patients outside of a clinical trial. The combination of ATRA and vaccination in AML/acute promyelocytic leukemia showed an increase of specific T-cell responses in vitro.31,32 The addition of ATRA to initial chemotherapy might contribute to the eradication of leukemic cells through down-regulation of NPM1, thus inducing apoptosis.25 Moreover, chemotherapy followed by a combination of immunotherapy and ATRA as maintenance therapy might facilitate the elimination of minimal residual disease in AML patients.
In conclusion, the results of the present study show that the simultaneous NPM1mut-triggered CD8+ and CD4+ T-cell immune response may result in a T-cell orchestration that could be responsible for the favorable prognosis of AML patients with NPM1mut, and this boosted NPM1mut immunity may also support enduring remission in NPM1mutAML patients.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cornelia Herbst, Vanessa Schneider, and Zhang Lu for excellent technical assistance.
This research was supported by grants from the German José Carreras Leukemia Foundation (DJCLS A 10/01, R 10/03, and R09/13) and the German Research Foundation (DFG GR2676/3-1).
Authorship
Contribution: J.G. designed and performed the research, analyzed and interpreted the data, and wrote the manuscript; Y.O. performed the research and analyzed and interpreted the data, S.H. performed the research, analyzed and interpreted the data, and reviewed the manuscript; A.S. interpreted the data and reviewed the manuscript; E.M. performed the research and reviewed the manuscript; M.G. performed the research, analyzed the data, and reviewed the manuscript; P.G. produced the tetramers; K.D. collected the data and reviewed the manuscript; J.M. analyzed the samples and reviewed the manuscript; H.D. designed the research, contributed vital reagents, and reviewed the manuscript; and M.S. designed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr med Jochen Greiner, Third Department of Internal Medicine, University of Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany; e-mail: jochen.greiner@uniklinik-ulm.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal