Abstract
Antigen-presenting cells (APCs) act as vehicles that transfer HIV to their target CD4+ cells through an intercellular junction, termed the virologic synapse. The molecules that are involved in this process remain largely unidentified. In this study, we used photoaffinity labeling and a proteomic approach to identify new proteins that facilitate HIV-1 transfer. We identified ectopic mitochondrial ATP synthase as a factor that mediates HIV-1 transfer between APCs and CD4+ target cells. Monoclonal antibodies against the β-subunit of ATP synthase inhibited APC-mediated transfer of multiple strains HIV-1 to CD4+ target cells. Likewise, the specific inhibitors of ATPase, citreoviridin and IF1, completely blocked APC-mediated transfer of HIV-1 at the APC-target cell interaction step. Confocal fluorescent microscopy showed localization of extracellular ATP synthase at junctions between APC and CD4+ target cells. We conclude that ectopic ATP synthase could be an accessible molecular target for inhibiting HIV-1 proliferation in vivo.
Introduction
Antigen-presenting cells (APCs), including dendritic and B cells, play a major role in HIV pathogenesis.1,2 These cells act as vehicles that transfer the virus to CD4+ lymphocytes, while simultaneously activating these cells to produce high levels of HIV replication. Using various imaging techniques, it has been shown that contact between monocyte-derived dendritic cells (MDDCs) and T cells facilitates transmission of HIV by locally concentrating virus, receptor, and coreceptor at the intercellular adhesion point, forming an infectious junction termed the virologic synapse.3,4 The cell-cell transfer of HIV-1 involves binding and internalization by the donor cell into intracellular compartments followed by release of virus and transfer across the viral synapse to the target cell resulting in infection. This phenomenon was extensively studied and reported in some excellent reviews.1,5-7 The “virologic synapse,” which is made of components of the immunologic synapse, explains the high efficiency with which HIV-1 infects target cells by cell-cell transfer. However, the mechanism of HIV internalization, synapse formation, and cell-cell transmission is not known. Moreover, the molecules within APCs and target cells that are involved in this process remain largely unidentified. The DC specific intercellular adhesion molecule grabbing nonintegrin (DC-SIGN) is the best studied C-type lectin on the DC surface that captures HIV-1 and transmits the virus to T cells.8-10 Nevertheless, DC-SIGN alone cannot account for the multistep process of viral transfer, and the possible involvement of other components has been proposed.9,11,12 In this work, we used a photoaffinity labeling and proteomic approach to identify proteins that facilitate APC-mediated transfer of HIV-1 to target cells. The ectopic ATP synthase was identified as a factor that controls APC-mediated HIV-1 transfer at the intercellular level.
Methods
Antibodies
Anti–DC-SIGN (clone DC-28) was a gift from Robert Doms from the Department of Microbiology University of Pennsylvania School of Medicine. Anti-ATP synthase (2 clones, mouse monoclonal ab5432, Abcam; and MS511, Mitoscience) and control mouse IgG Dye-conjugated antibodies against monocyte and iDC markers were from BD Biosciences. Virus isolates were produced from chronically infected cell lines as previously described13 and were generously supplied by the AIDS and Cancer Virus Program, SAIC Frederick Inc, Frederick MD. Pseudotyped HIV-Luciferase/AD8 viruses were propagated in human embryonic kidney cells (293 cells). Purified recombinant ATP synthase specific inhibitor IF1 was prepared by “Varniss” (Frederick, MD). The TZM-bl indicator cell line,13 obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, is a HeLa cell line derivative that expresses high levels of CD4 and CCR5 along with endogenously expressed CXCR4. TZM-bl cells contain HIV LTR-driven β-galactosidase and luciferase reporter cassettes that are activated by HIV tat expression. DC-SIGN–expressing Raji cells2 (DC-Raji) and HuT/CCR5 cells were generously provided by Vineet KewalRamani, from the HIV drug resistance program National Cancer Institute-Frederick, National Institutes of Health.
Monocyte derived DCs
The buffy coat fraction isolated from fresh donor blood was supplied by the National Institutes of Health clinical center blood bank. Monocytes were isolated by Percoll gradient centrifugation.14 Briefly, In a 50-mL conical tube, the buffy coat fraction was overlaid on a layer of 15 mL Histopaque (Sigma-Aldrich) and centrifuged for 30 minutes at 600g. The peripheral blood mononuclear cell band at the interface between the plasma and the histopaque was collected and washed 4 times in PBS and collected using 5-minute centrifugations at 500g. The peripheral blood mononuclear cells were resuspended in 25 mL HEPES-buffered RPMI, and 5-mL fractions were overlaid in 15-mL conical tubes on a 5-mL layer of 42% Percoll (GE Healthcare) preequilibrated to isotonicity with 10 times PBS using an osmometer. The cells were centrifuged for 30 minutes at 800g, and the monocyte fractions at the interface between the RPMI and the percoll were collected. The lymphocytes that separated in the pellet were collected as well for some experiments. The monocytes were washed 3 times and resuspended in RPMI 1640 + 10% FCS. The cells were incubated for 30 minutes at 37°C. The nonadherent cells were removed, and the adherent monocytes were 93% positive for the CD14 marker as determined by flow cytometry. Monocytes were differentiated to iDCs by incubating them at 1 × 106/mL in RPMI 1640 medium (RPMI 1640 + 10% FBS, 2mM glutamine, 25mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin) in the presence of rhGM-CSF (50 ng/mL) and rhIL-4 (50 ng/mL) at 37°C in a humidified CO2 (5%) incubator for 7 days while changing the medium every 48 hours. The resulting iDCs had the specific markers DC-SIGN, CD11b, and CD11c determined by flow cytometry.
HIV-1 isolate induced labeling of DC-Raji cells surface proteins by photoaffinity labeling. HIV-1 (MN) propagated in H9 T cells that included approximately 50% (weight/weight) microvescicle proteins15 was reacted with the trifunctional cross-linker sulfo-SBED (sulfo-N-hydroxysuccinimidyl-2-(6-[biotinamido]-2-(p-azidobenzamido)-hexanoamido)ethyl-1,3′-dithioproprionate; Pierce Chemical, Thermo Fisher Scientific; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The HIV-1 MN/H9 preparation (containing H9 cell microvesicles) in the amount of 0.35 mg total protein (0.16 mg viral capsid, with a TCID50 of 3.2 × 105/mL) was reacted with the succinimide ester moiety of sulfo-SBED in PBS in a probe-to-protein molar ratio of 30:1. After 1-hour incubation, the reaction was blocked by TBS and the virus was separated from the excess probe by size exclusion chromatography on a PD-10 column. The modified virus preparation was added to a total of 8 × 107 DC-Raji cells or MDDCs in RPMI and incubated at 37°C for 3 hours by rotation. The cells were washed twice with PBS and resuspended in 2 mL of the same buffer. The cells were irradiated with UV light for 15 minutes to activate the phenyl azide moiety and induce crosslinking between the bound virus and the proteins it binds to on the cells. The light source was a 100-W ozone-free Mercury arc lamp placed in a lamp house with collector lens. The cells were then washed and lysed by freeze thawing in hypotonic buffer. The membrane fraction was isolated by centrifugation of the lysate at 100 000g for 40 minutes and collection of the pellet. Preliminary experiments have shown that practically all the biotin-labeled proteins were associated with the membrane fraction of the cells.
Separation of biotinylated proteins by 2D electrophoresis
Proteins of the membrane fraction were separated by 2D electrophoresis using membrane solubilization buffers that contained DTT to cleave the S-S bond of the probe and thus deposit the biotinylated moiety on the target protein. Isoelectric focusing buffer in the first dimension consisted of 7M urea, 2M thiourea, 2% (weight/volume) amidosulfobetaine-14 (ASB14; Calbiochem) and 0.5% (volume/volume) Triton X-100, 20mM DTT, and 0.5% (volume/volume) ampholytes. After incubation at 23°C for 2-4 hours, bromophenol blue was added to 0.01% (weight/volume), and the sample was centrifuged in a microcentrifuge at top speed for 10 minutes to remove insoluble material. Samples were added to 11-cm pH 4-7 immobilized pH gradient strips (GE Healthcare) or pH 3-10NL (nonlinear) immobilized pH gradient strips (Bio-Rad). Immobilized pH gradient strips were rehydrated with sample and focused for 35 000 volt-hours (Protean-IEF; Bio-Rad). For the second dimension, the strips were equilibrated in SDS buffer according to the manufacturer's instructions and second dimension SDS-PAGE was carried out followed by colloidal Coomassie or silver staining. Two-dimensional separation of proteins was carried out in different pH-ranges in the first dimension and 10% acrylamide in the second dimension, with various gel sizes of 11, 18, and 24 cm. Gels were run in duplicates; one was stained with colloidal Coomassie and the other was transferred to nitrocellulose membrane for Western blotting. Biotinylated proteins were detected either by enhanced chemiluminescence after staining of the nitrocellulose membrane with streptavidin-peroxidase or by infrared imaging on staining with streptavidin-alexafluor-680 (supplemental Figure 2C).
Separation of biotinylated proteins by affinity capture
To obtain the full spectrum of the proteins labeled by HIV in solution, an extraction protocol was developed to solubilize all the biotinylated proteins without creating a denaturizing condition that will interfere with the subsequent affinity purification by streptavidin. The concentrated biotin-labeled membranes were solubilized in a minimal volume of 2% SDS buffer, boiled for 3 minutes, and then diluted with 20 volumes of RIPA buffer (50mM Tris-HCl, pH 7.4, 150mM NaCl, 0.25% deoxycholate, 1% NP-40, 1mM EDTA, and 100mM DTT). The final SDS concentration was 0.1%, and the solution was centrifuged at 14 000g for 20 minutes for removal of aggregates. A clear homogeneous solution was obtained without the formation of precipitates. The complete solution of biotinylated proteins was incubated with streptavidin-agarose overnight for affinity precipitation of the biotinylated proteins. The agarose-streptavidin beads were washed, and the biotinylated proteins were released by boiling the beads in SDS buffer (2% SDS, 50mM DTT, and 62.5mM Tris-HCl, pH 8.0), and proteins were identified by mass spectrometry (supplemental Figure 2B).
Identification of labeled proteins
Identification of isolated proteins was carried out by liquid chromatography tandem mass spectrometry (LC-MS/MS) as previously described.16 Gel bands stained with colloidal Coomassie were subjected to in-gel tryptic digestion and the resultant peptides extracted. Each peptide sample was desalted using C18 ZipTips as per the manufacturer's directions (Millipore), lyophilized, and resuspended in 16 μL of 0.1% formic acid. Each sample (6 μL) was loaded onto an Agilent 1100 nano-capillary HPLC system (Agilent Technologies) equipped with a 10-cm integrated μRPLC-electrospray ionization emitter column (made in house), coupled online to a LTQ XP mass spectrometer (Thermo Fisher Scientific) for μRPLC-MS/MS analysis.
Measuring transfer of HIV-1 to target cells by APCs
DC-Raji cells or DCs (5 × 105/group) were resuspended in 100 μL PBS. Different amounts of HIV-1, determined by a transfer assay calibration curve, were added and brought to a final volume of 400 μL in RPMI for each individual assay and then mixed with the cells and incubated at 37°C for 3 hours with rotation. The virus concentration used was the one that induced a transfer signal within the linear range as determined in separate calibration transfer assays carried out for each individual HIV-1 strain and lot (supplemental Figure 3). The APCs were washed twice in PBS and resuspended in 500 μL/group of DMEM containing 40 μg/mL diethylaminoethyl-dextran. The APCs were then added in triplicates to 96-well plates (150 μL/well) that were plated a day before with 2 × 104 TZM-bl cells/well. Infectivity of target TZM-bl was measured using the luciferase reporter gene assay.2 After 24-hour incubation at 37°C, the medium was aspirated and 50 μL of Steady-Glo Luciferase Assay substrate was added to each well. After 20- to 30-minute incubation at room temperature, the colorimetric signals were measured by a microplate luminometer. In summary, the transfer assay consists of the following consecutive steps: (1) mix APCs with HIV-1; (2) incubate at 37°C for 3 hours; (3) wash unbound virus twice and resuspend; (4) add the APCs to TZM or HuT/CCR5 target cells; and (5) after coculture for 24 hours at 37°C, luciferase substrate was added and the resulting luminescence was measured. For evaluating the effect of monoclonal antibodies on transfer activity, 2 clones of anti-ATP synthase β-chain were added simultaneously to enhance the effect to either the incubation step of HIV-1 and APCs (step 1) or to the step of incubation of the APCs with target cells (step 4). The specified antibodies were applied each in the amount of 10 μg/reaction (final concentration, 0.1 mg/mL). The ATP synthase inhibitors citreoviridin and recombinant IF1 were added as specified for the individual experiments.
Confocal microscopy
MDDCs were incubated 1 hour (37°C) with Cell Tracker green chloromethylfluorescein diacetate (Invitrogen), and HUT/CCR5 cells were incubated with Cell Tracker blue calcein AM at a dilution of 1:1000. After the incubation with the dyes, the cells were resuspended in fresh medium and washed with PBS. The MDDCs (5 × 105 cells) were incubated with HIV-1–MN for 3 hours at 37°C, washed (2 times) with PBS, and added to target TZM cells that were plated on poly-d-lysine–coated glass-bottom dishes (35 mm, MatTek). When HUT/CCR5 cells were used as target cells, the MDDC–HIV-1 were mixed with the target cells in a microcentrifuge tube. Mouse anti-ATP synthase β-chain antibody (10 μg, Abcam; and 10 μg Mitoscience) was added at the time of mixing between MDDCs and target cells. After incubation for 3 hours, the MDDC:HIV-1 plus target cell complex was washed and fluorescently modified. Goat antimouse IgG secondary antibody (1:2000, AlexaFlour-555; Invitrogen) was added for 1 hour (4°C). The samples were fixed with formaldehyde (4%, 15 minutes, 25°C) and then washed again with PBS. Image acquisition was carried out using a Zeiss LSM 510 and LSM710 (Carl Zeiss) confocal laser scanning microscope. A 100×/1.3 NA oil immersion objective lens was used with a zoom factor of 4.0. Ar+ laser line (488 nm) was used for Cell Tracker green. Chloromethylfluorescein diacetate excitation and emission light were collected with a 500-550 bandpass filter. AlexaFluor-555 was excited by HeNe 543-nm laser line (HFT UV/488/543/633, Mirror NFT 545) and emission light was collected by 565-615 bandpass filter. Data were corrected with background subtraction and analyzed by Zeiss LSM Image Version 4.2 software.
Results
Identification of proteins labeled by HIV-1 induced photoaffinity labeling of DC-Raji cellular proteins
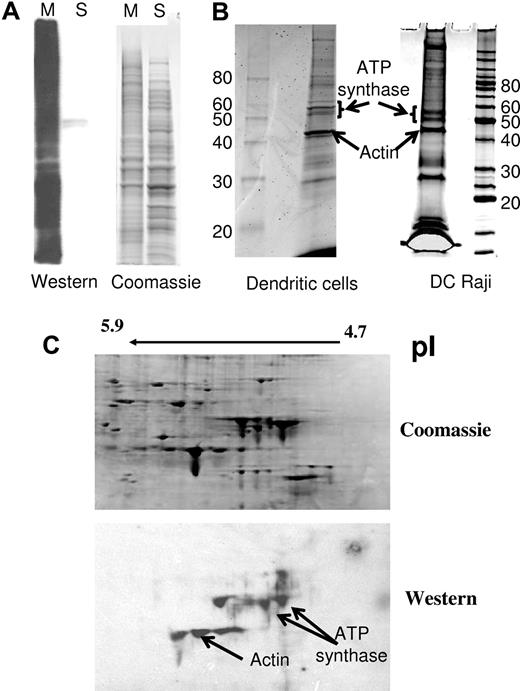
Photoaffinity labeling has been applied to identify receptors for protein ligands that were modified with a photoactivatable crosslinker. In this work, the photoactivatable crosslinker (SBED) was bound to the surface proteins of HIV-1 viruses and membrane vesicles derived from T cells that react with APCs in the context of viral transfer and the formation of the virologic synapse. The photoinduced crosslinked proteins on DC-Raji cells and immature MDDCs that became labeled with biotin were separated and subjected to mass spectrometry (MS) analysis. Two different methods for separating the biotinylated proteins were used (Figure 1): (1) Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) of the bulk membrane fraction followed by protein transfer to nitrocellulose membranes and affinity staining of the membrane with streptavidin conjugated to AlexaFluor and/or HRP; and (2) sodium dodecyl sulfate (SDS)–assisted total solubilization of the biotinylated proteins followed by affinity capture of the biotinylated proteins using streptavidin agarose beads. The captured proteins were released by boiling in SDS and separated by one-dimensional SDS-PAGE. Ten biotinylated protein spots were detected on the 2D-PAGE gel in the pI and molecular weight ranges of 5.5 and 45-60 kDa, respectively. These spots were excised and identified using LC-MS/MS. Likewise, 10 stained proteins from the affinity-captured proteins were also excised and analyzed. The identified proteins obtained from 2 different APCs (DC-Raji and monocyte-derived immature DCs) were selected based on the number of peptides identified for each and their presence within 4 replicative experiments. One major protein, ATP synthase β-chain, stood out in this analysis (Figure 1B-C).
Composite figure describing the different fractions isolated and analyzed after photoaffinity labeling. (A) After photoaffinity labeling, DC-Raji cells were lysed and the membrane fraction (M) was isolated from the cytosolic fraction (S) by centrifugation as described in “Methods” for separation of proteins. Samples from both fractions were subjected to Western affinity staining with streptavidin-HRP for the identification of the biotin-containing fraction. (B) The membrane fraction of APC was solubilized in solubilization buffer, and the biotinylated proteins were isolated by affinity capture using streptavidin-agarose beads. Ten bands were subjected to digestion/tandem MS identification. An arrow indicates the identified band containing ATP synthase. The distribution of the peptides was as follows: DC-Raji cells (2 bands): band 1, 18 peptides of ATP synthase β-chain, 10 unique 15 peptides of ATP synthase α-chain, 9 unique. Altogether, 12 unique peptides of ATP synthase β-chain (19 total). Band 2, 9 peptides of ATP synthase β-chain, 9 unique, 6 peptides of ATP synthase α-chain, 5 unique. Altogether, 9 unique peptides of ATP synthase α-chain (14 total). DCs (2 bands): band 1, 38 peptides of ATP synthase β-chain, 15 unique 11 peptides of ATP synthase α-chain, 8 unique. Altogether, 14 unique peptides of ATP synthase β-chain (43 total). Band 2, 5 peptides of ATP synthase β-chain, 3 unique 24 peptides of ATP synthase α-chain, 14 unique. Altogether, 15 unique peptides of ATP synthase α-chain (35 total). Vimentin was also highly represented in those DC samples. (C) The membrane fraction was solubilized and subjected to 2D electrophoresis as described in “Methods” for separation of proteins. ATP synthase was identified in the spots indicated by an arrow. Peptides recovered (4 spots): 519 peptides of ATP synthase β-chain, 24 unique.
Composite figure describing the different fractions isolated and analyzed after photoaffinity labeling. (A) After photoaffinity labeling, DC-Raji cells were lysed and the membrane fraction (M) was isolated from the cytosolic fraction (S) by centrifugation as described in “Methods” for separation of proteins. Samples from both fractions were subjected to Western affinity staining with streptavidin-HRP for the identification of the biotin-containing fraction. (B) The membrane fraction of APC was solubilized in solubilization buffer, and the biotinylated proteins were isolated by affinity capture using streptavidin-agarose beads. Ten bands were subjected to digestion/tandem MS identification. An arrow indicates the identified band containing ATP synthase. The distribution of the peptides was as follows: DC-Raji cells (2 bands): band 1, 18 peptides of ATP synthase β-chain, 10 unique 15 peptides of ATP synthase α-chain, 9 unique. Altogether, 12 unique peptides of ATP synthase β-chain (19 total). Band 2, 9 peptides of ATP synthase β-chain, 9 unique, 6 peptides of ATP synthase α-chain, 5 unique. Altogether, 9 unique peptides of ATP synthase α-chain (14 total). DCs (2 bands): band 1, 38 peptides of ATP synthase β-chain, 15 unique 11 peptides of ATP synthase α-chain, 8 unique. Altogether, 14 unique peptides of ATP synthase β-chain (43 total). Band 2, 5 peptides of ATP synthase β-chain, 3 unique 24 peptides of ATP synthase α-chain, 14 unique. Altogether, 15 unique peptides of ATP synthase α-chain (35 total). Vimentin was also highly represented in those DC samples. (C) The membrane fraction was solubilized and subjected to 2D electrophoresis as described in “Methods” for separation of proteins. ATP synthase was identified in the spots indicated by an arrow. Peptides recovered (4 spots): 519 peptides of ATP synthase β-chain, 24 unique.
The effect of anti-ATP synthase β-chain antibodies on APC-mediated transfer of HIV-MN
To confirm the involvement of ATP synthase in HIV-1 transfer, monoclonal antibodies against ATP synthase were added to DC-Raji or MDDC at different stages of the transfer assay as described in “Measuring transfer of HIV-1 to target cells by APCs.” Anti-ATP synthase antibodies had no effect on the transfer activity when added during incubation of the virus with APC (Figure 2A). In contrast, the anti-DC-SIGN antibody had a profound inhibitory effect when added at this step (Figure 2B). Because both the virus and the microvesicles are derived from T cells, ATP synthase β-chain may bind to a T cell–associated ligand involved in the interaction of the APC with the target cells through the formation of the virologic synapse. To examine this possibility, APCs (DC-Raji and MDDCs) were incubated with the virus, washed, and mixed with the target cells (TZM) in the presence of anti-ATP synthase antibody. As seen in Figure 2B and D, the anti-ATP synthase antibody had a profound inhibitory effect on viral transfer to the target cells, whereas the anti–DC-SIGN antibody had no effect. Similar results were obtained using pseudotyped viruses with the T-cell line Hut/CCR5 as target cells. Control experiments were carried out for testing possible inhibitory effects that the antibodies may have on HIV-1 infectivity of target cells (TZM), which is the basis of our transfer assay. No significant effect was detected on HIV-1 infectivity by any antibodies used in this study.
Anti-ATP synthase antibodies block APC-mediated transfer of HIV-1 at the cell-cell interaction step. APC-mediated HIV-1 transfer was carried out either with DC Raji cells (A-B) or with DCs (C-D). The indicated antibodies (20 μg/mL) were added either at the step of APC–HIV-1 interaction (A,C) or added at the step of incubation of the virus-primed APCs with target TZM cells (B,D). No Ab indicates no antibody added; IgG, nonimmune mouse IgG; DC-SIGN, anti–DC-SIGN; and anti-ATPsyn, anti-ATP synthase β-chain. All antibodies were at a concentration of 1.2 × 10−7M. Transfer activity was measured by viral infectivity given in luminescence arbitrary units. A total of 100% transfer activity was determined as the maximal activity obtained in the absence of added antibody (No Ab). Experiments were carried out at least 3 times, and bar graphs represent SEM of triplicate samples.
Anti-ATP synthase antibodies block APC-mediated transfer of HIV-1 at the cell-cell interaction step. APC-mediated HIV-1 transfer was carried out either with DC Raji cells (A-B) or with DCs (C-D). The indicated antibodies (20 μg/mL) were added either at the step of APC–HIV-1 interaction (A,C) or added at the step of incubation of the virus-primed APCs with target TZM cells (B,D). No Ab indicates no antibody added; IgG, nonimmune mouse IgG; DC-SIGN, anti–DC-SIGN; and anti-ATPsyn, anti-ATP synthase β-chain. All antibodies were at a concentration of 1.2 × 10−7M. Transfer activity was measured by viral infectivity given in luminescence arbitrary units. A total of 100% transfer activity was determined as the maximal activity obtained in the absence of added antibody (No Ab). Experiments were carried out at least 3 times, and bar graphs represent SEM of triplicate samples.
Anti-ATP synthase β-chain monoclonal antibodies inhibit DCs mediated transfer of various strains of HIV-1 at the step of the virologic synapse formation
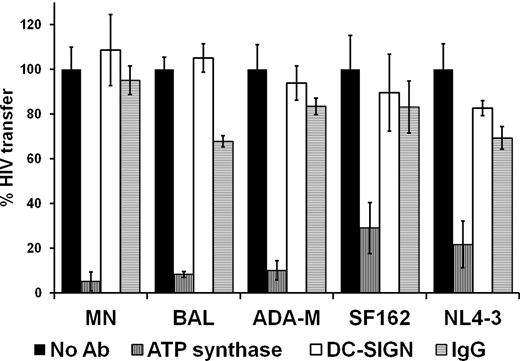
To evaluate whether the role of ATP synthase β-chain in the DC-mediated transfer of HIV-1 is general and common to other strains of HIV-1, MDDC-mediated HIV transfer was measured using different HIV-1 strains (BAL, ADA, SF162, NL4-3, and MN) that were propagated in one T-cell line (SUP-T1). The effect of the anti-ATP synthase antibody was measured for each strain. The control antibody, anti-DC-SIGN, had no significant effect on the transfer of any of the HIV-1 strains tested. Nonimmune mouse IgG was used as a negative control. Figure 3 conclusively shows that the antibody against ATP synthase β-chain blocks the MDDC-mediated transfer of all tested HIV-1 strains, indicating a general role for ATP synthase in the transfer of HIV-1 mediated by primary DCs at the cell-cell interaction step.
Anti-ATP synthase β-chain antibodies block DC-mediated transfer of multiple strains of HIV-1. The indicated HIV-1 strains were all propagated in the Sup-T1 cell line. Data are presented as in Figure 1. All antibodies used in this figure as well as in subsequent figures were at concentrations as specified in “Measuring transfer of HIV-1 to target cells by APCs” and for Figure 1.
Anti-ATP synthase β-chain antibodies block DC-mediated transfer of multiple strains of HIV-1. The indicated HIV-1 strains were all propagated in the Sup-T1 cell line. Data are presented as in Figure 1. All antibodies used in this figure as well as in subsequent figures were at concentrations as specified in “Measuring transfer of HIV-1 to target cells by APCs” and for Figure 1.
The inhibitory effect of anti-ATP synthase β-chain antibody on DC-mediated transfer of HIV-1 is not affected by the cell line in which the viruses were propagated
We next tested whether the role that ATP synthase has in HIV-1 transfer is dependent on the cell line in which the viruses were grown. Many cellular proteins have been identified that adhere to HIV-1 virions after budding,17,18 and these may play a role in the biology of the virus. For that purpose, anti-ATP synthase antibodies were tested for their ability to inhibit transfer of HIV-1 MN that was propagated in different cell lines. The T-cell lines examined were H9, SUPT1, Jurkat-TAT-CCR5, CEMX174, and H9-CL.4. Experiments were carried out essentially as described in Figure 2. The results presented in Figure 4 show that the antibody inhibits the MDDC-mediated transfer of HIV-1 from all tested T-cell lines. Likewise, anti-ATP synthase antibody also blocked the transfer of pseudotyped HIV-1 AD8 viruses that were expressed and propagated in 293 cells while using the T-cell line HuT/CCR5 as target cells.
The inhibitory effect of anti-ATP synthase β-chain antibodies on HIV-1 transfer is not affected by the cell line in which the virus is propagated. HIV-1 MN was propagated in the different T-cell lines as indicated. MDDC-mediated viral transfer activity to target TZM cells was measured as described in “Measuring transfer of HIV-1 to target cells by APCs.” A total of 100% transfer activity was determined for each cell line as the maximal transfer activity obtained in the absence of added antibody (No Ab).
The inhibitory effect of anti-ATP synthase β-chain antibodies on HIV-1 transfer is not affected by the cell line in which the virus is propagated. HIV-1 MN was propagated in the different T-cell lines as indicated. MDDC-mediated viral transfer activity to target TZM cells was measured as described in “Measuring transfer of HIV-1 to target cells by APCs.” A total of 100% transfer activity was determined for each cell line as the maximal transfer activity obtained in the absence of added antibody (No Ab).
Specific inhibitors of ATP synthase activity block HIV-1–mediated transfer by DCs
We next determined whether the activity of ATP synthase plays a role in the MDDC-mediated transfer of HIV-1 to target cells. Two specific inhibitors of the mitochondrial ATP synthase, citreoviridin a potent inhibitor produced by fungus,19 and the endogenous specific inhibitor IF120 were applied at the virologic synapse step. Citreoveridin completely inhibited virus transfer at concentrations levels where the toxin had no effect on cell viability or infectivity of the of HIV-1 (Figure 4A). Likewise, the endogenous inhibitor of ATP synthase, IF1, blocked APC-mediated transfer of HIV-1 in a dose-dependent manner at pH 6.5, the optimum pH for IF1 specific inhibition of ATP synthase in the mitochondria21 (Figure 5B-C).
ATP synthase inhibitors prevent APC-mediated HIV-1 transfer at the cell-cell interaction step. (A) The ATP synthase inhibitor, citreoviridin, was added to the transfer assay with DCs at the indicated concentrations either at step 1 (diamonds) or at step 4 (triangles) of the assay as described in “Measuring transfer of HIV-1 to target cells by APCs.” “Infectivity” represents the direct effect of the inhibitor on the infectivity of the virus as measured directly on TZM cells. The infectivity in the absence of citreoviridin was determined as 100%. (B) Dose-dependent inhibition of APC-mediated HIV-1 transfer by recombinant IF1 at different pH. Viral transfer was mediated by DC-Raji cells, and 25 μg (8.3 × 10−5M) of the endogenous inhibitor IF1 was added at the step of APC-target cell interaction. (C) Effect of different inhibitors on DC-mediated HIV-1 transfer at the step of APC-target cell interaction. Control indicates no inhibitors. The amount of protein used per assay was 10 μg (1.3 × 10−7M) for each antibody and 25 μg (8.3 × 10−5) for IF1. Experiments were repeated at least 3 times and were performed in triplicates. Bars represent the SEM of the individual samples in a group.
ATP synthase inhibitors prevent APC-mediated HIV-1 transfer at the cell-cell interaction step. (A) The ATP synthase inhibitor, citreoviridin, was added to the transfer assay with DCs at the indicated concentrations either at step 1 (diamonds) or at step 4 (triangles) of the assay as described in “Measuring transfer of HIV-1 to target cells by APCs.” “Infectivity” represents the direct effect of the inhibitor on the infectivity of the virus as measured directly on TZM cells. The infectivity in the absence of citreoviridin was determined as 100%. (B) Dose-dependent inhibition of APC-mediated HIV-1 transfer by recombinant IF1 at different pH. Viral transfer was mediated by DC-Raji cells, and 25 μg (8.3 × 10−5M) of the endogenous inhibitor IF1 was added at the step of APC-target cell interaction. (C) Effect of different inhibitors on DC-mediated HIV-1 transfer at the step of APC-target cell interaction. Control indicates no inhibitors. The amount of protein used per assay was 10 μg (1.3 × 10−7M) for each antibody and 25 μg (8.3 × 10−5) for IF1. Experiments were repeated at least 3 times and were performed in triplicates. Bars represent the SEM of the individual samples in a group.
HIV-1 induces the localization of ATP synthase β-chain at the junction of APC and target cells
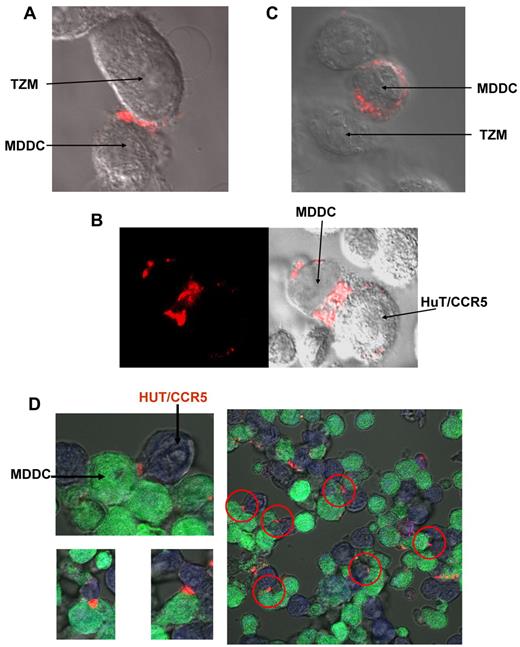
We wished to establish whether ATP synthase undergoes an HIV-1–assisted localization and sequestration at the APC-target cell junction, as implied by the biochemical data. An immunofluorescence experiment was carried out in which MDDCs were first incubated with HIV-1, washed, and then added and incubated with target cells (TZM or HUT/CCR5) in the presence of anti-ATP synthase antibody. For fluorescence microscopy measurements, APC and target cells were stained with distinct flluorescent probes and ATP synthase was visualized using a fluorescent anti–mouse IgG secondary antibody as described in “Confocal microscopy.” The cells were fixed, and fluorescence was monitored with a confocal microscope. The images presented in Figure 6 show that ATP synthase is concentrated at the junction between HIV-1–primed APCs and target cells.
Immunocytolocalization of ATP synthase at the junction between HIV-1–primed DCs and target cells. MDDCs were incubated with HIV-1 for 3 hours, washed, and added to target cells either TZM cells (A) or HUT/CCR5 cells (B,D) in the presence of anti-ATP synthase β-chain antibodies. (C) Same as in panel A, only that the incubation was done without the virus. (D) Same as in panel B, only that MDDCs were stained in green and HUT/CCR5 were stained in blue as described in “Confocal microscopy.” For all samples, staining was carried out with Alexa-555–anti–mouse IgG secondary antibody, the sample was fixed, and the pattern was visualized by confocal microscopy as described in “Methods.”
Immunocytolocalization of ATP synthase at the junction between HIV-1–primed DCs and target cells. MDDCs were incubated with HIV-1 for 3 hours, washed, and added to target cells either TZM cells (A) or HUT/CCR5 cells (B,D) in the presence of anti-ATP synthase β-chain antibodies. (C) Same as in panel A, only that the incubation was done without the virus. (D) Same as in panel B, only that MDDCs were stained in green and HUT/CCR5 were stained in blue as described in “Confocal microscopy.” For all samples, staining was carried out with Alexa-555–anti–mouse IgG secondary antibody, the sample was fixed, and the pattern was visualized by confocal microscopy as described in “Methods.”
Discussion
Mediator cells, such as APC, which bind HIV-1 and transfer the virus to its target CD4+ cells, are a major factor in the process of virus dissemination and infection in vivo. The discovery of the cell surface proteins that facilitate virus transfer can provide insights into the formation of the virologic synapse between the APC and the target T cell and identify potential molecular targets for intervention in this process. In this study, a proteomics strategy combining photoaffinity labeling and subfractionation was used to discover proteins on the APC that facilitate transfer of HIV-1. The crosslinker (SBED) was placed on the HIV-1 MN isolate from H9 T cells that consisted of a mixture of HIV particles and cellular microvesicles.15 Aside from the viral envelope proteins present exclusively of HIV, the vesicles and viruses both contain T cell ligands (eg, LFA-1 and ICAM-1) that are known to participate in the formation of the immunologic or virologic synapse.6,7,17,18 After crosslinking, the β-subunit of ATP synthase was consistently identified on both DC Raji cells and MDDCs as a dominant component in multiple targeted photolabeling experiments (Figure 1).
It is important to note that labeling of these proteins on DC-Raji cells was triggered at the end of a 3-hour incubation period at 37°C of a mixture of the virus and cells (step 3; see “Measuring transfer of HIV-1 to target cells by APCs”); therefore, any labeled protein may not necessarily represent the initial binding sites of the virus but rather those that the virus interacts with after some cellular trafficking, internalization, or endocytosis has taken place. Three main approaches were used to establish the relevance of the identified protein to the APC-mediated transfer of HIV-1: (1) measuring the effect of monoclonal antibodies against ATP synthase β-chain on HIV-1 transfer by DC-Raji cells and DCs; (2) evaluating the effect of specific inhibitors of ATP synthase activity on viral transfer by MDDC; and (3) cytoimmunolocalization of ATP synthase in the context of viral transfer. Antibodies against ATP synthase β-chain almost completely block viral transfer but only when added to the intercellular interaction step between the virus primed APC and target cell (TZM or HuT/CCR5; Figure 2B,D). The antibody had no or little effect when added at the initial step of interaction between the APC and the virus (Figure 2A,C). This finding indicates that the ligand or effector molecule that induced the photolabeling of ATP synthase β-chain on APC is probably a T-cell component that represents a target cell (T cell) contribution to the formation of the virologic synapse. This T cell–associated ligand may have come either from the T cell–derived microvesicles or the virus. The envelope proteins of the virus most probably did not contribute to the photolabeling, which is consistent with the finding that DC-SIGN was not present among the photolabeled proteins. This result is not entirely surprising because the heavily glycosylated envelope proteins gp-120/gp41 will not readily react with the succinimide moiety of SBED, which is selective toward amino side chains in proteins.
Anti-ATP synthase antibodies completely blocked transfer of multiple strains of HIV-1, indicating that the role of ATP synthase in the APC-mediated transfer of HIV-1 is a general phenomenon common to many strains of HIV-1 (Figure 3). Further support for this conclusion comes from the finding that the T-cell line in which the virus was grown had no effect on the inhibition of transfer induced by anti-ATP synthase antibody (Figure 4). HIV-1 transfer activity seems to be dependent on the enzymatic activity of ATP synthase because the specific inhibitors of this enzyme, the fungus derived cytreoviridin and the human endogenous inhibitor of mitochondrial F1F0 ATP synthase, IF1, block APC-mediated viral transfer at the cell-cell interaction step (Figure 5). The inhibitory effect of IF1 is conferred by specific binding of this protein to the F1F0 ATP synthase. The experiments carried out using antibodies or inhibitors were controlled by showing that those had no effect neither on the viability of the cells involved (APC or TZM) or on the infectivity of the virus as measured in target cells by the luciferase reporter gene assay (supplemental Figures 3 and 4).
Immunocytolocalization of extracellular ATP synthase β-chain shows that this protein is localized at junctions between DCs and target cells after priming of the DCs with HIV-1 (Figure 6). The immonolocalization was done on intact cells without permeabilizing agents, indicating that the binding of the antibodies was to an extracellular component of the enzyme. The localization of extracellular ATP synthase at the APC-target cell junction is consistent with the premise that the enzyme participates in the transfer of the virus from the APC to the CD4+ target cells. Localization of the virus at that junction has been previously demonstrated as the site for virus transfer.3-5 Our finding that ATP synthase is found at the junctions between APC and CD4+ target cells, even without the presence of the virus (Figure 5C), suggests that the virus is only using the enzyme at this location as a facilitator of the transfer. ATP sythase activity seems to be vital for this process as inhibitors of the ATPase component of ATP synthase, citreoviridin and IF1, inhibited HIV transfer from APC to CD4+ target cells. The involvement of ATP synthase β-chain in APC-mediated HIV-1 transfer and the sequestration of this enzyme at the junction of APC and target cells are not entirely surprising. Energy demanding remodeling of cell cytoskeletal proteins, like actin, has been shown to be essential for the formation of structural elements of the immunologic and virologic synapse.22 Previous studies have demonstrated the dynamic nature of the mitochondria that undergo migration and translocation to cellular “hot spots” where ATP is in high demand, including the immunologic and neurologic synapse.22-26 A more detailed structural study of the virologic synapse at the junction of dendritic and T cells shows membrane remodeling and cytoskeletal conduits that form by the contact between the 2 cells through which the virus migrates from one cell type to another.27 This process may also be triggered by T-cell antigens present on membrane microvesicles and/or HIV-1 particles leading to photoaffinity labeling of ATP synthase β-chain. Previous studies have shown that this protein, as part of the whole and active F1/F0 complex, is present on the surface of endothelial cells, tumor cells,28-30 and multiple cell lines.31-34 ATP synthase is a protein present on the inner membrane of the mitochondria. The mechanism by which it migrates to the surface of the cell is not understood. The significance of the topologic orientation of this enzyme as it pertains to this study is that, contrary to anti–HIV-1–neutralizing antibodies that do not block viral infection when applied at the virologic synapse,35,36 anti-ATP synthase β-chain antibody completely blocks APC-mediated infection of target cells at that step. This finding makes ATP synthase an attractive and accessible target for blocking the APC-mediated infection of HIV-1.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Vineet KewalRamani from the HIV drug resistance program National Cancer Institute-Frederick, National Institutes of Health; Julian Bess from the AIDS and Cancer virus program SAIC-Frederick Inc for HIV isolates and expert advice; Steven Lockett and Kim Peifley from the Optical Microscopy and Analysis Laboratory SAIC-Frederick for expert imaging assistance; and Dr Michael F. Marusich, from Mitoscience Inc for material support and expert advice on ATP synthase and oxidative phosphorylation.
This work was supported in whole or in part by the Frederick National Laboratory for Cancer Research, Intramural Research Program of the National Institutes of Health (contract HHSN26120080001E) and the Intramural AIDS Targeted Antiviral Program.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
National Institutes of Health
Authorship
Contribution: M.V., R.B., and Y.R. designed research; A.Y., M.V., Y.R., and E.H. performed research; J.M.W. and W.G. contributed new reagents; M.Z. and T.D.V. analyzed data; Y.R. wrote the manuscript; and M.V., R.B., and E.H. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yossef Raviv, Frederick National Laboratory for Cancer Research, Bldg 469, Room 213, PO Box B, Frederick, MD 21702; e-mail: ravivy@mail.nih.gov.
References
Author notes
A.Y. and M.V. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal