Arsenic in the form of arsenic trioxide (ATO) is used as a therapeutic drug for treatment of acute promyelocytic leukemia (APL). The mechanism by which this agent cures this disease was previously shown to involve direct interactions between ATO and the promyelocytic leukemia protein (PML), as well as accelerated degradation of the APL-associated fusion oncoprotein PML/retinoic acid receptor α (RARA). Here we investigated the fate of PML-generated nuclear structures called PML bodies in ATO-treated cells. We found that ATO inhibits formation of progeny PML bodies while it stabilizes cytoplasmic precursor compartments, referred to as cytoplasmic assemblies of PML and nucleoporins (CyPNs), after cell division. This block in PML body recycling is readily detected at pharmacologic relevant ATO concentrations (0.02-0.5μM) that do not cause detectable cell-cycle defects, and it does not require modification of PML by SUMOylation. In addition, PML and PML/RARA carrying mutations previously identified in ATO-resistant APL patients are impeded in their ability to become sequestered within CyPNs. Thus, ATO may inhibit nuclear activities of PML and PML/RARA in postmitotic cells through CyPN-dependent cytoplasmic sequestration.
Introduction
Arsenic trioxide (ATO) is a clinically approved cancer therapeutic drug that is effective in treatment of acute promyelocytic leukemia (APL) both as a single agent as well as in combination with all-trans retinoic acid (ATRA).1,,,–5 This is a targeted therapy as recent studies have demonstrated direct interactions between ATO and the promyelocytic leukemia protein (PML) moiety of the PML/RARA oncoprotein, the product of the APL-specific t(15;17) chromosomal translocation.6,7 At the molecular level this drug causes PML and PML/RARA SUMOylation and ubiquitination, biochemical modifications that promote degradation of these proteins by the proteasome.8,–10 In addition, ATO has been shown to stimulate lysosome-dependent degradation of PML/RARA through activation of autophagy.11 Such therapy-induced degradation of PML/RARA has been proposed to contribute to clinical remission through decreased self-renewal capacity as well as increased differentiation of the malignant APL cells.12,–14
The PML protein, expressed from its normal nonrearranged gene in APL or non-APL cells, is a tumor suppressor implicated in multiple cellular activities, including apoptosis, senescence, differentiation, and genome maintenance.15 A characteristic and unique feature of this protein is its ability to induce the formation of cellular compartments called PML bodies. These structures are readily detected within the nucleus of most mammalian cells by conventional immunofluorescence microscopy as 10 to 20 foci ranging in size from 0.1 to 1.0 μm.15,–17
PML bodies are highly dynamic structures that change their morphology, subcellular distribution, and protein composition in a cell cycle-dependent manner.15,–17 During interphase they primarily reside within the nucleus, where they dynamically recruit and release a multitude of proteins with diverse cellular functions.15,–17 On entry into mitosis, several PML body resident proteins, including SUMO, Daxx, and sp100, dissociate concomitant with increased aggregation of these structures into mitotic bodies referred to as mitotic assemblies of PML proteins (MAPPs).18,–20 At the end of mitosis the MAPPs are consistently excluded from the interior of progeny daughter nuclei,19,–21 and at entry into G1 they complex with nucleoporins (proteins that constitute nuclear pore complexes) to form cytoplasmic bodies that we refer to as cytoplasmic assemblies of PML and nucleoporins (CyPNs).21
Several observations suggest that CyPNs represent transient intermediates between MAPPs and nuclear PML bodies. Firstly, CyPNs gradually disappear during early G1 phase concomitant with repopulation of the nucleus with new PML bodies.18,,–21 Secondly, PML proteins carrying mutations within their nuclear import motif fail to support formation of CyPNs on expression in the cytoplasm, suggesting that formation of these structures is coupled to nuclear translocation of PML.21 Lastly, generation of PML bodies during G1 has been shown to occur in the absence of protein synthesis, showing that PML bodies from the previous cell cycle are recycled to the next generation of daughter nuclei to participate in construction of progeny PML bodies.19
In this study, we show that ATO causes CyPN stabilization concomitant with inhibition of nuclear PML body formation after progression of cells through mitosis, suggesting that this drug blocks the cell-cycle–dependent PML body circuit at the level of nuclear import. Notably, both PML and PML/RARA are recruited to CyPNs, and in contrast to ATO-induced protein degradation, stimulation of CyPN accumulation does not require PML SUMOylation. Finally, stabilization of CyPNs is primarily activated by relatively low ATO concentrations ranging from 0.01 to 1.0μM, and PML and PML/RARA carrying mutations associated with resistance to ATO-based therapy were found to have reduced affinity for these cytoplasmic compartments. Thus, postmitotic accumulation of CyPNs may represent a mechanism that operates in parallel with degradation to restrict nuclear activities of PML and PML/RARA in the presence of ATO.
Methods
Immunofluorescence and analysis of CyPNs
Adherent cells (HaCaT and HEF cells) were grown on coverslips and fixed in 4% paraformaldehyde in phosphate-buffered saline. Cells grown in suspension (NB4, T2, and human CD34+ cells) were subjected to cytospin centrifugation and air dried for 60 minutes before fixation in 4% paraformaldehyde in phosphate-buffered saline. After permeabilization with 0.3% Triton X-100, fixed cells were processed for immunofluorescence (IF) as previously described.22 The following antibodies were used: rabbit anti-PML (H-238; Santa Cruz Biotechnology), mouse anti-PML (PG-M3; Santa Cruz Biotechnology), nucleoporin-specific mouse Mab414 antibody (Abcam), mouse anti-Nup153 (Abcam), mouse anti–SUMO-1 (GMP-1; Invitrogen), mouse anti-FLAG (Sigma-Aldruch), and rabbit anti-RARA (C-20; Santa Cruz Biotechnology).
For quantification of CyPNs, we acquired confocal microscopy images using the LSM 110 confocal microscopy system equipped with a 40× lens on an Axiovert Observer Z1 microscope. For each sample, 6 to 10 different fields of view were selected randomly, and images were generated by projection of 6 to 10 z-scans that spanned the entire cell. Each cell in an image was manually inspected, and CyPNs were scored if PML and nucleoporins accumulated and colocalized within distinct foci. The same criteria were used for scoring of RARA/nucleoporin-positive foci, except that anti-RARA, instead of anti-PML, was used.
Lentivirus production and preparation of cell lines with stable transgene expression
Lentivirus-based vectors expressing shRNAs against PML and luciferase were previously described,23 and provided by Dr Roger D. Everett (Medical Research Council–University of Glasgow, United Kingdom). Dr Everett also provided pLNGY-PML.I, and the derived mutants KKK, ΔSIM, ΔRING, ΔBB1, ΔBB2, and ΔCC, which were previously described.24 The lentivirus vector pLKOneo-FLAG-PML.I, which expresses FLAG-tagged PML1 from a cytomegalovirus (CMV) promoter, was also generously provided by Dr Everett. The patient mutations were introduced into pLNGY-PML.I using the QuickChange mutagenesis kit (Stratagene). To obtain lentivirus vectors expressing wild-type (WT) and mutated PML/RARA, cDNAs containing these transgenes were placed into pLenti-PGK-Neo-DEST (Addgene) using Gateway cloning technology (Invitrogen). PGK-H2BmCherry (Addgene), which expresses mCherry-tagged histone H2B, was previously described.25
To produce lentivirus particles, 293T cells were cotransfected with one of the lentivirus constructs and the helper plasmids pVSV-G and pCMV-DR8.91 at a 2:1:1 ratio using the FuGENE6 transfection reagent (Roche). After infection with viruses containing PML or histone H2B-expressing vectors, HaCaT cells with stable transgene expression were grown in selection medium containing 1 mg/mL geneticin (Invitrogen) for 7 days, and then allowed to grow for at least 5 additional days without selection before performing an experiment. After infection with viruses containing the PML/RARA-expressing vectors, HaCaT cells were grown in selection medium containing 1 μg/mL puromycin for 2 weeks. In this case only a few cells survived the lentivirus transduction, possibly because of growth inhibition and toxicity caused by high levels of PML/RARA expression. Colonies that formed were allowed to grow for 4 weeks in the absence of puromycin before they were pooled.
Results
ATO stimulates CyPN accumulation
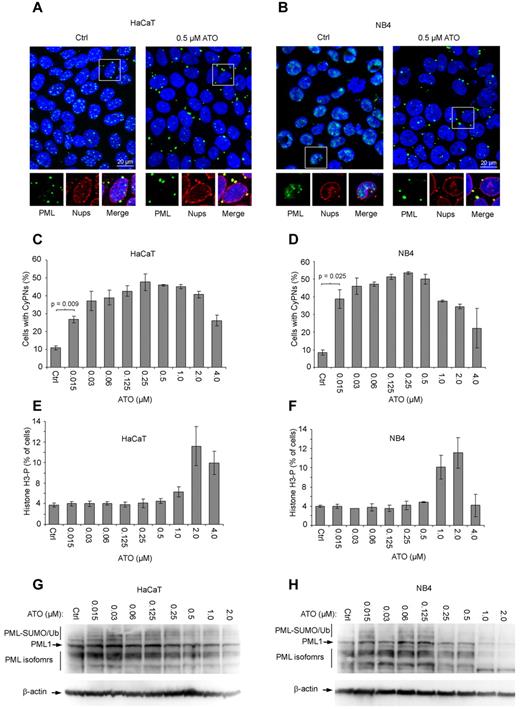
CyPNs are cytoplasmic compartments that form in G1 phase of dividing cells and are defined by their ability to sequester PML and nucleoporins.21 To analyze the behavior of these structures in the presence of ATO, the immortalized keratinocyte cell line HaCaT, as well as the APL cell line NB4 (2 cell lines with high proliferative capacity), were treated with different concentrations of this agent for 24 hours and subsequently analyzed by IF microscopy using antibodies against PML and nucleoporins. For both cell lines we observed a significant increase in the number of cells that contained CyPNs in ATO-treated compared with untreated samples (Figure 1A-B). Maximal stimulation was observed between 0.1 and 1.0μM (Figure 1C-D), a concentration range that roughly equals that observed in serum of APL patients receiving ATO-based therapy.26,–28 Concomitant with increased levels of cytoplasmic PML, we noted a decrease in the number and intensity of nuclear PML bodies (Figure 1A-B).
ATO stimulates formation of CyPNs. (A-B) IF of HaCaT and NB4 cells treated for 24 hours with or without 0.5μM ATO. Antibodies against PML (green) and nucleoporins (red; inset) were used. DAPI is shown in blue. Scale bar, 20 μm. (C-D) Quantification of CyPNs in HaCaT and NB4 cells treated with indicated ATO concentrations for 24 hours. For each sample more than 200 cells were analyzed. Data represent average of 2 independent experiments ± SD. (E-F) Determination of percentage cells in mitosis. HaCaT (E) and NB4 (F) cells treated with the indicated concentrations of ATO for 24 hours were analyzed by flow cytometry using phospho-histone H3 as a marker. Data represent average of 2 independent experiments ± SD. (G-H) Analysis of total PML expression levels by Western blotting. Proteins were extracted from HaCaT and NB4 cells treated for 24 hours with indicated ATO concentrations using a urea-based extraction buffer.
ATO stimulates formation of CyPNs. (A-B) IF of HaCaT and NB4 cells treated for 24 hours with or without 0.5μM ATO. Antibodies against PML (green) and nucleoporins (red; inset) were used. DAPI is shown in blue. Scale bar, 20 μm. (C-D) Quantification of CyPNs in HaCaT and NB4 cells treated with indicated ATO concentrations for 24 hours. For each sample more than 200 cells were analyzed. Data represent average of 2 independent experiments ± SD. (E-F) Determination of percentage cells in mitosis. HaCaT (E) and NB4 (F) cells treated with the indicated concentrations of ATO for 24 hours were analyzed by flow cytometry using phospho-histone H3 as a marker. Data represent average of 2 independent experiments ± SD. (G-H) Analysis of total PML expression levels by Western blotting. Proteins were extracted from HaCaT and NB4 cells treated for 24 hours with indicated ATO concentrations using a urea-based extraction buffer.
ATO was previously shown to cause oxidative stress in NB4 cells leading to arrest in mitosis at 1.0μM or higher concentrations.29 Accordingly, quantitation of mitotic HaCaT and NB4 cells by flow cytometry, using phospho-histone H3 as a marker, demonstrated cell-cycle arrest in mitosis at 1.0μM or higher levels of ATO (Figure 1E-F; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Interestingly, inhibition of cell-cycle progression correlated with decreased CyPN accumulation (Figure 1C-F). Thus, CyPN formation is preferentially stimulated by low ATO concentrations that do not cause detectable cell-cycle defects. Indeed, a significant increase in CyPN accumulation compared with control cells was observed even at 0.015μM ATO, the lowest concentration tested in these experiments (Figure 1C-D). We also detected ATO-induced CyPN accumulation in primary human CD34+ hematopoietic cells (and other cell lines), showing that the observed effect of ATO on PML bodies is not associated with the transformed phenotypes of HaCaT and NB4 cells (supplemental Figure 2).
Finally, we determined the total PML levels in HaCaT and NB4 cells by Western blotting after treatment with different ATO concentrations. Notably, these experiments were done using a urea-based extraction buffer to maximize solubilization of protein aggregates.11 In agreement with previous studies we observed reduced PML expression levels on treatment of cells with 0.25μM or higher concentrations of this drug.8,9,30 However, PML levels were generally observed to be stable or modestly reduced at concentrations ranging from 0.015 to 0.25μM (Figure 1G-H). Notably, ATO-induced degradation was observed to be less effective in HaCaT compared with NB4 cells. This could be because of a less active RNF4-dependent degradation pathway or a lower basal oxidation level in the keratinocyte compared with the APL cell line.
The low concentration of ATO sufficient to induce increased CyPN accumulation suggested that this activity is not caused by increased levels of reactive oxygen species (ROS), toxic derivatives of oxygen that are known to increase in arsenic-treated cells (especially at higher doses).31 In agreement with this we did not observe increased CyPN accumulation in cells treated with other ROS stimulating reagents such as H2O2 (supplemental Figure 3) or doxorubicin (data not shown). Conversely incubation in the presence of the antioxidant N-acetyl cysteine (NAC) did not lead to a detectable decrease in ATO-induced CyPN formation (supplemental Figure 3).
ATO blocks CyPN disintegration and PML body regeneration after cell division
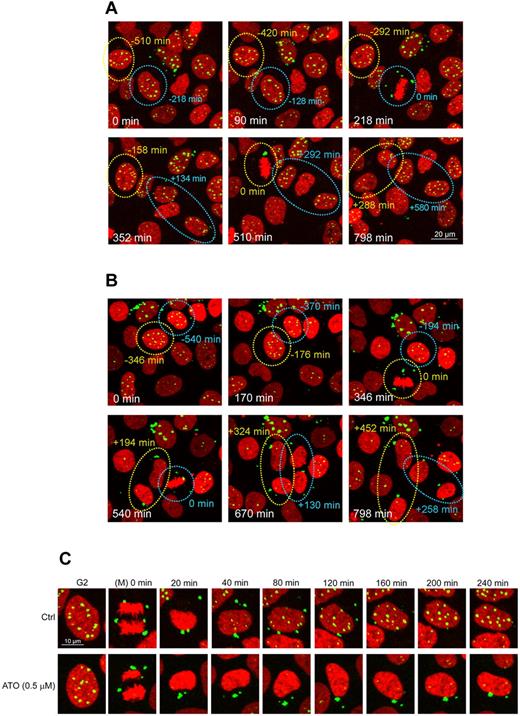
To better understand the mechanism by which ATO stabilizes CyPNs in the cytoplasm, we analyzed the behavior of PML bodies in the presence and absence of this agent in living cells. To achieve this, we stably transduced HaCaT cells with a lentivirus-based vector expressing an enhanced yellow fluorescent protein (EYFP)–tagged version of PML1, one of the largest and most abundantly expressed of the PML isoforms.32 Notably, the lentivirus-based plasmid construct used expressed EYFP-PML1 from a weak cytomegalovirus gD promoter to achieve close to endogenous expression levels.24 In addition, we also introduced a second lentivirus vector into these cells, which expressed mCherry-histone H2B, to visualize chromatin. Confocal microscopy time series of living cells were then generated by image acquisitions at 2 minute intervals for up to 20 hours, a time scale which allowed for analysis of multiple cells as they traversed through mitosis.
In untreated control cells (Figure 2A-C; supplemental Video 1), we observed release of PML bodies from chromatin followed by their aggregation into MAPPs at early stages of mitosis. At the end of mitosis, these structures were consistently excluded from the progeny daughter nuclei, and on entry into G1, new PML bodies were generated de novo within the nuclei, whereas CyPNs gradually disintegrated and disappeared within 3 to 4 hours after mitotic exit (Figure 2A-C; supplemental Video 1).
ATO stabilizes CyPNs and inhibits formation of nuclear PML bodies after progression through mitosis. Live imaging of mCherry-Histone H2B and EYFP-PML1 expressed in HaCaT cells was performed using 2 minutes intervals between image acquisitions for a total imaging period of approximately 20 hours. (A-B) Selected images of control treated cells (A) and cells treated with 0.5μM ATO (B). Circles highlight mitotic cell divisions, and the associated numbers indicate time in minutes before (−) and after (+) metaphase. Scale bar, 20 μm. (C) Illustration of single daughter cells undergoing PML body recirculation after cell division in the absence or in the presence of 0.5μM ATO. The first panels show the cells immediately before mitosis. Images have been synchronized at time 0 in anaphase. Only 1 of the resulting daughter cells is shown. Each of the images represents projections of 3 z-scans. Scale bar, 10 μm. The full movies can be viewed in supplemental Videos 1 and 2.
ATO stabilizes CyPNs and inhibits formation of nuclear PML bodies after progression through mitosis. Live imaging of mCherry-Histone H2B and EYFP-PML1 expressed in HaCaT cells was performed using 2 minutes intervals between image acquisitions for a total imaging period of approximately 20 hours. (A-B) Selected images of control treated cells (A) and cells treated with 0.5μM ATO (B). Circles highlight mitotic cell divisions, and the associated numbers indicate time in minutes before (−) and after (+) metaphase. Scale bar, 20 μm. (C) Illustration of single daughter cells undergoing PML body recirculation after cell division in the absence or in the presence of 0.5μM ATO. The first panels show the cells immediately before mitosis. Images have been synchronized at time 0 in anaphase. Only 1 of the resulting daughter cells is shown. Each of the images represents projections of 3 z-scans. Scale bar, 10 μm. The full movies can be viewed in supplemental Videos 1 and 2.
For cells grown in the presence of 0.5μM ATO (Figure 2B-C; supplemental Video 2), we did not observe a reduction in intensity or number of PML bodies before entry into the first mitosis after drug exposure. After completion of mitosis, however, the resulting CyPNs were found to be more stable within the cytoplasm compared with CyPNs in untreated cells. In fact, after these structures had formed, they consistently persisted without detectable reduction in size or intensity throughout the entire imaging period (Figure 2B; supplemental Video 2). In addition, generation of progeny nuclear PML bodies were observed to be delayed in ATO-treated cells compared with untreated control cells (Figure 2B-C; supplemental Videos 1-2). This experiment shows that the increase in cells containing CyPNs, as well as the depletion of PML bodies from the nucleus which occur on treatment with ATO, mainly is caused by CyPN stabilization and inhibition of nuclear PML body regeneration after mitotic cell division.
ATO-induced PML SUMOylation is counteracted by cell division
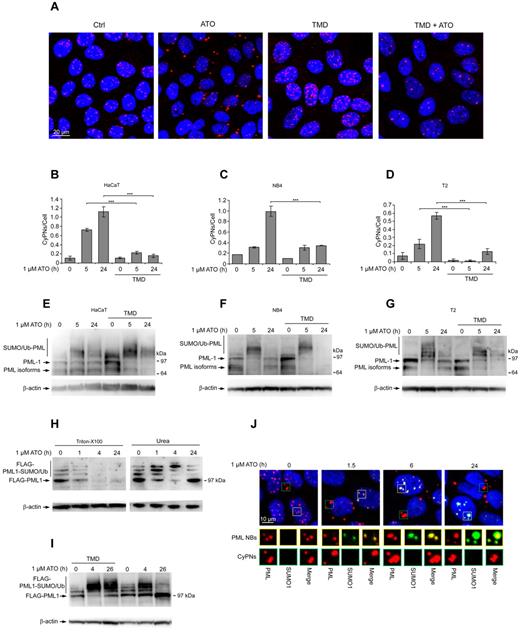
The experiments described in Figure 1 suggest that mitosis facilitates ATO-stimulated CyPN formation. To verify this, we assessed the ability of this drug to stimulate cytoplasmic PML accumulation in HaCaT cells treated with thymidine (TMD), a nucleoside analog that blocks cell-cycle progression in G1/S-phase. We found that the number of CyPNs per cell was strongly reduced on treatment for 12 hours with 1mM TMD before addition of 1.0μM ATO and continued incubation for 5 or 24 hours (Figure 3A-B). Similar results were also obtained using NB4 or T2 cells (Figure 3C-D, supplemental Figure 4). Thus, CyPN accumulation in response to ATO is strongly reduced in arrested versus cycling cells. It should be emphasized in this context that a weak but significant increase of these cytoplasmic compartments could also be seen in TMD arrested cells, indicating that ATO-induced de novo CyPN formation in the absence of cell division may occur, albeit at a significantly reduced rate compared with that observed in cycling cells.
ATO-induced SUMOylation is counteracted by cell division. (A) IF showing HaCaT cells treated with the indicated combinations of 1.0μM ATO and 1.0mM TMD. Cells were pretreated for 12 hours with TMD before administration of ATO. Incubation was then continued with both drugs present for an additional 24 hours. DAPI (blue) and PML (red) is shown. Scale bar, 20 μm. (B-D) Quantification of CyPNs in HaCaT (B), NB4 (C), and T2 (D) cells after treatment with 1.0μM ATO and/or 1.0mM TMD as aforementioned in panel A. More than 200 cells were analyzed per sample. Data represent average of 2 independent experiments ± SD (***P < .001). (E-G) Western blot showing PML expression in HaCaT (E), NB4 (F), and T2 (G) cells treated with 1.0μM ATO and/or 1.0mM TMD as aforementioned in panel A. β-actin was used as loading control. (H) HaCaT cells stably expressing FLAG-PML1 were treated with 1.0μM ATO and harvested for analysis by Western blotting at indicated time points using urea or Triton X-100–based extraction buffers. β-actin was used as loading control. (I) HaCaT cells expressing FLAG-PML1 were treated with 1.0μM ATO and/or 1.0mM TMD as in panel A, and subsequently harvested for analysis by Western blotting at the indicated time points. β-actin was used as loading control. (J) IF analysis of HaCaT cells treated with 1.0μM ATO as indicated using antibodies against PML (red) and SUMO1 (green). Yellow rectangles highlight nuclear PML bodies (PML/SUMO1 colocalization), whereas green rectangles highlight CyPNs (absence of PML/SUMO1 colocalization). DAPI staining is shown in blue. Scale bar, 10 μm.
ATO-induced SUMOylation is counteracted by cell division. (A) IF showing HaCaT cells treated with the indicated combinations of 1.0μM ATO and 1.0mM TMD. Cells were pretreated for 12 hours with TMD before administration of ATO. Incubation was then continued with both drugs present for an additional 24 hours. DAPI (blue) and PML (red) is shown. Scale bar, 20 μm. (B-D) Quantification of CyPNs in HaCaT (B), NB4 (C), and T2 (D) cells after treatment with 1.0μM ATO and/or 1.0mM TMD as aforementioned in panel A. More than 200 cells were analyzed per sample. Data represent average of 2 independent experiments ± SD (***P < .001). (E-G) Western blot showing PML expression in HaCaT (E), NB4 (F), and T2 (G) cells treated with 1.0μM ATO and/or 1.0mM TMD as aforementioned in panel A. β-actin was used as loading control. (H) HaCaT cells stably expressing FLAG-PML1 were treated with 1.0μM ATO and harvested for analysis by Western blotting at indicated time points using urea or Triton X-100–based extraction buffers. β-actin was used as loading control. (I) HaCaT cells expressing FLAG-PML1 were treated with 1.0μM ATO and/or 1.0mM TMD as in panel A, and subsequently harvested for analysis by Western blotting at the indicated time points. β-actin was used as loading control. (J) IF analysis of HaCaT cells treated with 1.0μM ATO as indicated using antibodies against PML (red) and SUMO1 (green). Yellow rectangles highlight nuclear PML bodies (PML/SUMO1 colocalization), whereas green rectangles highlight CyPNs (absence of PML/SUMO1 colocalization). DAPI staining is shown in blue. Scale bar, 10 μm.
The ATO and/or TMD-treated cells were also analyzed by Western blotting to determine the level of PML expression. As shown in Figure 3E through G, an appearance of higher migrating PML species was observed after 5 hours of ATO treatment, which is in agreement with previous studies demonstrating increased modification of PML by SUMO and/or Ub in the presence of ATO. Furthermore, we observed a decrease in PML modification concomitant with reappearance of unmodified PML species after 24 hours of ATO treatment (Figure 3E-G). This result may indicate that the covalent PML alterations become reversed on prolonged treatment with this drug. Alternatively, the nonconjugated PML species appearing at later time points may represent neosynthesized PML that has not yet been SUMOylated. When cells were pretreated for 12 hours with TMD, we observed an even more effective modification of PML by SUMO and/or Ub that reverted back to unmodified PML species at a considerably lower frequency compared with cells that had not been treated with TMD (Figure 3E-G). These experiments support previous observations showing that basal PML SUMOylation in cultured human cells is reduced on entry into mitosis,18,–20 and suggest that SUMOylation and degradation in response to arsenic may be counteracted by cell division. Interestingly, the presence of TMD appeared to cause a more effective PML depletion in some of our experiments (eg, NB4 cells in Figure 3F) suggesting that a block in cell-cycle progression leads to more effective PML degradation, possibly because of prolonged exposure of PML and PML bodies to the nuclear environment under these conditions.
To more precisely delineate the fate of SUMO/Ub-modified PML in ATO-treated cycling or noncycling cells, we used a HaCaT-derived cell line that stably expressed FLAG-PML1 from a lentivirus-based vector. Although the FLAG-PML1 protein failed to become significantly degraded in response to ATO in these experiments (possibly because of distortion of normal PML functions because of overexpression or the presence of an N-terminal tag), we observed efficient modification of this protein by SUMO/Ub conjugation (Figure 3H-I). Interestingly, a robust increase of high molecular weight FLAG-PML1 species was readily observed at 1 and 4 hours of ATO treatment, but at 24 hours we mainly detected nonconjugated PML species (Figure 3H). In contrast to untreated cells, the unmodified FLAG-PML1 species detected at 24 hours of ATO treatment were not extracted by a Triton X-100–based procedure, demonstrating biochemical alterations of PML into more insoluble and aggregated forms (Figure 3H). Furthermore, the transformation of SUMO/Ub-conjugated FLAG-PML1 into nonconjugated species was prevented in cells treated with TMD, suggesting that the reduction in SUMO-PML conjugates is promoted by cell-cycle progression (Figure 3I).
Lastly, we analyzed the subcellular localization of SUMO1 and PML in untreated and ATO-treated HaCaT cells by IF. This experiment revealed an increase in SUMO-positive PML bodies in the nucleus on treatment with ATO (Figure 3J). However, CyPNs consistently stained negative for SUMO1 both before and after ATO treatment (Figure 3J). Similar results were also obtained using an antibody that reacts with SUMO2 and SUMO3 (data not shown). In addition, inspections of mitotic cells revealed a considerably lower level of SUMO1 within MAPPs compared with the nuclear PML bodies in interphase cells. These results strongly support the notion that ATO-stimulated SUMOylation of PML primarily occurs in the nucleus. Furthermore, in the absence of effective RNF4-dependent degradation, these conjugates may be removed on progression through mitosis leading to sequestration of non-SUMOylated PML within CyPNs in the cytoplasm.
ATO-induced CyPN formation does not require SUMOylation and is prevented by PML mutations identified in therapy-resistant APL patients
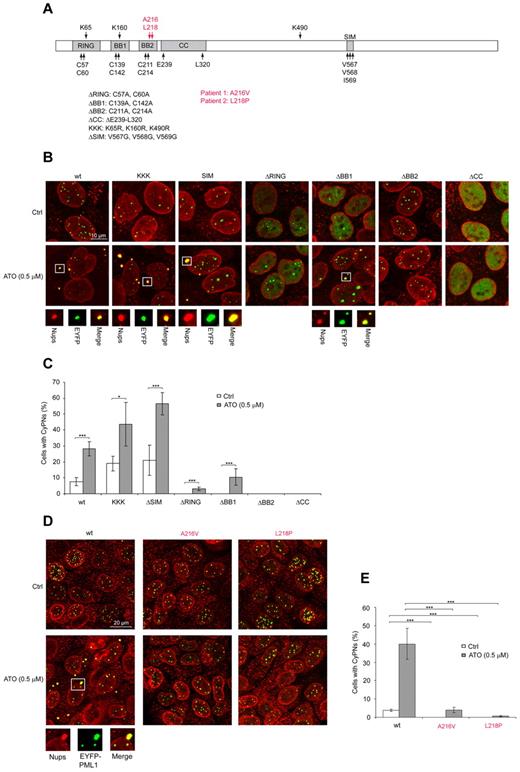
The PML protein is organized into a tripartite (TRIM) domain at its N-terminus, which comprises the 3 zinc-binding domains RING, B-box 1 (BB1), and B-box 2 (BB2) in addition to a coiled coil (CC).33 Furthermore, this protein harbors a SUMO interaction motif (SIM) at its central region and 3 different lysine residues (K65, K160, and K490) that can be modified by SUMOylation (Figure 4A).34 To determine the requirement for SUMOylation and the different PML substructures in ATO-induced CyPN accumulation, we used a previously described depletion-reconstitution approach.24 For these experiments PML bodies were first depleted from HaCaT cells using stable expression of shRNAs against PML, and PML expression and PML bodies were subsequently regenerated by a second lentivirus-based vector expressing shRNA-resistant EYFP-PML1. Importantly, these constructs were designed to produce close to endogenous levels of PML from a weak promoter.24
SUMOylation is dispensable whereas the BB2 motif and amino acids mutated in ATO-resistant APL patients are required for effective CyPN accumulation. (A) Schematic overview of PML structural organization and mutations used in this study. Mutations identified in PML/RARA transcripts isolated from ATO-resistant APL patients are shown in red. (B) IF showing nucleoporins (red) together with WT or mutated EYFP-PML1 (green) stably expressed in HaCaT/shPML cells. Representative images of untreated cells and cells treated with 0.5μM ATO are shown. Images are projections of 5 to 6 confocal z-scans. Scale bar, 10 μm. (C) Quantification of CyPNs regenerated by WT or mutated EYFP-PML1 in HaCaT/shPML cells incubated in the presence or absence of 0.5μM ATO for 24 hours. For each sample more than 80 cells were analyzed. Data represent average of 3 independent experiments ± SD (*P < .05, ***P < .001). (D) IF showing nucleoporins (red) together with WT EYFP-PML1 or EYFP-PML1 containing the patient derived mutations A216V and L218P (green) stably expressed in HaCaT/shPML cells. Representative images showing untreated cells or cells treated with 0.5μM ATO are shown. Images display projections of 5 to 6 confocal z-scans. Scale bar, 20 μm. (E) Quantification of CyPNs regenerated by WT or mutated EYFP-PML1 in HaCaT/shPML cells incubated in the presence or absence of 0.5μM ATO for 24 hours. For each sample more than 100 cells were analyzed. Data represent average of 3 independent experiments ± SD (***P < .001).
SUMOylation is dispensable whereas the BB2 motif and amino acids mutated in ATO-resistant APL patients are required for effective CyPN accumulation. (A) Schematic overview of PML structural organization and mutations used in this study. Mutations identified in PML/RARA transcripts isolated from ATO-resistant APL patients are shown in red. (B) IF showing nucleoporins (red) together with WT or mutated EYFP-PML1 (green) stably expressed in HaCaT/shPML cells. Representative images of untreated cells and cells treated with 0.5μM ATO are shown. Images are projections of 5 to 6 confocal z-scans. Scale bar, 10 μm. (C) Quantification of CyPNs regenerated by WT or mutated EYFP-PML1 in HaCaT/shPML cells incubated in the presence or absence of 0.5μM ATO for 24 hours. For each sample more than 80 cells were analyzed. Data represent average of 3 independent experiments ± SD (*P < .05, ***P < .001). (D) IF showing nucleoporins (red) together with WT EYFP-PML1 or EYFP-PML1 containing the patient derived mutations A216V and L218P (green) stably expressed in HaCaT/shPML cells. Representative images showing untreated cells or cells treated with 0.5μM ATO are shown. Images display projections of 5 to 6 confocal z-scans. Scale bar, 20 μm. (E) Quantification of CyPNs regenerated by WT or mutated EYFP-PML1 in HaCaT/shPML cells incubated in the presence or absence of 0.5μM ATO for 24 hours. For each sample more than 100 cells were analyzed. Data represent average of 3 independent experiments ± SD (***P < .001).
Reintroduction of EYFP-PML1.wt in HaCaT/shPML cells resulted in effective regeneration of PML bodies that redistributed to the cytoplasm as CyPNs on treatment with ATO (Figure 4B-C; supplemental Figure 5). Interestingly, the HaCaT/shPML cell line, which showed strongly reduced PML expression, completely failed to support accumulation of nucleoporins into cytoplasmic foci on ATO stimulation, an observation that demonstrates an important role of PML in CyPN integrity (supplemental Figure 5).
Expression of EYFP-PML1.KKK, which contained lysine-to-arginine substitutions at the 3 known PML SUMOylation sites, or EYFP-PML1.ΔSIM, which contained a nonfunctional SIM, were observed to support formation of CyPNs and increased stability of these structures on ATO treatment (Figure 4B-C). Thus, modification of PML by SUMOylation or the ability of this protein to interact with SUMO does not appear to play a critical role in CyPN regulation.
EYFP-PML1.ΔRING and EYFP-PML1.ΔBB1, which contained mutations in zinc-binding cysteines within the RING and BB1 motifs, respectively, produced a more diffuse nuclear distribution compared with EYFP-PML1.wt and did not support CyPN formation in the absence of ATO (Figure 4B-C). However, in the presence of 0.5μM ATO, the nuclear foci became slightly more intense in cells expressing EYFP-PML1.ΔRING, whereas EYFP-PML1.ΔBB1 responded by accumulating into more prominent nuclear PML bodies as well as becoming more effectively assembled into CyPNs (Figure 4B-C). Expression of EYFP-PML1.ΔBB2, which contained mutations in critical zinc-binding cysteine residues within the BB2 domain, retained the ability to form seemingly normal PML bodies in the nucleus, but was completely incapable of supporting formation of CyPNs, both in the absence and in the presence of ATO (Figure 4B-C). Finally, expression of EYFP-PML1.ΔCC, which contained a deleted CC, exhibited a completely diffuse staining pattern both in the absence and in the presence of ATO (Figure 4B-C). Thus, of the PML mutations analyzed, ΔBB2 was the only one that retained close to WT abilities of nucleating formation of nuclear bodies, while being completely defective in generating CyPNs. In addition, the observed irresponsiveness of PML1.ΔBB2 to ATO is in agreement with a recent study implicating this motif as an interaction site for ATO.6
Recently, 2 APL patients with clinical resistance to ATO-mediated therapy were found to harbor single amino acid substitutions that clustered proximal to the zinc-binding cysteines within the BB2 motif of PML/RARA.35 To assess whether these mutations affected CyPN assembly, we introduced them into the EYFP-PML1–expressing lentivirus-based plasmid construct and analyzed the localization of the expressed proteins in the PML-depleted HaCaT/shPML cell line. Similar to EYFP-PML1.ΔBB2, both EYFP-PML1.A216V and EYFP-PML1.L218P (which expressed EYFP-PML1 with amino acid substitutions derived from ATO-resistant patient 1 and 2, respectively) supported formation of seemingly normal PML bodies, but were severely impaired in their ability to support CyPN formation (Figure 4D-E). In the presence of ATO, we observed a few cells that contained small but detectable CyPNs for both of the patient-derived mutants, but this effect was markedly lower compared with the WT PML protein (Figure 4D-E). These results support the notion that the PML BB2 motif has a critical role in CyPN formation and stabilization in addition to its previously characterized role in promoting SUMOylation and degradation.6
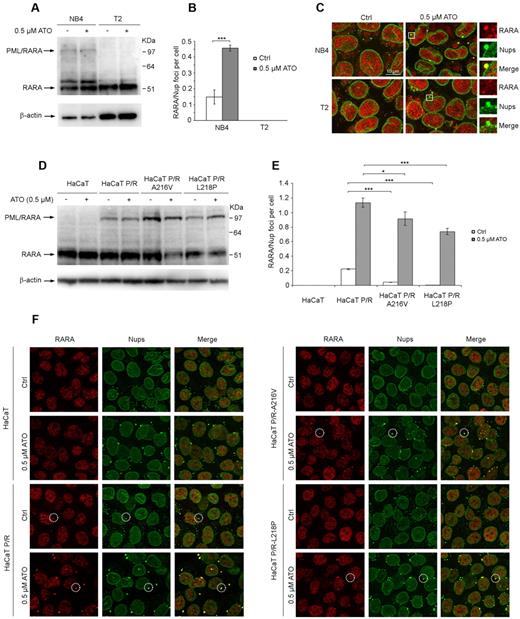
ATO promotes cytoplasmic sequestration of PML/RARA within CyPNs
In APL cells, PML bodies are reorganized into a more scattered nuclear pattern of smaller dots, referred to as microspeckles, because of expression of the PML/RARA oncoprotein.36,–38 However, PML/RARA does not appear to alter formation of MAPPs and CyPNs, as this oncoprotein was shown to accumulate within both these types of structures.21 To determine whether ATO promotes stabilization of PML/RARA within CyPNs, we first analyzed NB4 cells and the PML/RARA-negative lymphoma cell line T2. Western blot analysis of urea-extracted samples using RARA-specific antibodies revealed a higher migrating protein species with the size expected for PML/RARA in NB4 cells but not in T2 cells (Figure 5A). Under the condition used, we did not observe a dramatic reduction in the intensity of the PML/RARA-specific band after incubation of cells in medium containing 0.5μM ATO for 24 hours (Figure 5A). IF analysis revealed RARA/nucleoporin-positive foci in the cytoplasm of NB4 cells (but not in T2 cells) that increased in size and number on treatment with ATO (Figure 5B-C). To verify the specificity of the anti-RARA antibody used in IF experiments we demonstrated reduced antibody reactivity in U2OS cells treated with a RARA-specific siRNAs compared with untreated cells (supplemental Figure 6).
ATO stimulates increased accumulation of PML/RARA within CyPNs. (A) Western blot showing total RARA and PML/RARA expression in NB4 and T2 cells treated with or without 0.5μM ATO for 24 hours. Blots were probed using an anti-RARA antibody. β-actin was used as loading control. (B) Quantification of RARA-positive CyPNs in NB4 and T2 cells treated with or without 0.5μM ATO for 24 hours. For each sample more than 500 cells were analyzed. Data represent average of 3 independent experiments ± SD (***P < .001). (C) IF showing the localization of RARA (red) and nucleoporins (Nups; green) in NB4 and T2 cells treated with or without 0.5μM ATO for 24 hours. RARA-positive CyPNs are detected in NB4 cells, but not in T2 cells. Images represent projections of 5 to 6 confocal z-scans. Scale bar, 10 μm. (D) Western blot showing stable expression of WT and mutated (A216V and L218P) PML/RARA in HaCaT cells. Cells were treated with or without 0.5μM ATO for 24 hours and subsequently lysed in a urea-based extraction buffer. Blots were probed using an anti-RARA antibody. β-actin was used as loading control. (E) Quantitation of CyPNs in HaCaT cells stably expressing WT or mutated PML/RARA and treated with or without 0.5μM ATO for 24 hours. For each sample more than 100 cells were analyzed. Data represent average of 3 independent experiments ± SD (*P < .05, ***P < .001). (F) IF showing RARA (red) and nucleoporins (Nups; green) in HaCaT and HaCaT cells expressing WT or mutated PML/RARA (P/R). Selected foci containing Nups and RARA are highlighted. Scale bar, 20 μm.
ATO stimulates increased accumulation of PML/RARA within CyPNs. (A) Western blot showing total RARA and PML/RARA expression in NB4 and T2 cells treated with or without 0.5μM ATO for 24 hours. Blots were probed using an anti-RARA antibody. β-actin was used as loading control. (B) Quantification of RARA-positive CyPNs in NB4 and T2 cells treated with or without 0.5μM ATO for 24 hours. For each sample more than 500 cells were analyzed. Data represent average of 3 independent experiments ± SD (***P < .001). (C) IF showing the localization of RARA (red) and nucleoporins (Nups; green) in NB4 and T2 cells treated with or without 0.5μM ATO for 24 hours. RARA-positive CyPNs are detected in NB4 cells, but not in T2 cells. Images represent projections of 5 to 6 confocal z-scans. Scale bar, 10 μm. (D) Western blot showing stable expression of WT and mutated (A216V and L218P) PML/RARA in HaCaT cells. Cells were treated with or without 0.5μM ATO for 24 hours and subsequently lysed in a urea-based extraction buffer. Blots were probed using an anti-RARA antibody. β-actin was used as loading control. (E) Quantitation of CyPNs in HaCaT cells stably expressing WT or mutated PML/RARA and treated with or without 0.5μM ATO for 24 hours. For each sample more than 100 cells were analyzed. Data represent average of 3 independent experiments ± SD (*P < .05, ***P < .001). (F) IF showing RARA (red) and nucleoporins (Nups; green) in HaCaT and HaCaT cells expressing WT or mutated PML/RARA (P/R). Selected foci containing Nups and RARA are highlighted. Scale bar, 20 μm.
We also analyzed HaCaT cells stably expressing WT PML/RARA or PML/RARA containing the A216V or L218P amino acid substitutions associated with clinical arsenic resistance. To achieve this we transduced cells with lentiviral vectors expressing WT or mutated versions of the oncoprotein from a phosphoglycerate kinase (PGK) promoter. Interestingly, similar to the NB4 cells, the stable cell lines that we obtained exhibited relatively low PML/RARA versus RARA expression (Figure 5D), possibly because of a selective disadvantage of HaCaT cells with high PML/RARA levels (see “Lentivirus production and preparation of cell lines with stable transgene expression” for details). Consistent with the observations in NB4 cells, we did not detect a dramatic reduction in PML/RARA levels after incubation in the presence of 0.5μM ATO (Figure 5D). Furthermore, IF-labeling of PML revealed characteristic PML/RARA-induced microspeckled PML distribution in more than 70% of the cells, suggesting that most cells expressed the oncoprotein (supplemental Figure 7).
Using the anti-RARA antibody as a probe, we detected significantly higher basal levels of RARA/nucleoporin-positive foci in cells expressing the WT PML/RARA versus cells expressing PML/RARA with A216V or L218P amino acid substitutions (Figure 5E-F). On treatment with ATO we readily detected a strong increase in the number of RARA-positive CyPNs per cell both for WT and mutated PML/RARA. However, the HaCaT cells expressing WT PML/RARA were observed to generate a stronger RARA-specific fluorescence signal within CyPNs compared with HaCaT cells expressing the mutated proteins, and a significant lower number of RARA-positive CyPNs per cell were detected (Figure 5E-F; supplemental Figure 7). Thus, PML/RARA containing the patient-derived mutations appears to have reduced affinity for CyPNs compared with WT PML/RARA. Importantly, RARA-positive CyPNs were not detected in normal nontransduced HaCaT cells before and after ATO treatment showing that the fluorescent signal represented exogenously expressed PML/RARA and that the endogenous RARA protein expressed in these cells is not recruited to these cytoplasmic compartments (Figure 5E-F).
Discussion
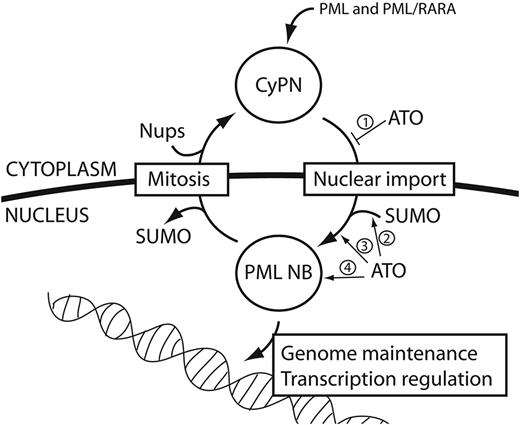
Previous studies have shown that ATO interacts directly with cysteine-rich motifs within the PML protein to promote its SUMOylation and subsequent ubiquitination and proteasomal degradation.6,,–9 Because this molecular pathway also affects the APL-associated PML/RARA oncoprotein, it is probable that it plays an important role in ATO-mediated clinical remission of this disease. The data presented in this study reveals an additional mechanism that does not involve SUMOylation, and instead of activating degradation, leads to redistribution of PML and PML/RARA from the nucleus to the cytoplasm (Figure 6). Several observations suggest that this pathway may also be implicated in the cure of APL. Firstly, the PML/RARA oncoprotein is thought to promote malignant transformation and tumor progression predominantly through its activity as a transcription repressor.12 Because this function requires direct contact between the protein and the genome, it is expected to be lost on sequestration of PML/RARA in the cytoplasm. Secondly, accumulation of CyPNs is observed predominantly at relatively low ATO concentrations and the activity peaks between 0.1 to 1.0μM, the same range of concentrations that is normally detected in the plasma of APL patients receiving ATO-based therapy.26,–28 Thirdly, the ability of PML to support CyPN formation is strictly dependent on cysteine residues within the BB2 motif of the PML protein, the same amino acids that recently have been shown to be important for ATO-PML interactions as well as the ATO-mediated cure of APL in mouse.6 Finally, single amino acid substitutions within the PML protein, which recently were identified in 2 APL patients with clinical resistance to ATO,35 severely reduced the ability of PML and PML/RARA to support CyPN formation both in the presence and in the absence of ATO. Because these mutations are also expected to cause reduced PML/RARA SUMOylation,6 they may have a double effect in causing arsenic resistance through both inhibiting RNF4-mediated degradation as well as postmitotic PML body recycling. However, an important point to consider is that the various PML bodies, including PML NBs, MAPPs, and CyPNs, may only contain a fraction of total PML proteins at any given time, and it is not clear from this study to what extent ATO-mediated inhibition of PML body recycling also affects “free” PML and PML/RARA that do not associate with these structures.
Effects of ATO on CyPNs and PML NBs. PML bodies are exported to the cytoplasm and transformed into CyPNs through mitotic cell division. After mitosis, CyPNs contribute to formation of new PML bodies through nuclear import of PML body components. SUMO-conjugates are lost from PML and PML NBs during progression through mitosis, whereas nucleoporins (Nups) are complexed to CyPNs at exit from mitosis. The model depicts 4 different consequences of direct PML and PML/RARA targeting by ATO: (1) inhibition of CyPN dissociation and nuclear import of PML body components after mitosis; (2) stimulation of PML and PML/RARA modifications by SUMOylation and disulphide formation; (3) stimulation of PML and PML/RARA aggregation into PML NBs; and (4) stimulation of RNF4-dependent degradation of PML and PML/RARA by the proteasome within PML NBs. Not shown in this model is the disruption of PML NBs that occur on expression of PML/RARA in APL cells.
Effects of ATO on CyPNs and PML NBs. PML bodies are exported to the cytoplasm and transformed into CyPNs through mitotic cell division. After mitosis, CyPNs contribute to formation of new PML bodies through nuclear import of PML body components. SUMO-conjugates are lost from PML and PML NBs during progression through mitosis, whereas nucleoporins (Nups) are complexed to CyPNs at exit from mitosis. The model depicts 4 different consequences of direct PML and PML/RARA targeting by ATO: (1) inhibition of CyPN dissociation and nuclear import of PML body components after mitosis; (2) stimulation of PML and PML/RARA modifications by SUMOylation and disulphide formation; (3) stimulation of PML and PML/RARA aggregation into PML NBs; and (4) stimulation of RNF4-dependent degradation of PML and PML/RARA by the proteasome within PML NBs. Not shown in this model is the disruption of PML NBs that occur on expression of PML/RARA in APL cells.
As authors of previous studies have reported extensive modification of PML by SUMOylation and/or ubiquitination on ATO treatment,8,9,30 we observed reduced levels of these modifications as cells progressed through mitosis. This finding is consistent with previous publications demonstrating loss of basal PML SUMOylation at entry into mitosis,19,20 and suggests that such protein modifications are counteracted by cell division and subsequent generation of CyPNs. This implies that the fate of PML in the presence of ATO may be influenced by the cells proliferative capacity. For example, SUMOylation and degradation may be expected to predominate in slowly dividing or quiescent cells, whereas effects caused by cytoplasmic sequestration within CyPNs may dominate in more actively proliferating cells. In this respect ATO-induced CyPN-accumulation may be more pronounced in rapidly proliferating cells in culture (such as the HaCaT and NB4 cells used in this study) compared with APL cells in the bone marrow of patients receiving ATO-based therapy. However, any cell that passes through mitosis in the presence of this drug (irrespective of whether this happens in vivo in an APL patient or in vitro in cell culture) will be expected to have reduced capacity of PML and PML/RARA nuclear import in G1 phase. Given the importance of this cell cycle stage for events, such as transcription complex assembly and cell fate determination, the arrest of PML and PML/RARA within CyPNs after progression through mitosis in the presence of ATO may, in part, explain the role of this drug in promoting APL cell differentiation.
While PML bodies within the nucleus represent the most extensively studied compartment formed by PML, little is known about the function of MAPPs and CyPNs that are produced in mitotic and newly formed G1 cells, respectively. Besides the ability of these cytoplasmic compartments to sequester PML and nucleoporins, it was shown that they migrate within the cytoplasm of the cell in a microtubule-dependent manner.21 Interestingly, PML was shown to functionally interact with other microtubule-binding cytoplasmic organelles, such as endosomes, mitochondria, and endoplasmatic reticulum to regulate growth signaling and calcium homeostasis, respectively.39,–41 It will be important to identify the cytoplasmic functions of CyPNs and a potential PML-mediated gain or inhibition of these functions in ATO-treated cells. Notably, low concentrations of arsenic (0.1μM to 0.5μM), which in this study was shown to cause a significant increase in CyPN accumulation, was previously shown to affect cell differentiation as well as calcium homeostasis.42,–44
In summary, we have identified and characterized a highly sensitive and specific response of PML, PML/RARA, and PML bodies to arsenic. In addition to providing important insight into ATO-mediated cure of APL, these findings may also contribute to the development of ATO-based treatment regiments that can be used for cancers other than APL. Finally, because PML is a tumor suppressor, our study may contribute to increased understanding of tumorigenesis related to long-term exposure to arsenic through drinking water contamination.45,46
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Roger D. Everett for generously providing the lentivirus plasmid constructs expressing EYFP-PML1, and Agnete Svendsen for help with flow cytometry. Dr Alex Rowe is acknowledged for help with the making of videos.
This work was supported by the Norwegian Research Council (FRIBIO) and the Norwegian Cancer Society.
Authorship
Contribution: E.L. performed experiments, analyzed data, and drafted parts of the paper; A.G. performed experiments and analyzed data; S.P. performed experiments; Ø.B. provided cell lines and reagents; A.S. supervised and analyzed data; R.B. supervised and analyzed data; M.B. supervised and analyzed data; S.O.B. designed the study, performed experiments, and wrote the paper; and all authors read and approved the final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stig Ove Bøe, Dept of Microbiology, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, NO-0424 Oslo, Norway; e-mail: stig.ove.boe@rr-research.no.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal