Blast crisis (BC) remains the major challenge in the management of chronic myeloid leukemia (CML). It is now generally accepted that BC is the consequence of continued BCR-ABL activity leading to genetic instability, DNA damage, and impaired DNA repair. Most patients with BC carry multiple mutations, and up to 80% show additional chromosomal aberrations in a nonrandom pattern. Treatment with tyrosine kinase inhibitors has improved survival in BC modestly, but most long-term survivors are those who have been transplanted. Patients in BC should be treated with a tyrosine kinase inhibitor according to mutation profile, with or without chemotherapy, with the goal of achieving a second chronic phase and proceeding to allogeneic stem cell transplantation as quickly as possible. Although long-term remissions are rare, allogeneic stem cell transplantation provides the best chance of a cure in BC. Investigational agents are not likely to provide an alternative in the near future. In view of these limited options, prevention of BC by a rigorous and early elimination of BCR-ABL is recommended. Early response indicators should be used to select patients for alternative therapies and early transplantation. Every attempt should be made to reduce or eliminate BCR-ABL consistent with good patient care as far as possible.
Introduction
Blast crisis (BC) is the major remaining challenge in the management of chronic myeloid leukemia (CML). The introduction of an inhibitor targeted at the BCR-ABL tyrosine kinase (imatinib) has fundamentally changed treatment of CML.1 BCR-ABL expression can be reduced by imatinib to very low or nondetectable levels in the majority of patients.2 Median survival in chronic phase (CP) is estimated at a median of 25 to 30 years. Progress to advanced phase CML or BC has been reduced to 1% to 1.5% per year1 compared with more than 20% per year in the pre-imatinib era.3 Prevalence of CML is estimated to increase by a factor of approximately 10 within the next 40 years.4 Once BC has appeared, however, the prognosis of imatinib-treated patients is not much better than that after conventional therapy.5 Median survival after diagnosis of BC currently ranges between 7 and 11 months compared with 3 to 4 months in the pre-imatinib era. Very few long-term survivors after diagnosis of BC have been reported. Most of these represent recipients of transplants during a second CP. The therapeutic dilemma of BC has recently been well summarized.6 More research is needed to fully understand the mechanisms underlying progression to BC. It is distressing that in CML BC a true malignancy evolves under our eyes. The 2 current burning questions in CML are: How can we best manage patients who progress to BC despite appropriate treatment? How can we best prevent BC?
How I define and diagnose BC
First attempts at the definition of BC date back more than forty years.7 The generally used definition, which underlies virtually all current clinical CML trials and the European LeukemiaNet management recommendations, rests on at least 30% blasts in blood or marrow or the demonstration of extramedullary blastic infiltrates.8 The more recent World Health Organization definition proposes a blast count of 20% in analogy to the definition of acute myeloid leukemia (AML).9 Both definitions are not supported by biologic evidence. A new definition would regroup approximately 10% of patients.10 Patients with 20% to 29% blasts have significantly better prognoses than patients with more than 30% blasts. Because most clinicians and trialists would probably use the definition based on their own data and experience, I suggest awaiting the results of clinical and biologic research for a new evidence-based definition of BC.
To diagnose BC, I do complete blood and differential counts and a bone marrow analysis with cytogenetics (Table 1). Cytogenetic evolution is the most consistent predictor of blast transformation. Flow cytometry or cytochemistry is needed to determine the type of BC (myeloid or lymphoid). Molecular genetics with mutation analysis are needed to choose the appropriate tyrosine kinase inhibitor (TKI). Consensus recommendations when to perform mutation analyses have been published on behalf of the European LeukemiaNet.11 A donor search for allogeneic stem cell transplantation (allo-SCT) should be started immediately.
BC diagnostics
| . | Test rationale . |
|---|---|
| Test at diagnosis of BC | |
| CBC with differential and bone marrow | Proportions of blasts, promyelocytes, and basophils? |
| Flow cytometry and/or cytochemistry | Myeloid or lymphoid phenotype? |
| Cytogenetics | Clonal evolution? |
| Molecular genetics | Mutation profile? Choice of TKI |
| Donor search (if applicable) | Allo-SCT |
| Follow-up under therapy | |
| Blood count and differential | Return to CP? |
| Bone marrow and cytogenetics | Ascertainment of second CP |
| Molecular genetics | Monitoring of BCR-ABL transcript levels under TKI and after allo-SCT |
| In lymphoid BC: CSF cytology | Intrathecal instillation for neuroprophylaxis |
| . | Test rationale . |
|---|---|
| Test at diagnosis of BC | |
| CBC with differential and bone marrow | Proportions of blasts, promyelocytes, and basophils? |
| Flow cytometry and/or cytochemistry | Myeloid or lymphoid phenotype? |
| Cytogenetics | Clonal evolution? |
| Molecular genetics | Mutation profile? Choice of TKI |
| Donor search (if applicable) | Allo-SCT |
| Follow-up under therapy | |
| Blood count and differential | Return to CP? |
| Bone marrow and cytogenetics | Ascertainment of second CP |
| Molecular genetics | Monitoring of BCR-ABL transcript levels under TKI and after allo-SCT |
| In lymphoid BC: CSF cytology | Intrathecal instillation for neuroprophylaxis |
BC indicates blast crisis; CP, chronic phase; CSF, cerebrospinal fluid; CBC, complete blood count; and TKI, tyrosine kinase inhibitor.
What are the clinical and laboratory features observed in BC? Do they play a role in prognostic prediction?
Clinically, BC may present with night sweats, weight loss, fever, bone pain, or symptoms of anemia. An increased risk of infections and of bleeding is also observed. The common laboratory features include high white blood cell and blast counts, decreased hemoglobin values, and platelet numbers and, in up to 80% of BC patients, additional cytogenetic aberrations (ACAs) in addition to the Philadelphia (Ph)–chromosome. Most frequent are the so called “major route” ACA (trisomy 8, additional Ph-chromosome, isochromosome (17q), trisomy 19), which are nonrandom and considered relevant for the pathogenesis of BC.12,–14 Less frequent are the so-called “minor route” cytogenetic aberrations involving chromosome 3 aberrations, loss of the Y-chromosome, and other rarer aberrations. Minor route ACAs are less likely involved in BC pathogenesis and may mainly indicate genetic instability. The impact of major route ACA at diagnosis on progression and survival has been shown.15
A variety of mutations has been associated with progression to BC. Mutations of the BCR-ABL tyrosine kinase domain have been observed in up to 80% of patients.11,16 ABL mutations in late CP with upfront imatinib resistance have been associated with a greater likelihood of progression to BC.17 Other mutations associated with BC include p53 mutations in approximately 24% of myeloid BC, p16 mutations in approximately 50% of lymphoid BC,18,19 and more recently characterized mutations, such as RUNX-1, IKZF1 (Ikaros), ASXL1, WT1, TET2, IDH1, NRAS, KRAS, and CBL in 3% to 33% of myeloid and/or lymphoid BC.20,–22 In addition, a profoundly altered gene expression profile has been reported in CD34+ BC cells compared with CP cells.23,24 Genes overexpressed, down-regulated, or deregulated in BC include SOCS2, CD52, HLA antigens, PRAME, JunB, Fos, FosB, and Il8 and genes of the Wnt/β-catenin pathway.25
Several features have been associated with an unfavorable prognosis, such as clonal evolution, more than 50% blast cells, high platelet counts, short duration of the CP, and extramedullary disease.26,–28 Although nonrandom, chromosomal individuality of each clonal evolution is a characteristic feature of BC similar to other cancers, which has been compared with speciation in evolution.29,30 The most important predictor of a poor prognosis is an unsatisfactory response to initial therapy.
What is the rationale for treating BC?
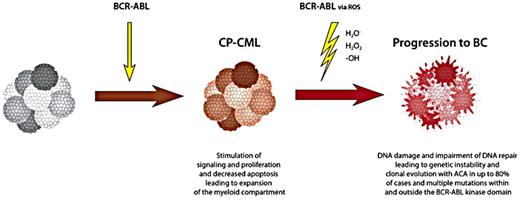
Treatment of BC is guided by our understanding of BC pathogenesis. Good in-depth reviews on the biology of BC have been published.31,–33 According to current evidence, BC is the direct consequence of continued BCR-ABL activity,31 possibly via oxidative stress and reactive oxygen species,34,35 causing DNA damage and impaired DNA repair36 (Figure 1) and, in a vicious circle, genomic instability by more mutations, gene doublings, translocations, and chromosomal breakage.37 The latter effect of BCR-ABL would explain what is observed during clonal evolution and progression to BC. BCR-ABL has been shown to produce reactive oxygen species in hemopoietic cells.38
Mechanisms of BCR-ABL activity in CML and blast crisis, leading to stimulation of proliferation and to induction of genetic instability, DNA damage, and impaired DNA repair. Reactive oxygen species induced by BCR-ABL are thought to mediate DNA damage and genetic instability. Data are from Skorski,34 Melo and Barnes,31 Radich,32 and Perrotti et al.33
Mechanisms of BCR-ABL activity in CML and blast crisis, leading to stimulation of proliferation and to induction of genetic instability, DNA damage, and impaired DNA repair. Reactive oxygen species induced by BCR-ABL are thought to mediate DNA damage and genetic instability. Data are from Skorski,34 Melo and Barnes,31 Radich,32 and Perrotti et al.33
This consideration underlies the therapeutic principle in CML to hit “hard and early” to reduce the BCR-ABL–positive cell pool as early and as deep as possible and to thereby achieve the best possible outcome.39 The validity of this principle may be limited by quiescent CD34+ CML cells, which evade currently available pharmacotherapy40 or by a speculative preexisting genetic instability responsible for the generation of BCR-ABL.41 The clinical improvement by TKI treatment in parallel to BCR-ABL reduction and the postponement (or prevention) of BC in most patients with TK-inhibition (8-year incidence of BC in IRIS1 < 8% under standard imatinib) support the conclusion that BCR-ABL is the driving force behind disease progression. The transient nature of response to TK inhibition in BC demonstrates that most cells are still sensitive to BCR-ABL inhibition but that BCR-ABL independence has been achieved in some cells, which then have a growth advantage. It follows that the most effective management of BC would be its prevention by early reduction of tumor burden and elimination of BCR-ABL.
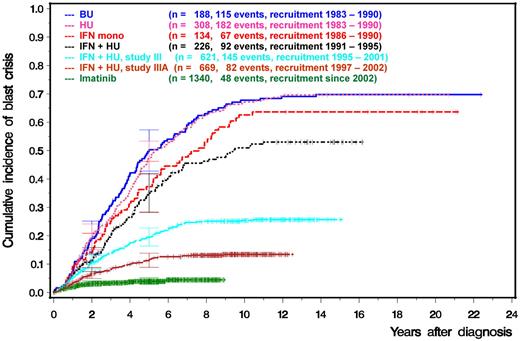
This is confirmed by experience of the German CML Study Group (Figure 2). The cumulative incidence of BC, as a consequence of more effective treatment early on, has decreased from close to 70% after 8 years 25 years ago to currently approximately 5% in CML Study IV under an optimized dose of imatinib.42
Prevention of BC by more effective treatment in early CP as shown by the cumulative incidence of blast crisis (German CML Study Group experience 1983-2011). CML study I compared busulfan versus hydroxyurea (HU) versus interferon-α (IFN) monotherapy, CML study II IFN in combination with HU versus HU alone, CML study III and IIIA IFN in combination with intensive chemotherapy versus allo-SCT and CML study IV imatinib 400 mg versus imatinib in combination with low-dose cytosine arabinoside versus imatinib in combination with IFN versus imatinib after IFN failure versus imatinib at 800 mg.42
Prevention of BC by more effective treatment in early CP as shown by the cumulative incidence of blast crisis (German CML Study Group experience 1983-2011). CML study I compared busulfan versus hydroxyurea (HU) versus interferon-α (IFN) monotherapy, CML study II IFN in combination with HU versus HU alone, CML study III and IIIA IFN in combination with intensive chemotherapy versus allo-SCT and CML study IV imatinib 400 mg versus imatinib in combination with low-dose cytosine arabinoside versus imatinib in combination with IFN versus imatinib after IFN failure versus imatinib at 800 mg.42
Management of BC: what we have learned from the pre-imatinib era
In the late 1960s/early 1970s, attempts were made to treat BC with treatment protocols designed for acute leukemia (AL). It was observed that 30% of the patients responded to a combination of vincristine and prednisone as used for acute lymphoblastic leukemia (ALL), whereas 70% did not.43,–45 The cells of the responding BC frequently showed features of lymphoid morphology and were TdT+.46 These observations have led to the distinction of lymphoid and myeloid variants of BC. The response rates to vincristine and prednisone and other drugs used for ALL, such as 6-thioguanine, 6-mercaptopurine, cytosine arabinoside, and methotrexate, ranged between 15% and 50%. Response was only of short duration. Responders survived a median of 3 to 10 months compared with 1 to 5 months in nonresponders.
In the 1980s and 1990s, AML-type induction therapies were applied, including various combinations of anthracyclines, cytosine arabinoside, 5-azacytidine, etoposide, carboplatin, fludarabine, and decitabine.47 In a series of 162 patients with nonlymphoid BC, 31 patients treated with decitabine showed a trend for better survival at lower toxicity.48 In total, a return to CP was observed in approximately 10%, opening a window for transplantation. No cures in the absence of stem cell transplantations were observed.
Overall, treatment of BC turned out to be less successful than that of AL despite considerable intensity (and toxicity), but the chance offered by a second CP for allo-SCT was recognized.
What progress in the management of BC is offered by the availability of TKI?
Once BC has been diagnosed and without clear targets available for inhibition, management depends on previous therapy and type of leukemia (myeloid or lymphoid). Best results are achieved for the few patients who return to CP and are successfully transplanted.
1. If the patient has been pretreated with conventional therapy (IFN or hydroxyurea, meanwhile the exception), a TKI (imatinib 600-800 mg/d, dasatinib 140 mg once daily or nilotinib 2 × 400 mg/d according to mutation profile) should be given and allo-SCT planned. Outcomes of trials with imatinib and other TKIs in BC are summarized in Table 2. Imatinib and dasatinib have been approved for all phases of CML, including BC by the Food and Drug Administration and the European Medicine Agency.
Treatment of BC by BCR-ABL TKI
| Drug . | Patients . | CR, % . | Survival . | |
|---|---|---|---|---|
| MBC/LBC . | 12 mo, % . | Median, mo . | ||
| Imatinib | ||||
| 300-600 mg28 | 58 (20 LBC) | 12 | NA | NA |
| 400-600 mg49 | 229 (MBC only) | 16 | 30 | 6.9 |
| 300-1000 mg50 | 75 (10 LBC) | 16 | 22 | 6.5 |
| 600 mg51 | 30 | 13 | 36 | 10 |
| 600 mg52 | 92 (20 LBC) | 17 | 29 | 7 |
| Dasatinib | ||||
| 50-100 mg bid54 | 33 (10 LBC) | 52/90 | ∼ 22* | ∼ 6 |
| 70-100 mg bid55 | 157 (48 LBC) | 35/56† | 49/30 | 11.8 (5.3) |
| 70 bid vs 140 mg qd56 | 210 (61 LBC) | 25-28/40-50 | 34-39/39-46 | 8 (10) |
| Nilotinib | ||||
| Up to 1200 mg58 | 33 (9 LBC) | 18 | NA | NA |
| 400-600 mg bid59 | 136 (31 LBC) | 40 | 42 | 10 |
| Drug . | Patients . | CR, % . | Survival . | |
|---|---|---|---|---|
| MBC/LBC . | 12 mo, % . | Median, mo . | ||
| Imatinib | ||||
| 300-600 mg28 | 58 (20 LBC) | 12 | NA | NA |
| 400-600 mg49 | 229 (MBC only) | 16 | 30 | 6.9 |
| 300-1000 mg50 | 75 (10 LBC) | 16 | 22 | 6.5 |
| 600 mg51 | 30 | 13 | 36 | 10 |
| 600 mg52 | 92 (20 LBC) | 17 | 29 | 7 |
| Dasatinib | ||||
| 50-100 mg bid54 | 33 (10 LBC) | 52/90 | ∼ 22* | ∼ 6 |
| 70-100 mg bid55 | 157 (48 LBC) | 35/56† | 49/30 | 11.8 (5.3) |
| 70 bid vs 140 mg qd56 | 210 (61 LBC) | 25-28/40-50 | 34-39/39-46 | 8 (10) |
| Nilotinib | ||||
| Up to 1200 mg58 | 33 (9 LBC) | 18 | NA | NA |
| 400-600 mg bid59 | 136 (31 LBC) | 40 | 42 | 10 |
CR indicates cytogenetic response (includes complete, partial, minimal, and minor response when available); LBC, lymphoid blast crisis; NA, not available; MBC, myeloid blast crisis; bid, twice a day; and qd, daily.
At 18 months.
Only complete and major cytogenetic response listed. Updated from Hehlmann and Saussele.5
Imatinib
Five studies on 484 patients, 50 with lymphoid BC, showed hematologic remission rates of 50% to 70% (70% in patients with lymphoid BC), cytogenetic response rates of 12% to 17% (all responses), a 1-year survival of 22% to 36%, and a median survival of 6.5 to 10 months.28,49,,–52
2. If BC evolves under imatinib, treatment with a second-generation TKI (dasatinib 140 mg or nilotinib 2 × 400 mg according to mutation profile) combined with chemotherapy as necessary should be given and allo-SCT planned as quickly as possible. In case of V299L, T315A, or F317L/V/I/C mutations, nilotinib is probably more effective than dasatinib. In case of Y253H, E255K/V, or F359V/C/I mutations, dasatinib is probably more effective than nilotinib.11 In case of the T315I mutation, an investigational approach (eg, with ponatinib) should be tried.53 Cytopenias may necessitate TKI dose reduction or treatment interruption, substitution of erythrocytes and platelets, or, in case of neutropenia, treatment with G-CSF.
Dasatinib
Three studies on 400 BC patients pretreated with imatinib, including 119 with lymphoid BC, showed hematologic remission rates of 33% to 61% (lymphoid BC, 36%-80%), major cytogenetic remission (MCR) rates of 35% to 56%, a 1-year survival of 42% to 50%, a 2-year survival of 20% to 30%, and a median survival of 8 to 11 months.54,–56
The largest of the studies, a randomized open label phase 3 study on 214 patients with 61 in lymphoid BC, tried to optimize the dose-schedule of dasatinib, stratified for lymphoid and myeloid BC, and compared dasatinib at 140 mg once daily with 70 mg twice daily. The study yielded similar efficacy and improved tolerability for the once-daily regimen.56 Pleural effusion, which is observed in up to one-third of dasatinib-treated BC patients, may necessitate dose reduction, diuretics, and, in some cases, corticosteroids.
Dasatinib crosses the blood-brain barrier and shows long lasting responses in Ph+ CNS disease.57 It is speculated that these effects, which are different from imatinib, are the result of the dual specific SRC/BCR-ABL TK-inhibitory property of dasatinib. Dasatinib maintenance is recommended in responders not suitable for allo-SCT.
Nilotinib
Two studies have been published on 169 patients, including 40 with lymphoid BC58,59 reporting hematologic response rates of 60% (lymphoid BC 59%), major cytogenetic response rates of 38% (myeloid BC), and 52% (lymphoid BC), a 1-year survival of 42%, a 2-year survival of 27%, and a median survival of 10 months (7.9 months for lymphoid BC). Hyperglycemia, which is observed in up to 40% of nilotinib-treated patients, requires monitoring and may necessitate dose adaptation. Nilotinib has been approved for treating CP and accelerated phase (AP) CML, but not yet BC.
The outcomes with dasatinib and nilotinib are similar to those with imatinib.
Bosutinib, a third second-generation TKI, shows in preliminary analyses similar activity in advanced phase CML as dasatinib and nilotinib.60 Bosutinib has not yet been approved for CML.
3. If TKIs fail, conventional approaches remain an option, such as AML induction protocols with anthracyclines and cytosine arabinoside in myeloid BC or a trial with vincristine and prednisone (combined with dasatinib) in lymphoid BC, or third-generation TKI within a clinical trial.
In summary, survival after BC is better after treatment with TKI than after conventional therapies, but with a median survival of less than 1 year, outcome is still unsatisfactory.
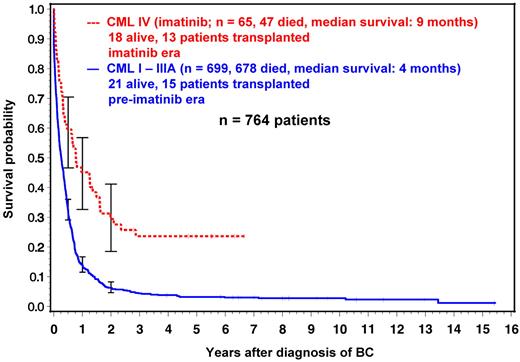
The modest survival progress that is achieved by TKI after BC is illustrated by the experience of the German CML Study Group in Figure 3. Median survival has increased from 4 months in the pre-imatinib era (n = 699) to 9 months under imatinib (n = 65).
Survival with BC in the preimatinib and imatinib eras. Most long-term survivors (72%) are transplant recipients. German CML Study Group experience (1983-2011). Data are from the German CML-studies I to IV.42
Survival with BC in the preimatinib and imatinib eras. Most long-term survivors (72%) are transplant recipients. German CML Study Group experience (1983-2011). Data are from the German CML-studies I to IV.42
When I recommend allo-SCT
If a return to CP or a complete remission has been achieved, I proceed to allo-SCT as quickly as possible, given that the patient can tolerate the procedure and has a donor. The search for a donor should be instituted as early as possible. The best outcome continues to be observed in patients after transplantation, although allo-SCT is successful in only a minority of BC patients mostly after prior return to a second CP. In an overview of the European Group for Blood and Marrow Transplantation from 1980 to 2003, 2-year survival rates are 16% to 22%.61 Most patients were transplanted in the pre-imatinib era. In a recent report from the German CML Study Group, the 3-year survival of 28 imatinib-pretreated patients transplanted in advanced phases (25 in BC) was 59%.62 The data show convincingly that allo-SCT represents the best chance of long-term remission or cure in BC. Current experience recommends allo-SCT in primary BC after an attempt has been made with a suitable TKI selected according to mutation profile in combination with chemotherapy as needed to achieve a second CP. In lymphoid BC, dasatinib should be combined with vincristine and prednisone.
In BC after imatinib failure, a second-generation TKI (according to mutation profile) has to be weighed against other options, such as AL-type therapy (also in combination with TKI) to give the best chance of a return to CP or cytoreduction. If patients carry the T315I mutation, this has to be considered in choosing the appropriate regimen (investigational agents; eg, ponatinib, AL-type therapy) followed by allo-SCT.63 Transplantation should be performed with an HLA-identical related or matched unrelated donor and an EBMT score 0 to 4.64 Standard conditioning with busulfan and cyclophosphamide or total body irradiation should be used. Reduced intensity conditioning is not recommended in this situation outside studies. Sudden-onset BC under imatinib is a rare event, but full disease eradication by allo-SCT may be successful65 and is warranted. Posttransplantation maintenance with TKI appears reasonable. Maintenance with dasatinib is recommended in lymphoid BC for neuroprophylaxis as it is known to cross the blood-brain barrier.57 Monitoring of BCR-ABL transcript levels should be done at regular intervals (3 months initially, 6 months later on, if transcripts are not detectable or stable).
What is the promise of new investigational approaches?
A number of investigational approaches are under exploration. A selection is shown in Table 3. Some agents are in clinical trial and can be tried after conventional treatments (TKI and AL-type therapy) have failed. Some approaches may be suitable for BC prevention.
Investigational approaches (selection)
| Mode of action . | Agent(s) . | Phase . | Target(s) . |
|---|---|---|---|
| Third-generation TKI | Ponatinib53 | II | Pan-BCR-ABL including T315I |
| DCC-203672 | I | Abl-switch pocket | |
| PP2A activation | Fingolimod (FTY720)75 | Preclinical | PP2A |
| SET antagonist OP44976 | Preclinical | SET | |
| CIP2A inhibitor74 | Preclinical | CIP2A | |
| Survival of LSCs | BCL6 + TK inhibitors78 | Preclinical | BCL6 + BCR-ABL |
| HIF1α inhibitor80 | Preclinical | HIF1α | |
| IL1 RAP antibodies86 | Preclinical | IL1 RAP | |
| Smoothened inhibitors in combination with TKI83 (dasatinib, nilotinib) | Preclinical | Smoothened (hedgehog pathway) + BCR-ABL | |
| Jak2 inhibitor + dasatinib85 | Preclinical | Jak2 + BCR-ABL, LSC | |
| Activation of apoptosis | BCL2-inhibitor ABT-73788 | Preclinical | Antiapoptotic proteins |
| Triptolide87,88 | Preclinical | Antiapoptotic proteins | |
| Dual-kinase inhibitor ON04458091 | Preclinical | BC, T315I | |
| MEK inhibitor PD184352 + farnesyltransferase inhibitor BMS-21466289 | Preclinical | MEK1, MEK2, RAS | |
| Others | Omacetaxine92 | II / III | BCR-ABL, T315I, BC |
| Mode of action . | Agent(s) . | Phase . | Target(s) . |
|---|---|---|---|
| Third-generation TKI | Ponatinib53 | II | Pan-BCR-ABL including T315I |
| DCC-203672 | I | Abl-switch pocket | |
| PP2A activation | Fingolimod (FTY720)75 | Preclinical | PP2A |
| SET antagonist OP44976 | Preclinical | SET | |
| CIP2A inhibitor74 | Preclinical | CIP2A | |
| Survival of LSCs | BCL6 + TK inhibitors78 | Preclinical | BCL6 + BCR-ABL |
| HIF1α inhibitor80 | Preclinical | HIF1α | |
| IL1 RAP antibodies86 | Preclinical | IL1 RAP | |
| Smoothened inhibitors in combination with TKI83 (dasatinib, nilotinib) | Preclinical | Smoothened (hedgehog pathway) + BCR-ABL | |
| Jak2 inhibitor + dasatinib85 | Preclinical | Jak2 + BCR-ABL, LSC | |
| Activation of apoptosis | BCL2-inhibitor ABT-73788 | Preclinical | Antiapoptotic proteins |
| Triptolide87,88 | Preclinical | Antiapoptotic proteins | |
| Dual-kinase inhibitor ON04458091 | Preclinical | BC, T315I | |
| MEK inhibitor PD184352 + farnesyltransferase inhibitor BMS-21466289 | Preclinical | MEK1, MEK2, RAS | |
| Others | Omacetaxine92 | II / III | BCR-ABL, T315I, BC |
LSC indicates leukemia stem cell; and MEK, mitogen-activated protein kinase kinase.
Imatinib in combination
Several small studies have focused on the combination of imatinib at 600 mg to 800 mg with chemotherapy or other agents. In a phase 1/2 trial on 16 BC patients, imatinib 600 mg daily was combined with mitoxantrone/etoposide.67 Hematologic response rate was 81% with a 1-year survival of approximately 50%, including 6 patients after allo-SCT. Another study combined imatinib 600 mg with decitabine in 10 patients and reported a median survival of 15 weeks.68 The combination of imatinib 600 mg with low-dose cytosine arabinoside and idarubicin in 19 patients with myeloid BC showed hematologic remissions in 47%. Median survival was 5 months.69 In a phase 1 study with the combination of the farnesyltransferase inhibitor lonafarnib with imatinib, 2 of 3 BC patients showed hematologic improvement.70 A study on 12 patients combining imatinib and homoharringtonine after priming with G-CSF reported hematologic or cytogenetic response in all patients.71 None of these studies has provided convincing evidence that the combinations are superior to imatinib alone.
Third-generation TKIs
New third-generation TKIs, such as the pan-BCR-ABL inhibitor ponatinib,53 show promise because, in addition to recognizing the T315I mutation, ponatinib also shows efficacy in BC and Ph+ ALL. A phase 2 study on 449 ponatinib-treated patients, 94 in BC or Ph+ ALL, showed after a median follow-up of approximately 5 months, complete cytogenetic remission (CCR) and major molecular remission (MMR) rates in BC of 27% and 22%, respectively.53 No data on survival were reported yet. Similarly, the ABL switch pocket inhibitor DCC-2036 showed efficacy against T315I and in BC in a phase 1 study.72 These TKIs may be the best choice of investigational agents in clinical trials.
PP2A activation
A new target of interest is the tumor suppressor protein phosphatase 2A (PP2A), which shows decreased activity in BC73 through up-regulation of its inhibitors suppressor of variegation, enhancer of zeste and trithorax (SET),73 and cancerous inhibitor of PP2A (CIP2A).74 The PP2A activator fingolimod (FTY720) induces apoptosis in CML-BC and Ph+ ALL progenitors33,75 and may be a candidate for BC treatment and prevention. Likewise, a novel SET antagonist (OP449) is selectively cytotoxic to CML cells and restores PP2A's tumor suppressive function.76 In addition, CIP2A inhibition increases PP2A activity.74
Self-renewal of leukemia stem cells
Another target potentially relevant for BC management or prevention is the self-renewal of leukemia stem cells (LSCs) in vivo or leukemia-initiating cells in vitro. BCL6 has been identified as a critical effector of the BCR-ABL downstream target FoxO in self-renewal signaling of CML initiating cells.77 Pharmacologic inhibition of BCL6 in combination with BCR-ABL inhibition is proposed for eradication of leukemia-initiating cells in CML.78 Dual inhibition of BCL6 and BCR-ABL is an interesting approach that merits exploration for application to BC, but BCL6 inhibitors are not yet available for clinical use.79
A similar role for survival maintenance of CML stem cells has been reported for the hypoxia-inducible factor 1α, a master transcriptional regulator of the cellular and systemic hypoxia response.80 Inhibition of the hypoxia-inducible factor 1α pathway may provide another strategy for eradicating LSCs in CML.
Clinical studies are ongoing to explore antagonists of the transmembrane protein smoothened, which plays a role in the hedgehog pathway and is essential for the maintenance of LSCs,81,82 such as cyclopamine, GDC-0449 (Genentech), LDE225 (Novartis), BMS833923, or PF0444913 (Pfizer), in combination with second-generation TKI for activity against BC-LSC and self-renewal.83 GDC-0449 has shown activity in basal cell carcinoma (18 of 33 patients responded)84 and in medulloblastoma. Similarly, the Jak2-inhibitor SAR503 in combination with dasatinib significantly reduced LSC, suggesting abolishment of LSC self-renewal capacity.85
A new cell surface biomarker, IL1 receptor accessory protein (IL1 RAP), has been specifically identified on CML stem cells and might offer a new therapeutic target in the future.86
Induction of apoptosis
Preclinical studies are investigating the activation of apoptosis in BC cells by various drugs and combinations. The BCL2 inhibitor ABT-737 combined with imatinib or with the diterpenoid triptolide reduces antiapoptotic proteins, thereby inducing apoptosis and cell death in K562 cells and in cells from BC patients.87,88 The MEK inhibitor PD184352 combined with the farnesyltransferase inhibitor BMS-214662 similarly induces apoptosis in K562 cells and CD34+ CML stem cells.89 In addition, p53 stabilization with the novel compound MI-219, which inhibits human homolog double minute 2, induces apoptosis in cell line and primary BC cells.90 And recently, the dual Jak2/Abl kinase inhibitor ON044580 was shown to induce apoptosis in cells from BC patients and in imatinib-resistant cells, including T315I.91
More drugs are in clinical and in preclinical evaluation. These drugs include omacetaxine (a semisynthetic derivative of homoharringtonine),92 arsenic trioxide, which showed synergy with imatinib, histone deacetylase (HDAC) inhibitors, aurora kinase inhibitors alone or in combination (eg, with TK or HDAC inhibitors), HSP90 inhibitors, mTOR inhibitors (rapamycin), and other substances.4,93,–95
None of these approaches is likely to provide a breakthrough in the near future; because of the numerous blastic genotypes and their instability, no single therapeutic approach can soon be expected to be successful in all patients.
Can BC be prevented? Is early prediction possible?
The low progression rates of CML under TKIs indicate that BC can be prevented (Figure 2). In addition, it is well known that very low or undetectable BCR-ABL transcripts after allo-SCT correlate with low relapse rates.96,97 Imatinib-treated patients who have achieved MMR enjoy durable responses with virtually no progression to AP or BC up to now.42,98 Patients who have achieved stable complete molecular remission experience, in approximately 40% of cases, continued remissions even in the absence of treatment.99 The challenge is how to identify early those patients who are at risk to proceed to BC to be able to offer alternative treatment to this patient group.
At diagnosis, risk scores provide information on the likelihood of progression.100,101 The EUTOS score, which was developed from imatinib-treated patients, has a predictive value of not reaching a CCR by 18 months of 34% and recognizes a small group of high-risk patients (∼ 12%), with a significantly higher progression rate. [The EUTOS score uses 2 variables at diagnosis (spleen size in centimeters below costal margin and percentage basophils) and separates 2 risk groups. It is calculated by the formula: EUTOS score = (7 × basophils) + (4 × spleen size). A score of > 87 indicates high risk.]102 In addition, distinct markers such as major route ACA,15 p190BCR-ABL,103 and signs of acceleration may be suitable for early prediction of progression (Table 4). In addition, BMI1 and CIP2A levels at diagnosis have been reported predictive of BC.74,104
Early prediction of progression
| Study . | n . | Baseline . | 3 mo . | 6 mo . | 12 mo . | End point . |
|---|---|---|---|---|---|---|
| Historical | ||||||
| Mahon et al (IFN)121 | 116 | NA | CHR | NA | NA | MCR |
| Baccarani et al (imatinib, review)8 | NA | NA | CHR | NA | CCR | OS |
| Baseline | ||||||
| Hasford et al (EUTOS)102 | 2060 | High risk | NA | NA | NA | CCR* |
| Fabarius et al15 | 1151 | Major route ACA | NA | NA | NA | OS |
| Verma et al103 | 1292 | P190BCR-ABL | NA | NA | NA | PFS |
| Clonal evolution | ||||||
| Baccarani et al (review)8 | NA | NA | NA | Any time | NA | OS |
| Response | ||||||
| Hanfstein et al122 | 692 | NA | MR 10%, MCR | MR 1%, CCR | NA | OS |
| Hehlmann et al42 | 1014 | NA | NA | NA | MMR (MR 0.1%) | OS |
| Marin et al123 | 282 | NA | MR 9.84% | MR 1.67% | MR 0.53% | OS |
| Jabbour et al124 | 435 | NA | MCR | CCR | NA | OS |
| Study . | n . | Baseline . | 3 mo . | 6 mo . | 12 mo . | End point . |
|---|---|---|---|---|---|---|
| Historical | ||||||
| Mahon et al (IFN)121 | 116 | NA | CHR | NA | NA | MCR |
| Baccarani et al (imatinib, review)8 | NA | NA | CHR | NA | CCR | OS |
| Baseline | ||||||
| Hasford et al (EUTOS)102 | 2060 | High risk | NA | NA | NA | CCR* |
| Fabarius et al15 | 1151 | Major route ACA | NA | NA | NA | OS |
| Verma et al103 | 1292 | P190BCR-ABL | NA | NA | NA | PFS |
| Clonal evolution | ||||||
| Baccarani et al (review)8 | NA | NA | NA | Any time | NA | OS |
| Response | ||||||
| Hanfstein et al122 | 692 | NA | MR 10%, MCR | MR 1%, CCR | NA | OS |
| Hehlmann et al42 | 1014 | NA | NA | NA | MMR (MR 0.1%) | OS |
| Marin et al123 | 282 | NA | MR 9.84% | MR 1.67% | MR 0.53% | OS |
| Jabbour et al124 | 435 | NA | MCR | CCR | NA | OS |
Patients at increased risk of progression can be detected by baseline markers, clonal evolution, and early molecular or cytogenetic response indicators. Failure to reach the defined response landmarks at 3, 6, and 12 months identifies a group of high risk patients with higher progression risks (25%-33% of patients at 3 months122,123 ) who might benefit from an early change of therapy. Percentages are according to international scale.130
CHR indicates complete hematologic remission; MCR, major cytogenetic remission; NA, not applicable; OS, overall survival; ACA, additional cytogenetic aberrations; PFS, progression-free survival; and MR, molecular response.
CCR at 18 months.
Another indicator of progression risk is clonal evolution (ie, the acquisition of ACA in the course of the disease).105,,–108 The relevance of clonal evolution has not changed in the imatinib era.109,,–112 Mutations may be associated with clonal evolution.113 The pattern of chromosome abnormalities is not altered by TKI treatment.114 The prognostic impact of ACA may depend on the type of ACA.112 Some ACA types (major route, complex karyotypes) appear to imply poorer prognosis than others that may only indicate genetic instability.115 Acquired ACAs are high-risk features by European LeukemiaNet definition and indicate treatment failure if they appear under therapy.8 The prognostic relevance of rare clonal evolution in Ph-negative cells (observed in < 5% of cases) remains uncertain.116,,–119 The evolution of gene expression profiles may also allow to diagnose disease progression.120
Early response indicators are probably the best predictors of progression.8,121 These include cytogenetic and molecular responses determined by monitoring all patients. Failure to achieve defined landmarks will detect high-risk patients as early as 3 months after diagnosis.122,–124 Table 4 summarizes the response levels and time points for response categorization.42,122,–124 Patients who do not respond satisfactorily and are classified as high risk need alternative approaches, such as early second-generation TKI, treatment intensification, or an early allo-SCT.8,125 If the patients have a donor and have no medical contraindications, the risk of progression to BC has to be weighed against the risk of early transplantation and of chronic GVHD. With the current progress in donor selection and posttransplantation management, the risk of transplantation seems acceptable if compared with the risk of BC. If the patients are too old or have other medical contraindications that preclude allo-SCT or have no donor, investigational agents can be tried (Table 3).
Conclusion: how I manage CML-BC
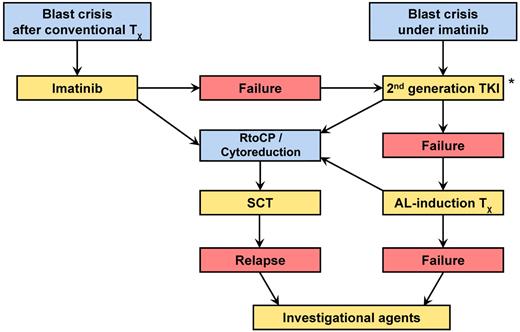
The algorithm in Figure 4 gives an overview on how I approach management of a patient with BC. The treatment goal is the return to CP or the induction of a remission. Mainstays are TKIs taking into account the type of mutation and allo-SCT as quickly as possible. If TKIs alone are not sufficient, AL-type induction therapy should be tried, cytosine arabinoside and anthracyclines for myeloid BC, vincristine and prednisone in lymphoid BC, or TKI in combination with AL-type induction therapy. Management of primary BC follows the same principle, except that imatinib should be tried first in myeloid BC. Treatment decisions have to be adapted to the individual patients' situations and needs as required. Hematologic, cytogenetic, and molecular monitoring are mandatory (Table 1). Cytopenias may necessitate dose adaptation, substitution therapy, and treatment with G-CSF. In lymphoid BC, intrathecal neuroprophylaxis may be indicated. Investigational approaches are recommended only after all other options have failed. Allo-SCT without prior return to CP or at least cytoreduction is a high-risk procedure and discouraged. An option is transplantation in aplasia without waiting for marrow recovery.
Management algorithm of CML-BC. Mainstays are TKI and rapid allo-SCT. *2nd generation TKI and AL-induction therapy may be combined.
Management algorithm of CML-BC. Mainstays are TKI and rapid allo-SCT. *2nd generation TKI and AL-induction therapy may be combined.
In view of the limited therapeutic options once BC has been diagnosed, the best management of BC is probably its prevention by a rigorous and early reduction to low levels or elimination of BCR-ABL. Regular molecular monitoring is required (Table 4). The current understanding of pathogenesis of CML-BC as a consequence of continued BCR-ABL activity provides the rationale for this approach. Patients with high-risk features at diagnosis,100,–102 unsatisfactory response to therapy (eg, no major cytogenetic response or < 90% BCR-ABL reduction by 3 months),122,–124 or signs of progression under therapy, such as clonal evolution, should receive more intensive therapies to prevent progression and BC. With the availability of optimized imatinib protocols42,126,127 and second-generation BCR-ABL inhibitors first line,128,129 which induce deeper remissions faster, I recommend every attempt to eliminate BCR-ABL as early as possible. I expect that more efficacious therapies and early treatment intensification in patients with high-risk features or unsatisfactory responses will further reduce progression and transformation to BC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The author thanks colleagues A. Hochhaus, M. C. Müller, S. Sauβele, M. Baccarani, and R. S. Silver for reading the manuscript; A. Gratwohl, R. Schwerdtfeger, and H.-J. Kolb for advice on transplantation in blast crisis; G. Bartsch and U. Böhm for technical support; and all members of the German CML Study Group for their continued patient care and cooperation.
This work was supported by the German CML Study Group, Deutsche Krebshilfe (106642), Novartis Germany, Kompetenznetz für Akute und Chronische Leukämien (BMBF 01GI0270), José-Carreras Leukämiestiftung (DJCLS H09/01f, H06/04v, H03/01), the European Commission (LSHC-CT-2004-503216), and Roche and Essex (now MSD).
Authorship
Contribution: R.H. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Rüdiger Hehlmann, Medizinische Klinik, Medizinische Fakultät Mannheim der Universität Heidelberg, Pettenkoferstr 22, 68169 Mannheim, Germany; e-mail: sekretariat.hehlmann@medma.uni-heidelberg.de.