The development of alloantibodies or inhibitors is the most serious complication a patient with severe hemophilia can experience from treatment with clotting factor concentrates. Although common in previously untreated patients, inhibitor development is rare in multiply exposed, well-tolerized patients. There has been a nonevidence-based reluctance to change concentrate because of a perceived greater inhibitor risk after the switch, even though most patients are now likely to be using a concentrate on which they did not begin. Inhibitors in previously treated patients are observed in approximately 2 per 1000 patient/years, which makes it difficult to study and compare rates among different products. Because the baseline inhibitor risk in previously treated patients may vary over time, it is important to compare the risk in patients switching to a new product with that in a parallel control group of nonswitching patients or within a case-controlled study. The study designs imposed by regulators are suboptimal in detecting immunogenicity signals. The issue of immunogenicity of new products is likely to gain more relevance in the near future, with a call for effective postmarketing surveillance studies for all of the new engineered factor VIII concentrates with prolonged half-lives that are likely to enter clinical practice.
Why do we need to care about switching treatment in hemophilia?
Hemophilia and inhibitors: definitions
Hemophilia A and B are inherited disorders caused by the respective deficiency of factor VIII (FVIII) and FIX. These deficiencies occur in 1 per 10 000 and in 1 per 50 000 male births, respectively. Severely affected persons with hemophilia (PWH) tend to bleed spontaneously into their joints, which can be treated or prevented by administering the relevant clotting factor concentrate. The major problem in caring for PWH in countries that have factor concentrates available is the occurrence of neutralizing alloantibodies, collectively referred to as inhibitors.1
The inhibitor literature spans more than 4 decades, and several definitions and assays have been used during this time, making comparisons among publications difficult. An exposure day (ED) is a 24-hour period during which a dose of concentrate has been administered, irrespective of size and frequency. Patients with < 20-50 EDs are defined as previously untreated patients (PUPs), whereas those exposed to > 75-150 EDs are referred to as previously treated patients (PTPs).2 Inhibitors are IgG polyclonal alloantibodies to FVIII, which neutralize clotting factor activity, and they usually target the A2 and C2 domains of the FVIII molecule.1 Inhibitor titers are best assessed with the Nijmegen modification of the Bethesda assay.3 A level of > 0.6 Bethesda units (BU) is considered to be positive, and levels < 5 BU are known as low-inhibitor titers, whereas those > 5 BU are defined as high-inhibitor titers.4 Once inhibitors develop, they have an adverse outcome both in terms of morbidity and mortality5 and, almost invariably, patients with inhibitor titers > 5 BU will require bypassing agents.6 Inhibitors are more likely to occur in the first 50 EDs in patients with severe hemophilia, but a baseline low risk remains through a patient's life.7 The cumulative inhibitor risk in PUPs is approximately 30%, whereas in PTPs it is 2-3 per 1000 patient/years.8,–10
Hemophilia treatment and risk of inhibitor development: facts and hypotheses
FVIII concentrates are available as plasma-derived or recombinant products. Plasma FVIII is prepared by fractionating up to 30 000 plasma donations, and products undergo dual viral inactivation.11 Despite their similarities, plasma-derived FVIII concentrates can have significant differences, primarily reflecting purity and viral inactivation procedures. Intermediate-purity concentrates contain high levels of VWF, whereas high-purity products are virtually devoid of VWF (Table 1).11 Many viral inactivation procedures have been used and, in the past, some were shown to affect the structure of the FVIII molecule, leading to inhibitor formation.12 Recombinant FVIII concentrates are produced by expression vectors carrying the human FVIII gene in cell lines. As with plasma-derived products, significant differences occur among recombinant products, in terms of the cell line being used (eg, Chinese hamster ovary or baby hamster kidney), FVIII gene length (full-length vs B-domain deleted), and product formulation, namely, with albumin (first generation), without albumin (second generation), and without human or animal proteins during the production steps and final formulation (third generation; Table 1).
The licensed recombinant FVIII concentrates
| Cell line . | Baby hamster kidney cells . | Chinese hamster ovary cells . | |
|---|---|---|---|
| . | . | ||
| FVIII gene length | Full length | Full length | B-domain deleted |
| First generation | Kogenate | Recombinate | — |
| Helixate | |||
| Second generation | Kogenate FS | — | Refacto |
| Helixate FS/ | |||
| Helixate NextGen | |||
| Third generation | — | Advate | Xyntha/Refacto AF |
| Cell line . | Baby hamster kidney cells . | Chinese hamster ovary cells . | |
|---|---|---|---|
| . | . | ||
| FVIII gene length | Full length | Full length | B-domain deleted |
| First generation | Kogenate | Recombinate | — |
| Helixate | |||
| Second generation | Kogenate FS | — | Refacto |
| Helixate FS/ | |||
| Helixate NextGen | |||
| Third generation | — | Advate | Xyntha/Refacto AF |
— indicates no available concentrate.
Inhibitors develop as a result of risk factors, which have been evaluated mostly in PUPs. Some factors are genetic,13 including genotype, race, family history, and the presence of several immune response genes.14,15 Others are environmental,13 either nontreatment-related (such as bleeding frequency, site and intensity, and intercurrent events, including surgery or trauma) or treatment-related. The latter may be further categorized into regimen-related (ie, on demand, high- or low-dose prophylaxis) and concentrate-related. Regarding concentrate-related risk factors, considerable debate exists as to whether plasma-derived and recombinant FVIII concentrates are associated with different risks of developing inhibitors and whether the risk varies among different recombinant molecules. Although greater rates were reported with some of the recombinant FVIII products, those, in general, were recorded by the authors of more recent studies in more intense inhibitor testing was performed, and it is possible that the reported difference is the result of confounders.10
The ongoing trial (Survey of Inhibitors in Plasma-Product–Exposed Toddlers, ie, SIPPET; http://www.sippet.org), in which investigators randomize PUPs to plasma-derived or recombinant FVIII, may possibly provide a definite answer, provided that the difference is relatively large. Similarly, a recent meta-analysis of published reports suggested that B-domain–deleted recombinant FVIII was associated with a 10-fold greater risk in inhibitor development relative to full-length products.16 There are no ongoing or planned trials to address this issue, but several methodologic considerations will be provided in this article. Finally, it can be noted that black patients have a greater inhibitor risk, and it has recently been suggested that those patients may have a different haplotype from that of the cell line used to manufacture the recombinant FVIII,17 emphasizing how genetic and environmental risk factors can interact with each other. The risk of inhibitor is a multicausal phenomenon,18 in which individual factors may be neither necessary nor sufficient—singly—but rather their combined effects will produce an effect. Notwithstanding, removing any of the single contributing factors might result in a decreased rate of inhibitor development.
In contrast to the high rate of inhibitors in PUPs, the rate in PTPs is much lower. However, inhibitors are not absent. It is important to recognize that there is a baseline low, yet definite, risk of inhibitor development in all severe PWH over time. Because the inhibitor risk in PTPs is so low, PTPs have historically been proposed as the ideal hemophilic subset to test the immunogenicity of new clotting factors,19,20 and regulatory authorities require that registration studies be performed in PTPs before PUPs are exposed to new products.2,21,22 Unfortunately, the number of patients enrolled in registration studies is so small (Table 2 23,,,,–28 ) that these studies are unlikely to detect anything but a large increase in inhibitor risk (Table 2), which makes comparisons among products even more difficult and may result in dismissing a potentially valuable new product.16
Inhibitors assessment in PTPs enrolled in prelicensure trials
| Author . | Brand . | Sample . | FVIII level . | ED . | Inhibitors . |
|---|---|---|---|---|---|
| White et al23 | Recombinate | 69 | < 0.05 | NR | 0 |
| Schwartz et al24 | Kogenate | 86 | NR | NR | 1 |
| Abshire and Brackmann25 | Kogenate-FS | 73 | < 0.02 | > 100 | 0 |
| Courter and Bedrosian26 | Refacto | 113 | < 0.02 | > 30/y | 1 |
| Tarantino et al27 | Advate | 108 | < 0.01 | > 150 | 1 |
| Recht et al28 | RefactoAF | 204 | < 0.02 | > 150 | 3 |
| Author . | Brand . | Sample . | FVIII level . | ED . | Inhibitors . |
|---|---|---|---|---|---|
| White et al23 | Recombinate | 69 | < 0.05 | NR | 0 |
| Schwartz et al24 | Kogenate | 86 | NR | NR | 1 |
| Abshire and Brackmann25 | Kogenate-FS | 73 | < 0.02 | > 100 | 0 |
| Courter and Bedrosian26 | Refacto | 113 | < 0.02 | > 30/y | 1 |
| Tarantino et al27 | Advate | 108 | < 0.01 | > 150 | 1 |
| Recht et al28 | RefactoAF | 204 | < 0.02 | > 150 | 3 |
ED indicates exposure day; NR, not reported; and PTP, previously treated patients.
Switching factor concentrates
Traditionally, on the basis of very little evidence, there has been a reluctance to change the type of concentrate that PWH are using. The main trigger was initially the epidemics of blood-borne infections, where having switched was an obstacle to track back the infection to the culprit concentrate. In addition, PWHs often develop a strong psychologic link with the product they use, and, particularly when previously hit by blood-borne diseases, they are firm in their reluctance to change their current product. A similar barrier to switch often was from hemophilia physicians, who were reluctant to propose a switch to their patients, more so when the patient had already contracted HIV or hepatitis C virus.
After products became safer, the theoretical reluctance to switch remained, even though the frequency of real-world switching is underappreciated. It is extremely rare, if not impossible, for adult PWH in most countries worldwide to have used the same concentrate throughout their lives. Inadvertently, the main reason not to switch became avoiding the development of new inhibitors. Although in countries in which there is a choice of available concentrates it may be worth keeping at least PUPs on the same product until 50 EDs, in many countries with national contracting avoiding a switch may not be feasible. Switching can be a reasonable choice for several reasons (Table 3), and as patients switch products more readily, the question of whether the switch will induce new inhibitors is a common one. In this perspective, we review the evidence and address the question of whether switching products will induce new inhibitors, and we outline and discuss the many confounding factors that play a role.
Reasons for switching clotting factor concentrates
| Improved safety (real or perceived) |
| Less risk of infection |
| Less inhibitor risk |
| Fewer side-effects (eg, allergic reactions) |
| Newer generation of product |
| Price |
| National contracting |
| Volume of final product |
| Mixing and administration device |
| Storage advantage |
| Patient/family preference |
| Longer half-life |
| Participation in a clinical trial of a new product/formulation |
| Research study participation that specifies product to be used |
| Improved safety (real or perceived) |
| Less risk of infection |
| Less inhibitor risk |
| Fewer side-effects (eg, allergic reactions) |
| Newer generation of product |
| Price |
| National contracting |
| Volume of final product |
| Mixing and administration device |
| Storage advantage |
| Patient/family preference |
| Longer half-life |
| Participation in a clinical trial of a new product/formulation |
| Research study participation that specifies product to be used |
Immunologic considerations
In the paragraphs to follow, we examine the biologic rationale behind the immunogenicity of FVIII. Our reasoning is built on experimental evidence from other proteins, and our aim is to discuss the robustness of the explanatory biologic hypothesis we might want to test with the available epidemiologic data (which are shown below). One principle of demonstrating causality is to have a plausible biologic rationale because a weak hypothesis often is associated with a high chance of spurious findings.29
Immunogenicity of therapeutic proteins
In understanding the immunogenicity of replacement proteins, both the nature of the protein as an immunogen and the ability of the host to sense the immunogen are critical components.30 These components are relevant both on priming (when the immunogen is first encountered by the host) and on reexposure (as occurs in secondary responses, usually triggered by a cross-reactive epitope, as is the case for patients previously treated with a FVIII product and then switched to another). The factors influencing the immunogenicity of therapeutic proteins in humans can be subdivided into 2 main categories, namely, those directly affecting the immune response (protein structure, immunomodulatory effect of the protein, formulation, contaminants and impurities, posology, MHC genotype of the host, associated diseases, and concomitant therapy) and those affecting the measured immune response (timing and frequency of sampling, assay methods, and expression of titers). Regarding the protein structure of a FVIII product, any native form of FVIII should theoretically be less likely to act as an immunogen because most antibodies against therapeutic proteins are directed to portions of the amino acid backbone protected by glycosylation or deeply buried within a native protein (“bystander” epitopes). However, this does not guarantee per se a reduced risk of inhibitor formation to plasma-derived FVIII because dose, route, form (complex/particulate vs simple/soluble), interaction with the host MHC, and particularly aggregation may all tip the balance between immunity and tolerance in favor of immunity, even in plasma-derived products. Surprising as it may appear to hemophilia clinicians, from a purely immunologic viewpoint, the risk of inhibitor development in a FVIII-naive patient, albeit high, is expected to be at the lowest range with the selective administration of a single, antigenically defined product, such as a properly engineered recombinant molecule.
The lower immunogenicity of recombinant versus native proteins has been demonstrated in several instances, most clearly for IFN-α,31 and there is evidence in that direction for FVIII as well. A most likely mechanism of immunogenicity is aggregation, something that all therapeutic proteins, including FVIII, tend to do.32 On aggregation, the recombinant protein loses its conformation and will no longer induce inhibitors (ie, antibodies capable of recognizing the recombinant, not aggregated molecule), whereas the native protein changes its conformation, thus inducing effective inhibitors. As clearly epitomized by Pisal et al, “protein concentrates, including FVIII products, tend to aggregate and nonnative aggregates may induce potent antibody responses, but these are not expected to include a large population of inhibitory antibodies because the native structure of the protein is lost on aggregation. In contrast, native-like aggregates have also been shown to be highly immunogenic and capable of producing inhibitory antibodies. Inhibitory antibodies frequently target regions of macromolecular interactions that are sensitive to conformational changes.”33
In the face of the possible occurrence of bystander epitopes in bioengineered FVIII products, several manipulations introduced in the recombinant protein might minimize the risk of immunogenicity,34 including modifying protein folding; using smaller peptides (ideally with a molecular weight < 2500) in lieu of full proteins, thus reducing the number of potential epitopes—a theoretical advantage for all truncated or deleted molecules35,36 ; and extending the half-lives of coagulation proteins, thus reducing the frequency of administration and avoiding repeatedly boosting memory responses.37 As an example, PEGylation may shield bystander epitopes38 and protect the molecule from epitope-specific antibodies in inhibitor-positive subjects.
The effects of protein structure manipulations might, however, be manifold. B-domain deletion was thought to reduce the risk of immunogenicity by reducing the number of epitopes on the molecule.36 Yet, should the manipulation result in a decreased half-life, one might obtain the opposite result, as indeed recently suggested.16,37 If these preliminary data are confirmed by subsequent studies, it could be appropriate to further manipulate the molecule to yield a fusion or conjugate protein (ie, with albumin or with the Fc fragment of an antibody) with improved resistance to proteolysis, possibly yielding a more favorable impact on the immune system, both in terms of prolonged half-life and antigen shielding. Advanced molecular engineering, such as site-specific mutagenesis, which may eliminate epitopes,39 or exon shuffling,37 which may eliminate antigenic determinants, might be eventually used to directly manipulate the immunogenic profile of new drugs.
Immunogenicity considerations related to switching
When specifically dealing with the question of whether switching a patient from 1 FVIII product to a different one may increase the risk of inhibitor formation, there is no a priori predicting the effect of the switch, which is the net result of several variables acting together, including host-extrinsic immunogenicity of each factor and host immune status. However, 2 general considerations might apply that should be carefully considered when generating hypotheses to be tested in epidemiologic observations or to provide clinical guidelines. First, the lower the intrinsic immunogenicity of a second FVIII product, the greater the probability of not breaking tolerance to epitopes shared between products.40 Second, the host could be conditioned to mount a tolerogenic response on receiving the new product in the form of a negative vaccination strategy.41
From a theoretical, immunologic point of view, the claim of safety issues on switching from a plasma-derived product to a molecularly defined bioengineered one, or on changing biodrug, appears to be weak. Similarly, the claim that a plasma concentrate may be safer because it is antigenically heterogeneous42 (as it should divert the host's immune system from mounting high-titer–specific responses) is hardly tenable because the very heterogeneity of the mixture can be thought to repeatedly boost anamnestic responses and sustain high-level inhibitor occurrence, as it is commonly observed with alloantibodies recognizing polymorphic haplotypes. Inhibitors induced by plasma-derived concentrates have been found to be of lesser occurrence, yet to be more persistent and higher in titers.11 In contract, introducing a molecularly defined recombinant product, particularly if “protected” by PEGylation or by other means, may boost an antibody response that is expected to be quantitatively lower and of limited specificity and duration in PTPs. Even when strong new antigens were introduced in clinical practice, only transient inhibitor production was observed.43
Reviewing the available evidence
A premise: hemophilia and the assessment of baseline risk of events
Epidemiologically, the risk of inhibitor development in PTPs is defined as the baseline risk for inhibitor development.44,45 In the multicausality framework, assessing the effect of a single risk factor, such as switching, requires measuring the baseline risk of events in patients not exposed to the specific risk factor, that is, not switching. The baseline risk for an event is a critical issue in the assessment of treatment-related effects.46,47 Usually, the baseline risk of the outcome of interest is measured in the control arm of a large pragmatic randomized control trial or in the nonexposed arm of a prospective cohort study.48
It has been demonstrated that most of the outcomes of interest in medicine are subject to temporal trends and geographic variability, which depends on a multiplicity of societal, environmental, and treatment-related factors.49,50 This leads to a consistent risk of error in evaluating treatment-related outcomes (such as rate of inhibitors associated with administering a new drug) if the baseline risk changes over time.51,–53 For example, if the risk of developing inhibitors is related to the average dose of factor concentrate being administered (in terms of IU/kg), and in 20-year timeframe the amount of factors administered as a prophylactic dose has been doubled, the rate of inhibitors eventually measured will necessarily be greater, irrespective of any specific contribution by a newly introduced concentrate. Unfortunately, in assessing the immunogenicity of new factor concentrates we usually do not contextually measure the baseline risk of inhibitor in patients not exposed to the drug.
Two major questions are thus relevant: First, is there any reliable direct or indirect estimate of the baseline or spontaneous rate of inhibitor development in PTPs? Second, is there any evidence for inhibitor development in PTPs undergoing a concentrate switch?
The baseline risk of inhibitor in PTPs
Several reports spanning more than 2 decades5,7,54,–56 provide the incidence and prevalence of inhibitors in large and well-characterized populations (Table 4). Unfortunately, none of these publications provides inhibitor rates in the subgroup of patients who stayed on a single-factor concentrate throughout their lives, which likely implies that for several years there has been no clear perception of any risk associated with switching. Although these reports provide a measure of the risk of inhibitors in PTPs that is reliable, consistent, and reproducible, they incorporate any additional risk contributed by switching from one drug to another (technically, this is called the risk of inhibitors “attributable” to switching, as would be the case for any other risk factor). As we await robust assessment of inhibitor rates in PTPs who did not switch at all, these data remain the best estimate on which we can rely.
Estimates of the risk of inhibitors development in previously treated hemophilia patients
| Year . | Author/reference . | Study design . | Sample . | Follow-up, mo . | Inhibitors . | Rate, × 1000 patient/years . | Age, y . |
|---|---|---|---|---|---|---|---|
| 1988 | McMillan et al54 * | Prospective | 1306 | 48 | 31 | 0.0080 | |
| 1995 | Colvin et al55 † | Cross-sectional | 2160 | 48 | 32 | 0.0015 | |
| 2004 | Darby et al5 ‡ | Registry | 6078 | 24 | 133 | 0.0020 | ≥ 15 |
| 42 | 0.0029 | 5-14 | |||||
| 2006 | Kempton et al56 § | Prospective | 838 | 48 | 7 | 0.0021 | |
| 2011 | Hay et al7 ‖ | Registry | 2258 | 144 | 106 | 0.0053 | 10-49 |
| 11 | 0.0052 | 50-59 |
| Year . | Author/reference . | Study design . | Sample . | Follow-up, mo . | Inhibitors . | Rate, × 1000 patient/years . | Age, y . |
|---|---|---|---|---|---|---|---|
| 1988 | McMillan et al54 * | Prospective | 1306 | 48 | 31 | 0.0080 | |
| 1995 | Colvin et al55 † | Cross-sectional | 2160 | 48 | 32 | 0.0015 | |
| 2004 | Darby et al5 ‡ | Registry | 6078 | 24 | 133 | 0.0020 | ≥ 15 |
| 42 | 0.0029 | 5-14 | |||||
| 2006 | Kempton et al56 § | Prospective | 838 | 48 | 7 | 0.0021 | |
| 2011 | Hay et al7 ‖ | Registry | 2258 | 144 | 106 | 0.0053 | 10-49 |
| 11 | 0.0052 | 50-59 |
Patient of all severities. A total of 14 inhibitors were in patients > 75 ED; 11 of 14 were low titer, and 6 of 14 were transient. Total patients with > 75 ED not reported but rate likely estimated at .0023.
Only patients with factor VIII < 0.03 U/mL were studied. A total of 13 inhibitors were in patients > 10 years of age. Total patients > 10 years not reported, but the rate was definitely < 0.002.
Patients of all severities. 95% confidence interval for ≥ 15 was 0.0017-0.0023. Rate was 0.0052 in severe patients 5-14 years of age and 0.0038 in those ≥ 15 years of age.
Patients of all severities with negative titer before and at enrollment in UDC. All had > 50 ED. Two additional transient inhibitor were reported; 6 of 7 inhibitors were low titer.
Severe patients only. Of the 106 inhibitors in patients 10-49 years of age, 54 were high and 28 were low titer. Of the 11 in patients 50-59 years of age, 9 were high and 9 were low titer.
Interestingly, the incidence and cumulative rates observed over time have progressively increased by 3.5 times, from 0.0015 to 0.0053.5,7,55 This effect might be a spurious one because of increased awareness and more accurate and frequent inhibitor testing; it might, alternatively, reflect more the widespread use of prophylaxis, greater factor consumption, and more frequent switching; it might finally parallel the temporal trends toward more frequent allergic and autoimmune disorders.
Published evidence about inhibitors related to product switching
What evidence do we have for patients who underwent switch and then continued follow-up for inhibitor development? There are 2 reports about the outbreaks of inhibitors in the 1990s,12,37,57,58 2 reports from Canada,59,60 1 from the United Kingdom,61 2 from Ireland62,68 and some others64,65 (Table 5).66 The single robust conclusion is that there is no clear signal of increase in inhibitor development when switching to and from the currently available factor concentrates. If any minor effect is present, this cannot be superior to a fraction of the overall 2-3 per 1000 patient/years rate, and no clustering of inhibitors soon after the switch has been reported. On the contrary, the Canadian experience showed how much preexisting, locally undiagnosed inhibitors (7.9% on centrally retested samples) may confound the assessment of the effect of switching from plasma to recombinant FVIII.59,60 Recently, 2 large populations of nearly 600 severe PTPs (a much larger sample size than in any registration trial; Table 2) switched mostly to a B-domain–deleted molecule as a result of national contracting in the United Kingdom and Australia. Patients who do not switch are followed up in parallel and will provide a valid comparator, the absence of which is the first criticism raised67,68 to the recent meta-analysis suggesting that B-domain–deleted FVIII is more immunogenic than the native full-length molecule,16 the second being that most of the available evidence falls in the category of the uncontrolled case series, by far the weakest study design.
Estimates of the risk of inhibitors development after product switch
| Year . | Author/reference . | Design . | Sample . | Follow-up, mo . | Inhibitors . | Rate, × 1000 patient/y . | Notes . |
|---|---|---|---|---|---|---|---|
| 1988 | Giles et al59 * | Prospective | 478 | 12 | 18 | 0.019 | |
| 339 | 24 | 17 | 0.030 | ||||
| 2007 | Singleton et al63 † | Retrospective | 94 | ≤ 20 | 4 | 0.042 | All patients |
| 77 | ≤ 20 | 1 | 0.013 | (−) history | |||
| 2007 | Gouw et al66 ‡ | Retrospective | 316 | (> 50 ED) | NR | ||
| 2008 | Rubinger60 § | Prospective | 225 | 12 | 0 | 0 | |
| 189 | 24 | 0 | 0 | ||||
| 2009 | Rea et al61 ‖ | Retrospective | 33 | > 3 | 1 | 0.033 | |
| 2011 | Siegmund et al65 ¶ | Retrospective# | 118 | NA | 0 | ||
| 2011 | Bacon et al62 # | Retrospective | 113£ | Up to > 100 ED | 1 | 0.009 |
| Year . | Author/reference . | Design . | Sample . | Follow-up, mo . | Inhibitors . | Rate, × 1000 patient/y . | Notes . |
|---|---|---|---|---|---|---|---|
| 1988 | Giles et al59 * | Prospective | 478 | 12 | 18 | 0.019 | |
| 339 | 24 | 17 | 0.030 | ||||
| 2007 | Singleton et al63 † | Retrospective | 94 | ≤ 20 | 4 | 0.042 | All patients |
| 77 | ≤ 20 | 1 | 0.013 | (−) history | |||
| 2007 | Gouw et al66 ‡ | Retrospective | 316 | (> 50 ED) | NR | ||
| 2008 | Rubinger60 § | Prospective | 225 | 12 | 0 | 0 | |
| 189 | 24 | 0 | 0 | ||||
| 2009 | Rea et al61 ‖ | Retrospective | 33 | > 3 | 1 | 0.033 | |
| 2011 | Siegmund et al65 ¶ | Retrospective# | 118 | NA | 0 | ||
| 2011 | Bacon et al62 # | Retrospective | 113£ | Up to > 100 ED | 1 | 0.009 |
BDD indicates B-domain deleted; ED, exposure day; NR, not reported; PTP, previously treated patients; and NA, not available.
Rate of inhibitor positivity before switch was 0.079. When these patients were not excluded, rates were 0.038 at 12 months and 0.050 at 24 months.
A total of 17 patients had history of previous inhibitors, of which 3 of developed a recurrence. All 4 inhibitors were transient, the only 1 de novo was secondary to surgery. At study completion, 51 patients had > 100 ED, and 24 had 20-100 ED.
The study enrolled PTPs and reported the RR for inhibitor development in 54 patients switching versus those not switching. The adjusted RR was 0.9 (95% confidence interval 0.5-1.6).
A total of 274 patients were tested at baseline, of which 4 were positive (0.014).
Cases observed over 8 years and switched from full-length to BDD factor VIII, the observed inhibitor was transient and secondary to surgery.
The cases were observed over 14 years, and switched from plasma-derived to recombinant factor VIII. A total of 101 patients had severe disease.
The observed inhibitor was in a PTP. No recurrent inhibitors were observed after switching 16 patients with a positive inhibitor history.
A joint immunology and epidemiology perspective to address some relevant clinical questions
Why do inhibitors develop in PTPs? We do not actually know. The mechanistic understanding of this occurrence is limited. The event is very rare, ie, in the order of 2-3 per 1000 patient/years. As already noted, even with large transnational (pooled) datasets, it would be almost impossible to perform a multivariable analysis powerful enough to identify risk factors for inhibitor development.67,69 Actually, in a prospective uncontrolled cohort, to detect a 2-fold increase in the rate measured by Hay (Table 2) with a power of at least 80% and an α level of 0.05, we would need 2589 patients, whereas the power of detecting the same increase in a registration trial of 100 patients would be 2%; an observational controlled study would require 5065 patients per arm.
Is it likely that the same risk factors that play a role in PUPs also do so in PTPs? The answer is also unknown, but it is likely that the gene mutation and the family history of inhibitor likely do not, whereas the occurrence of a trigger event probably does (by acting on the adaptive immune system and the related tolerance mechanisms), the genetic asset of the immune system possibly does.
When dealing with PTPs, how long after switching is the new product responsible for the inhibitor development? And, for PTPs, do we measure exposure in terms of calendar days or EDs? Are all EDs the same? Do the number of days in which the exposure was consistently high or, rather, crossing a given boundary of dose/time matter? We are not aware of any useful evidence to answer these questions in PTPs. We must possibly abandon our propensity to count all the inhibitors occurring after a switch as a consequence of the switching, independently of the time elapsed after switching. Ideally, a timeframe of 4-6 weeks could be considered to be reasonable from an immunologic point of view (provided that a patient is at least minimally treated during that period, which is very likely for patients on prophylaxis). In the multicausality framework discussed previously, the role for switch is very likely to wane off quickly, and other risk components might become more relevant.
What about the role for the specific factor concentrate? Ideally, if a patient is thought to have developed an inhibitor because of the switch to a new source of FVIII, which is deemed immunologically different from the one to which the patient was tolerized, we should expect that this specific inhibitor would minimally cross-react and this would possibly allow the return to the previous concentrate. Unfortunately, there are not many supportive data to this concept, and we cannot exclude that a new protein can cause loss of the tolerance to a cross-reacting one. The issue is not without practical interest. If in a hypothetical country all patients on product A were switched to product B, could the patients be safely switched back to product A at a later time? As far as we know, the answer is yes, but the evidence is minimal.61
Do we expect in PTPs the same relative distribution of high- and low-responding inhibitors found in PUPs? We can reasonably answer “no” to this important question: it must be appreciated that even after the outbreak of inhibitors caused in the 1990s in The Netherlands and in Belgium, the majority of the inhibitors detected were transient.12,43,57,58
Discussion
Perhaps the need for adopting more efficient clinical trial designs when investigating alternative, enhanced, or advanced treatment options is insufficiently recognized by the community of hemophilia scientists. On proposing the use of a PTP population to assess the immunogenicity potential of new factor concentrates,20 it was assumed that the inhibitor rate from historical controls should be taken as the most practical and economic estimate of the baseline risk of inhibitors. In the absence of a parallel group of not switching control patients, the concept of baseline risk of inhibitor development has then slowly turned into a sort of “unavoidable” minimum risk of inhibitors associated with switching, understating the role for multicausality of inhibitor development. One possible explanation why not having a control group, today as well as in the past, is the inappropriate extension of the concept that introducing an untreated control group is unnecessary, if not unethical, when assessing the efficacy of substitution therapies. In this specific case, indeed the goal is showing that functionality of the defective mechanism is restored, and this can be accomplished by showing that a drug's pharmacokinetic profile is similar to that of the native protein, that the drug is effective in arresting ongoing bleeding or in preventing surgical bleeding. This assumption is legitimate even by the most rigorous evidence-based medicine, and it may represent a condition whereby the principles of economics and ethics have resulted in unnecessary randomized trials.70,71 This concept, however, has erroneously been extended to different situations, such as assessing safety (in terms of adverse event rates, like inhibitors) or comparative effectiveness (as in comparing 2 different regimens or 2 different formulations). Under these circumstances, randomized controlled trials, the highest-level study design—intended to minimize confounding and bias—are needed, much like they are for any other drug trials.
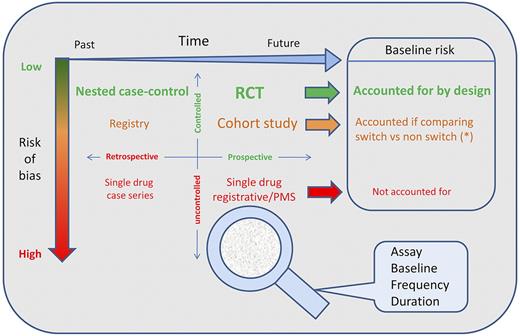
The proper methodology to address the issue of the comparative immunogenicity of different products and or associated with switching could and can be addressed with a more powerful and tailored approach (Figure 1). As it has been discussed, we cannot rely on uncontrolled cohorts or their univariate meta-analysis67 ; on the contrary, we need to assess the baseline risk, we need to take into account the attributable risk fraction, and we need to have control groups as unconfounded as possible.23,67 These steps can be accomplished in a retrospective fashion with large-sized rigorous nested-case control studies embedded in prospective registries, or with large perspective controlled observations. The European Hemophilia Surveillance Scheme (EUHASS) project72 is an example of the latter. The National Institutes of Health inhibitor study73 is an example of the former.
Knowledge framework to study and appraise the immunogenicity related to switch. The figure classifies the designs for the studies that can provide evidence about the immunogenicity of switching. The x-axis represents time (flowing left to right). The y-axis represents risk of bias, from low (top, in green) to high (bottom, in red). The space results partitioned into 4 quadrants by combination of study perspective (retrospective/prospective) and rigor of observation (controlled/uncontrolled). The panel on the right hand side describes how the baseline risk for inhibitors is accounted for. Randomization is the main mechanism to reduce risk of bias and control for the baseline risk of events. Multivariable analysis might be a good adjunct if size permits, and it is optimal for nested case control studies, which sum up more events by design). The risk of bias in registries and cohort studies lies in the nonrandomized assignment to “active” or control group and in incomplete follow up data. Complete data collections are indeed more powerful then nested case control studies, but are rarely available. The control group for prospective studies might be historical (studies across the switch, prone to secular variation in rate of events) or parallel. An ongoing example of prospective parallel controlled cohort is the EUHASS study. For each and any of the study designs, a much better insight might be obtained using as a “magnifier glass” a proper combination of baseline assessment for preexisting inhibitors; standardized assay methodology; observation time frame; and testing frequency.
Knowledge framework to study and appraise the immunogenicity related to switch. The figure classifies the designs for the studies that can provide evidence about the immunogenicity of switching. The x-axis represents time (flowing left to right). The y-axis represents risk of bias, from low (top, in green) to high (bottom, in red). The space results partitioned into 4 quadrants by combination of study perspective (retrospective/prospective) and rigor of observation (controlled/uncontrolled). The panel on the right hand side describes how the baseline risk for inhibitors is accounted for. Randomization is the main mechanism to reduce risk of bias and control for the baseline risk of events. Multivariable analysis might be a good adjunct if size permits, and it is optimal for nested case control studies, which sum up more events by design). The risk of bias in registries and cohort studies lies in the nonrandomized assignment to “active” or control group and in incomplete follow up data. Complete data collections are indeed more powerful then nested case control studies, but are rarely available. The control group for prospective studies might be historical (studies across the switch, prone to secular variation in rate of events) or parallel. An ongoing example of prospective parallel controlled cohort is the EUHASS study. For each and any of the study designs, a much better insight might be obtained using as a “magnifier glass” a proper combination of baseline assessment for preexisting inhibitors; standardized assay methodology; observation time frame; and testing frequency.
Although the newly released European Medicines Agency Committee for Medicinal Products for Human Use guidelines on investigations of FVIII and FIX products21,22 opportunely give the sponsor the duty to collect, in a standardized fashion, evidence for the immunogenic potential of the new molecule in PTP switching from a previous molecule, they continue to ask for uncontrolled case series (ie, the weakest design in the hierarchy of evidence), underpowered to properly assess the risk of inhibitor development. This would indeed be possible in more meaningful postregistration trials, in which the relative cost could be shared between the society (providing the factor for the control group as per standard practice) and the company producing the new drug (providing the drug for the experimental arm and covering the expenses for data collection in both arms). Alternatively, it could be accomplished by integrating a similar mandatory data collections in large pharmacovigilance programs, allowing meaningful comparisons among different brands and between patients switching or continuing on older products. Another perspective to foster research in the field is the science of small clinical trials, aiming at developing more powerful study designs to be used in the assessment of treatments for rare diseases.74 These 2 perspectives are complementary and synergistic, and combining the effort of sponsors and treaters in potentiating and enhancing the quality of data collection and evidence production is mandatory. Hierarchical Bayesian appraisal and multivariable analysis will then shed much brighter light on our knowledge about inhibitor rates.49,70,75
Clinical implications
We consider that there is no good evidence to suggest that switching factor concentrate in PTPs will have any significant effect on the development of clinically relevant inhibitors. Wherever a switch presents added value for the patient or the society, this should be seriously considered and safely adopted.
Of course, treaters and patients should be aware that absence of evidence for a risk of inhibitor is not the same as evidence of no risk; we can reasonably exclude that switching can be cause of a large risk of developing an inhibitor, on the basis of the uncontrolled observation of several thousands of switches that did not raise any major concerns; however, we should not lose the opportunity to perform a prospective standardized monitoring of future switches to corroborate this finding and build around our estimates progressively stricter confidence (or more opportunely “credibility”) intervals. We recommend that all hemophilia centers and countries planning to switch patients to new FVIII concentrates enroll both switching and nonswitching patients in registries, test for inhibitors prospectively immediately before the switch and at a minimum for at least 1-2 months after the switch,54 and formally report their data either singly or in collaboration.
Authorship
Contribution: All authors drafted the first version of different sections of the manuscript and all critically revised the final manuscript.
Conflict-of-interest disclosure: A.I. has served on the speaker's bureau of Bayer, Biogen Idec, Novo Nordisk, and Pfizer. He has served on the advisory boards of Novo Nordisk and Pfizer. He has received research funds from Biogen Idec, Novo Nordisk, and Pfizer. M.M. has served on the speaker's bureau for Baxter, Biotest, Bayer, CSL Behring, Novo Nordisk, Pfizer, and SOBI. He has been a consultant to CSL Behring and has served on the advisory boards of Baxter, CSL Behring, and Novo Nordisk. M.M. is the project leader for the EUHASS registry, which receives funding from Baxter, Bayer, Biotest, CSL Behring, Grifols, LFB, Novo Nordisk, Octapharma, and Pfizer. The remaining author declares no competing financial interests.
Correspondence: Mike Makris, Department of Cardiovascular Science, University of Sheffield, Royal Hallamshire Hospital, Sheffield S10 2JF, United Kingdom; e-mail: m.makris@sheffield.ac.uk.